Abstract
Objective(s):
Neuroprotective effect of creatine (Cr) against β-amyloid (Aβ) is reported in an in vitro study. This study investigated the effect of Cr supplementation on β-amyloid toxicity in vivo.
Materials and Methods:
Thirty two, male Wistar rats were divided into 4 groups. During ten weeks of study, control group went through no surgical or dietary intervention. At the 4th week of study Sham group had a hippocampal normal saline injection, while Aβ and AβCr groups had an β-amyloid injection in the hippocampus. AβCr group were fed by Cr diet during the study. After 10 weeks, Morris water maze (MWM) test was administered to measure learning ability and memory retrieval. Animals were sacrificed for TUNEL anti apoptotic assay and staining of amyloid plaques by Thioflavin-T.
Results:
There was a significant retention deficit among AβCr and Aβ group while the escape latency and the distance traveled to the platform were significantly higher in AβCr group compared to Aβ group. AβCr group had same percent of TUNEL positive neurons compared to Aβ group.
Conclusion:
Cr supplementation before and after β-amyloid injection into the CA1 area of hippocampus deteriorates the learning and memory impairment of rats and it does not protect neuronal apoptosis caused by β-amyloid.
Keywords: Alzheimer, Apoptosis, Creatine supplementation, Learning, Memory
Introduction
Creatine (Cr) is endogenously produced from the biosynthesis of glycine, arginine, and methionine in kidneys, liver, pancreas, and possibly the brain. It can also be obtained from high-protein foods (1). Nowadays, Cr dietary supplement is widely used by athletes to enhance their physical performance (2). There are pieces of evidence on the protective effects of Cr on muscles, heart (3), nervous system, and brain function (4).
Elevated Cr level in human and rodents’ brain have been reported following oral supplementation of Cr (5-7). There are also studies on changes in serotonin and dopamine regulators of athletes’ brain (8) and in depression-like behavior of rodents after a period of Cr supplementation (1). Some human studies have confirmed better cognitive performance following the supplementation of Cr in adequate doses (4, 9-11). According to the literature, oral Cr supplementation may impose neuroprotective effects on experimental animal models of Huntington’s disease (6, 12, 13).
Alzheimer’s disease (AD) is the main cause of dementia in elderly (14). Two core features of the AD are neurofibrillar tangles, which are intracellular aggregates of hyperphosphorylated tau protein, leading to neuronal malfunction (15, 16), and amyloid plaques, which are heterogenous aggregates found in extra-cellular space mainly containing β-amyloid (Aβ) peptide (17). Accumulation of amyloid inside neurons may contribute to the development or exacerbation of the AD (18). Cr is responsible for energy homeostasis in neurons and can protect the neurons exposed to neurotoxins by promoting their energy levels. It may be the probable mechanism behind neuroprotective role of Cr against the toxic effects of β-amyloid peptide in cell culture of hippocampal neurons (19). Furthermore, the discovery of Cr deposits in the brain of transgenic AD mice and in the hippocampus of AD patients indicates an association between cellular energy levels, β-amyloid, Cr metabolism, and AD (20). In this respect it may be speculated whether Cr supplementation at an early time point of the disease can prevent or delay the course of AD-related neurodegeneration (21).
Despite the numerous efforts and studies conducted in the field, there is no clinical or in vivo study to provide adequate proofs about the neuroprotective effects of Cr supplementation on the AD. Considering the neuropro-tective effect of Cr against β-amyloid toxicity in vitro and positive effects of Cr supplementation on improving the cognitive performance in healthy individuals, the present study was performed to investigate the effects of oral supplementation of Cr on β-amyloid toxicity in vivo.
Materials and Methods
Study design
Thirty two male Wistar rats (4 month of age and weighting of 200-250 g) were obtained from Pasteur Institute of Iran, Research, Production and Education centre, (Tehran, Iran). They were caged at a constant temperature of 21±1 °C with controlled 12 hr/12 hr light-dark cycles, and ad libitum access to food and water. The rats were given at least one week to habituate to the facilities, and then experimental procedures began. All the guidelines of the Committee of Care in the Use of Experimental Animals were followed, and the study procedures were approved by the Ethics Committee of Tehran University of Medical Sciences. Efforts were made to minimize animal suffering and reduce the number of animals used.
The animals were divided into four groups (n=8 per group): control, sham, Aβ (β-amyloid injection, no Cr supplementation), and AβCr (β-amyloid injection, Cr supplementation). The control group had no surgical or dietary intervention during the study. AβCr group received Cr monohydrate powder mixed in their chow (2% Cr/diet) and other groups received normal chow diet for four weeks. Then animals in the sham, Aβ, and AβCr groups underwent stereotaxic surgery. Those in the Aβ and AβCr groups were given a bilateral β-amyloid peptide injection in the CA1 hippocampus (0.5 µg/µl), while the sham group received a normal saline injection in the same area of the brain. After surgery, the AβCr group continued receiving 2% Cr diet while the other groups maintained their normal chow diet (Figure 1).
Figure 1.
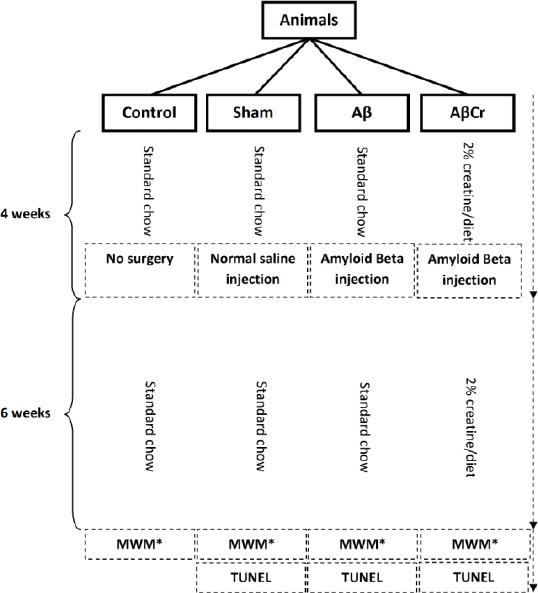
Study design
Aβ: β-Amyloid; Cr: Creatine
*Morris Water Maze
Surgery procedure
Human β-amyoid peptide 1-42 (Sigma-Aldrich, USA) was solved in 0.1 M phosphate buffer saline (PBS; pH=7.4) and then aliquoted and stored at -70 °C until use. Every aliquot was incubated at room temperature for 48 hr before injection. The animals were anesthe-tized with intra peritoneal ketamine (100 mg/kg) and xylazine (10 mg/kg) and then injected bilaterally under stereotaxic Conditions with β-amyloid or normal saline into the CA1 hippocampus (AP=3.9 mm, LR=2.2 mm, D=2.7 mm). Injections were performed at a rate of 0.5 µl/min using a Hamilton syringe attached to the stereotax apparatus. Four microliters of β-amyloid (0.5 µg/µl) solution was injected in every hippocampus, and the needle was kept in place for one min after injection before being slowly retracted (Figure 1).
Supplementation
Two percent of Cr (Sigma-Aldrich, USA) was mixed into the normal chow using an electric mill according the previous studies (6, 12, 13). After adding some water and cutting the paste, the Cr and chow mixture were being dried in a 30-45 min exposure to a continuous warm air stream. Animals in the AβCr group had freely access to this mixed food (Figure 1).
Morris Water Maze (MWM) Apparatus, Habituation and Procedure
The Morris water maze procedure was performed 6 weeks after the β-amyloid injection. Water maze consisted of a pool (155 cm in diameter) filled with water (21±1°C) to a level 10 cm from the edge of the tank. A transparent Plexiglas platform (10 cm diameter) was located 1.5 cm below the surface in the eastern quadrant of the tank (target quadrant). Climbing onto the platform was the only way to escape from water. The walls surrounding the pool were decorated with distinct extra maze spatial cues, which were kept in fixed positions during the entire experiment to allow the animals to find the hidden platform. The animals’ movements were recorded by a CCD camera (Panasonic Inc, Japan) hanging from the ceiling above the MWM apparatus, and locomotion tracking was measured using Ethovision software (version XT7, the Netherlands), a video tracking system for automated analysis of animal behavior.
Twenty-four hr before starting the hidden platform training, rats were given 60 sec to swim in the tank without the platform in order to adapt to the environment.
The reference spatial learning and memory tests were carried out based on the procedure previously conducted in our laboratory with some modifications in training; the platform was submerged in the eastern quadrant of the pool. Its place remained unchanged throughout training, but animals were released into the water (while facing the tank wall) from different locations chosen randomly from the south, north, west, northwest and southwest. Rats were given one training session per day for three consecutive days. Each session consisted of four trials with 1-min inter-trial intervals. The animals were allowed to swim for 60 sec to localize the platform’s position in the tank. The rats which did not find the platform within 60 sec, were directed into it and were allowed to rest on it for 10 sec. Twenty-four hr after the third session; the spatial probe test was performed. During this test, the platform was removed, and rats were allowed to swim for 60 sec before getting removed. Animals were released into the water at a location near the platform. Behavior was recorded with a video tracking system. Escape latencies, time spent in target quadrant, and swim speed were wholly recorded for subsequent analysis.
Histological tests
The rats were anesthetized and their brains were fixed through a transcardial perfusion (normal saline and 4% paraformaldehyde solution) within 24 hr after the spatial probe test. After that, the brains were kept in a 4% paraformaldehyde solution. In preparation for the histological staining, the brains were embedded with paraffin, cut into consecutive 5 µm transverse sections by a microtome, and placed on the poly-D-lysine-coated glass slides. Alternate sections were used for Thioflavin-T and TUNEL staining. In order to detect β-amyloid plaques in brain sections Thioflavin-T (Sigma-Aldrich, USA) was used for staining. Brain sections were deparaffinized in xylene, rehydrated, covered with Thioflavin-T solution (0.5% Thioflavin-T in 0.1N HCl), and incubated in dark room at room temperature for 10 min. Slides were immersed in distilled water twice before being observed through a fluorescent microscope.
In order to detect apoptotic neurons in brain sections, the TUNEL staining protocol was performed using TUNEL Apoptosis Detection Kit, (Millipore, UK). Brain sections were deparaffinized in xylene, rehydrated, immersed in PBS, and then incubated at 37 °C for 30 min. Out of PBS, sections were covered with diluted protein kinase and incubated at 37 °C for 20 min. To halt protein kinase activity, sections were washed in PBS solution (4×2 min). Each brain section was covered with 50 µl TdT (Terminal deoxynucleotidyl transferase) buffer for 10 min. The TdT buffer was removed using a sampler, and then each section was covered with 50 µl TdT mixture solution (90% TdT buffer, 5% dUTP-biotin, 5% TdT) and incubated for 60 min at 37 °C. Slides were washed for 5 min by immersing in TB buffer at room temperature and then dipping in PBS (4×2 min). After the washing process, sections were covered with 50 µl of blocking solution and incubated for 20 min at room temperature. After removing the blocking buffer with a sampler, 50 µl Avidin-FTC mixture (1 part Avidin FTC, 9 parts blocking solution) was used for each section, and slides were incubated at 37 °C for 30 min in a dark room. To obtain the mean percentage of apoptotic neurons in the hippocampus area, 3 brain sections were chosen randomly from each subject and the number of TUNEL positive and negative cells was counted in three adjacent 400X microscopic fields.
Statistical analysis
The data were presented as mean±SE and processed by the Statistical Package for Social Sciences (version 16.0; SPSS Inc., Chicago, Illinois, USA). Mean escape latency, distance traveled to the platform, time spent in target quadrant in MWM test and also mean percentage of apoptotic cells were analyzed. All the groups were compared using one-way ANOVA test. Comparing sham and Aβ and also Aβ and AβCr was carried out using one-way ANOVA, least significant difference (LSD) post hoc comparison. P-value<0.05 was considered as significance level for all comparisons. Study design is presented in Figure 1.
Results
According to the obtained results, β-amyloid plaques were found in the brain sections of the animals in either AβCr or Aβ groups, except for the sham-operated group and the control group (Figure 2).
Figure 2.
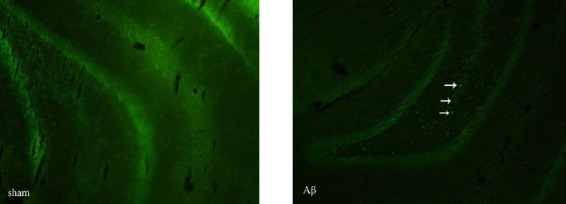
Thioflavin-T staining of amyloid plaques. Arrows show the amyloid plaques in the brain section derived from β-amyloid injected (without creatine supplementation) rats. No amyloid plaque is visible in the brain section of the rat from sham group. Obiective × 10
Although the rats in Aβ group had higher mean escape latency than the sham (27.9±0.53 vs 20.9±0.99, P=0.003) and control (27.9±0.53 vs 17.25±1.19, P=0.0001) groups, however, they showed better performance compared to the AβCr group (27.9±0.53 vs 33.22±1.56, P= 0.009). Overall, the results indicated learning impairment in both AβCr and Aβ groups, but, Cr supplementation imposed a deterioration effect on learning function of rats after β-amyloid injection (Figure 3). This was confirmed by comparing the average distance traveled by the rats to find the platform in training trials (Figure 4).
Figure 3.
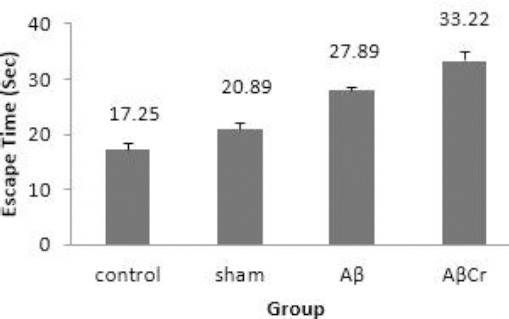
Mean escape latency during three days of training in study groups
Aβ: Aβ injection, no creatine supplementation. AβCr: Aβ injection, creatine supplementation before and after Aβ injection. Sham: Sham surgery with no creatine supplementation. Control: Control group with no surgical and nutritional intervention; Results are presented as mean±SE
Figure 4.
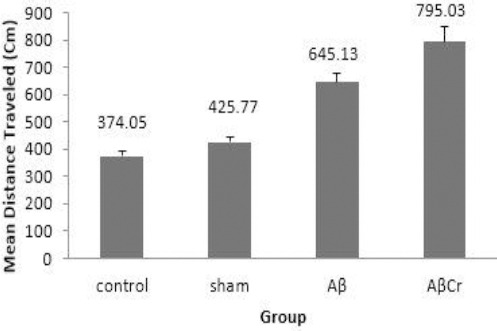
Mean distance traveled to the platform during three days of training in study groups
Aβ: Aβ injection, no creatine supplementation. AβCr: Aβ injection, creatine supplementation before and after Aβ injection. Sham: Sham surgery with no creatine supplementation. Control: Control group with no surgical and nutritional intervention; Results are presented as mean±SE Comparing Sham & Control groups (P=0.444), Sham & Aβ groups (P=0.003) and Aβ & AβCr groups (P=0.019) was carried out using one-way ANOVA, least significant difference (LSD) post hoc comparison
During the probe test, animals in Aβ group spent less time in the target quadrant (31.99±0.78) than those in the sham (35.9±0.91) and control (33.57±2.58) groups but these differences were not statistically significant (P=0.16 & P=0.56 respectively). However, AβCr group members spent less time in the target quadrant (27.67±1.55) compared to sham group (P=0.003) and the Aβ group, but the difference between Aβ and AβCr groups did not reach statistical significance (P=0.083). This reflects the negative effect of oral Cr supplementation on memory retrieval ability of rats after β-amyloid injection (Figure 5). As mentioned earlier, the TUNEL staining protocol was performed to detect apoptotic neurons in the brain sections (Figure 6). As the Figure suggests, the brain sections of the rats in the AβCr and Aβ groups contained more TUNEL-positive neurons than those in the sham group. The percentage of TUNEL-positive neurons counted in the brain sections of the rats in the Aβ (24.00±4.45 percent) and AβCr (25.33±5.04 percent) groups was significantly higher than those in the sham (4.83±1.33 percent) group (P=0.001 & P=0.001 respectively). Comparing to the Aβ group, the mean percentage of TUNEL-positive neurons was not significantly different in the AβCr group (P=0.799). Based on the foregoing, Cr supplementation had no influence on apoptotic effects of β-amyloid in neurons.
Figure 5.
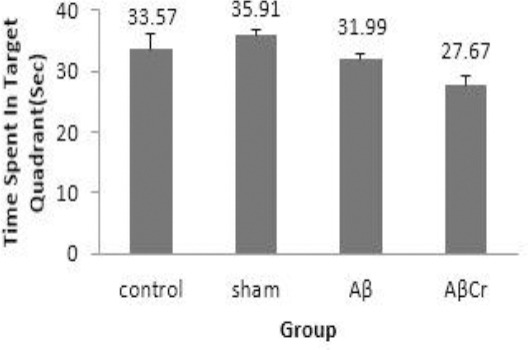
Mean time spent in target quadrant in probe test in different groups
Aβ: Aβ injection, no creatine supplementation. AβCr: Aβ injection, creatine supplementation before and after Aβ injection. Sham: Sham surgery with no creatine supplementation. Control: Control group with no surgical and nutritional intervention; Results are presented as mean±SE
Figure 6.
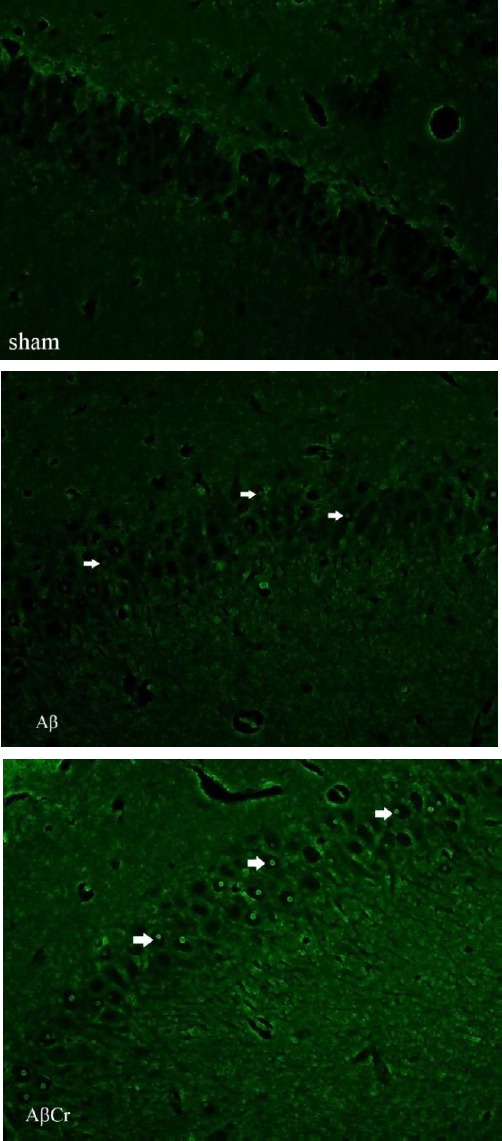
TUNEL staining. Arrows show the TUNEL positive neurons in the picture of the brain section
β-amyloid injected rats (Aβ & AβCr groups) have more TUNEL positive neurons compared to the rats in the sham group. Objective × 40
Aβ: β-amyloid injection, no creatine supplementation. AβCr: β-amyloid injection, creatine supplementation before and after β-amyloid injection. Sham: Sham surgery with no creatine supplementation
Discussion
The results of this study revealed a deficit in rats’ ability to orientate in the Morris Water Maze following a single injection of β-amyloid 1-42 in the hippocampal CA1 area. Many studies have examined the behavioral consequences of injecting or infusing different β-amyloid fragments in various brain areas of rodents.
Despite few studies that have found no behavioral changes after a low dose single injection of β-amyloid (22), the results of the present study revealed that a single 2 µg/side hipocampal CA1 area injection of prefibrillar β-amyloid 1-42 (incubated for 48 hr in room temperature) (23), could induce spatial short-term memory impairment, when tested six weeks after the injection.
Due to limited permeability of the blood-brain barrier to Cr, the increase of brain Cr followed by oral supplementation would be a slow process (21). For better metabolic support at the time of injection, supplementation of Cr was started 4 weeks before the β-amyloid injection and continued up to six weeks. Contrary to previous studies on healthy individuals, the results of the present study revealed negative effects of Cr supplementation on learning and memory retrieval (4, 9-11). Furthermore, the TUNEL-positive neurons counted in the brain sections showed no protective effect of Cr. This contrasts with the results of those studies that emphasize on protective effects of Cr in cell culture (19).
Given the same rate of TUNEL-positive neurons in either group, more severe memory impairment in the group received Cr can be for any reason; a temporary inflammation or non-apoptotic death of neurons. Although the final judgment in this case cannot be achieved based solely on the results of this research, however, by putting together the results of this study and previous literature, a mechanism could be expected for this phenomenon.
Gallant et al detected soluble Cr deposits in autopsy of transgenic mice and AD brain sections (20). In an earlier study on these TgCRND8 mice, the inflammation and microglial activation of their brain was reported by Wyss and Kaddurah-Daouk (21). Gallant et al attributed this inflammation to the detected Cr deposits that might be formed due to the low energy levels of neurons and declined creatine kinase (CK) activity (20). In the present study, CK activity was not measured, which may be one of the constraints of this research. However, there is some evidence to suggest that, β-amyloid promotes protein oxidation and causes irreversible inhibition of brain CK (BB-CK) at concentrations toxic to cultured neurons. This evidence is in line with the findings of Yatin and his colleagues in 2000 on modification of BB-CK in AD (24-26).
So, it would not be irrational to conclude that the activity of BB-CK may be declined in Aβ rats and in consequent; more Cr supply (AβCr) caused more Cr deposition in the neurons, resulting in more inflammation and/or cell death.
Similar studies are needed to determine the relationship between declined BB-CK activity, Cr supply of the neurons, and Cr deposition in neurons based on the measurement results of BB-CK activity and Cr deposits after β-amyloid injection.
Conclusion
Oral Cr supplementation in rats, before and after β-amyloid injection into the CA1 area of hippocampus, can deteriorate learning and memory impairment and does not protect neuronal apoptosis induced by β-amyloid. However, further research is needed to reveal more details.
Acknowledgment
The results described in this paper were part of student thesis. The authors are grateful for the grant by Tehran University of Medical Sciences, Vice Chancellor for Research, Tehran, Iran. Also, we are thankful to Professor Fereshteh Motamedi for her helpful cooperation in this study.
References
- 1.Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology. 2009;35:534–546. doi: 10.1038/npp.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarnopolsky MA. Caffeine and creatine use in sport. Ann Nutr Metab. 2010;57:1–8. doi: 10.1159/000322696. [DOI] [PubMed] [Google Scholar]
- 3.Poortmans JR, Francaux M. Adverse effects of creatine supplementation: fact or fiction? Sports Med. 2000;30:155–170. doi: 10.2165/00007256-200030030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res. 2002;42:279–285. doi: 10.1016/s0168-0102(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 5.Dechent P, Pouwels PJW, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am J Physiol Regul Integr Comp Physiol. 1999;277:R698–R704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- 6.Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF, et al. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. J Neurochem. 2003;85:1359–1367. doi: 10.1046/j.1471-4159.2003.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan JW, Takahashi K. Cerebral energetic effects of creatine supplementation in humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1745–R1750. doi: 10.1152/ajpregu.00717.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjicharalambous M, Kilduff L, Pitsiladis Y. Brain serotonin and dopamine modulators, perceptual responses and endurance performance during exercise in the heat following creatine supplementation. J Int Soc Sports Nutr. 2008;5:14. doi: 10.1186/1550-2783-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychology, development, and cognition Section B. Aging Neuropsychol Cogn. 2007;14:517–528. doi: 10.1080/13825580600788100. [DOI] [PubMed] [Google Scholar]
- 10.Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double–blind, placebo–controlled, cross–over trial. Proc Biol Sci. 2003;270:2147–2150. doi: 10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawson ES, Lieberman HR, Walsh TM, Zuber SM, Harhart JM, Matthews TC. Creatine supplementation does not improve cognitive function in young adults. Physiol Behav. 2008;95:130–134. doi: 10.1016/j.physbeh.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, et al. Neuroprotective effects of creatine in a transgenic mouse model of huntington’s disease. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, et al. Neuroprotective Effects of creatine and cyclocreatine in animal models of huntington’s disease. J Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pluta R. Unresolved questions concerning etiology of Alzheimer’s disease: Hypometabolism. Nutr. 2011;27:1–2. doi: 10.1016/j.nut.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem. 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed A, Posse de Chaves E. AβInternalization by Neurons and Glia. Int J Alzheimers Dis. 2011;2011:17. doi: 10.4061/2011/127984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry RD, Katzman R. Senile dementia of the Alzheimer type. Ann Neurol. 1983;14:497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- 19.Brewer GJ, Wallimann TW. Protective effect of the energy precursor creatine against toxicity of glutamate and β-amyloid in rat hippocampal neurons. J Neurochem. 2000;74:1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- 20.Gallant M, Rak M, Szeghalmi A, Del Bigio M, Westaway D, Yang J, et al. Focally elevated creatine detected in amyloid precursor protein (APP) transgenic mice and alzheimer disease brain tissue. J Biol Chem. 2006;281:39576. doi: 10.1074/jbc.C500244200. end of article. [DOI] [PubMed] [Google Scholar]
- 21.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 22.Chambon C, Wegener N, Gravius A, Danysz W. Behavioural and cellular effects of exogenous amyloid-βpeptides in rodents. Behav Brain Res. 2011;225:623–641. doi: 10.1016/j.bbr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Tamagno E, Bardini P, Guglielmotto M, Danni O, Tabaton M. The various aggregation states of β-amyloid 1–42 mediate different effects on oxidative stress, neurodegeneration, and BACE-1 expression. Free Radic Biol Med. 2006;41:202–212. doi: 10.1016/j.freeradbiomed.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. J Neurochem. 2000;74:2520–2527. doi: 10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- 25.Hensley K, Carney J, Mattson M, Aksenova M, Harris M, Wu J, et al. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yatin SM, Aksenov M, Butterfield DA. The antioxidant vitamin E modulates amyloid β-peptide-induced creatine kinase activity inhibition and increased protein oxidation: implications for the free radical hypothesis of alzheimer’s disease. Neurochem Res. 1999;24:427–435. doi: 10.1023/a:1020997903147. [DOI] [PubMed] [Google Scholar]


