Abstract
Objective(s):
The study aimed to investigate the effects of resveratrol on colorectal cancer HCT116 cells, including cell viability, apoptosis, and migration, and the partial mechanisms focused on hedgehog/gli-1 signaling pathways.
Materials and Methods:
We chose the appropriate time and concentration of recombinant human Sonic hedgehog (Shh) stimulation by cell viability. The proportion of cell apoptosis was detected by flow cytometry; HCT116 cell migration was measured by scratch test; the expression of Ptch, Smo, and Gli-1 was measured by Western blot analysis.
Results:
Shh signaling increased HCT116 cell viability and migration, inhibited cell apoptosis, and upregulated the expression of Ptch, Smo, and Gli-1. Resveratrol obviously inhibited HCT116 cell viability and migration, promoted cell apoptosis, and suppressed the protein of Ptch, Smo, and Gli-1. Furthermore, the effects of resveratrol and Shh on human colorectal cancer HCT116 cells were in a dose- and time-dependent manner.
Conclusion:
The inhibitory effect of resveratrol on HCT116 cells may be mediated by hedgehog/gli-1 signaling pathways.
Keywords: Colorectal cancer, Hedgehog/gli-1, Resveratrol, Signaling pathways
Introduction
Colorectal cancer is the most common digestive system cancer, and is the third leading cause of cancer death, following breast/prostate, and lung and bronchus cancer (1, 2). The treatment of colorectal cancer requires a multimodal approach, which comprises surgical resection of the tumor followed by chemotherapy and/or radiation therapy. Despite substantial progress being made in the therapy of colorectal cancer, emerging incidences of drug resistance often lead to chemotherapy failure, and impose high toxicity to bone marrow, the gastrointestinal tract, and the skin (3, 4). Therefore, it is needed for improving the treatment and finding a new target signaling pathways (5).
Proteins of the hedgehog (Hh) signaling pathway are important regulators of human embryonic development. Genetic mutations of the Hh signaling molecules in cancer tissues and their expression appears to correlate with the occurrence, develop-ment and biological behavior of tumor cells (6). Hh signaling molecules in mammals include three ligands (Sonic hedgehog—Shh, Indian hedgehog—Ihh, and Desert hedgehog—Dhh), two receptors (Ptch), a key signal transducer, and smoothened (Smo), and transcription factors (Gli-1, Gli-2, and Gli-3). If there are not ligands, the function of Smo is suppressed by some transmembrane protein, such as Patched (Ptch). Upon binding of an active Hh ligand, the activated Smo then relocalizes to the primary cilia and initiates an intracellular signaling cascade that eventually leads to the activation of the Gli transcription factor and the upregulation of the downstream target genes (7). Among the three Gli family members, Gli-1 results in the activation of the Hh pathway downstream target genes, whereas Gli-3 is a repressor of the signaling, and Gli-2 acts as either a repressor or an activator, depending on specific circumstances (8). Although aberrant activation of the Hh pathway has been reported in the oncogenesis of esophageal, small-cell lung, gastric, and pancreatic cancers, its role in colorectal cancer is not fully understood (9).
In recent years, bioactive plant constituents have attracted the interest of researchers for their efficacy and low toxicity. Resveratrol (Res; 3,5,4´-trihydroxystilbene) is a naturally occurring poly-phenolic compound present in almost 70 plant species, including the skin of red grapes, peanuts, and berries (10). Res can be used as an inhibitor for platelet aggregation, a cardiac protector, and an anticancer agent. In recent years, some findings indicated that Res could inhibited several cancers, such as lymphoma, breast cancer, and colon cancer, by different signaling pathway (11, 12). For colon cancer cells, the anti-proliferation and apoptosis-inducing effects of Res have already been validated, but the mechanism underlying these activities remains unclear.
In the present study, we assessed the effects of Res on human colorectal cancer HCT116 cells stimulated by Shh, and discussed Res’s therapeutic mechanism involving hedgehog/Gli-1 signaling-related proteins. This study provides an experimental basis for a better understanding of Res molecular mechanisms, better treatment outcomes, and increases survival rates as an anticancer agent.
Materials and Methods
Reagents
High purity Res was purchased from Sigma (USA). Res was dissolved in dimethyl sulfoxide (DMSO), which was added into the culture medium to obtain a 1 mmol/l stock solution; recombinant human Shh was purchased from Sigma (USA) and dissolved in PBS at the appropriate concentration; Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) from Gibco Co, Ltd (USA); the Cell Counting Kit-8 was purchased from Dojindo Molecular Technologies, Inc., (USA); the Annexin V-FITC and PI-PE apoptosis kit was purchased from Becton, Dickinson and Company (USA); the primary antibodies Ptch, Smo and Gli-1 were purchased from Santa Cruz Co, Ltd (USA); enhanced chemiluminescence reagent was purchased from Pierce Biotechnology Inc (USA).
Cell culture
The HCT116 human colon cancer cell line was purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences, Shanghai Institute of Cell Biology, Chinese Academy of Sciences, (China). Cells were maintained in the DMEM with 10% FBS, 100 U/ml of penicillin, and 100 µg/ml of streptomycin at 37 °C in 5% CO2.
Administration and cell viability assay
HCT116 cells were stimulated with Shh at different concentrations (0.25, 0.50, 1.0 µg/ml) and different times (12 hr, 24 hr, 36 hr, 48 hr). Each group of HCT116 cells (104/well) were plated in 96-well plates, in six replicates, and then incubated at 37 °C with 5% CO2 for 24 hr. To determine cell viability, Cell Counting Kit-8 (CCK-8) solution (20 µl) was added to each well for 4 hr, followed by absorbance measurements at 450 nm. CCK-8 was used in accordance with the reagent’s instructions. The absorbance was detected by Multiscan Spectrum (BioTek Co, Ltd, USA). The results were described as the average of 6 counts.
According to the results of cell viability, we chose the appropriate time and concentration of Shh. In the next experiment, Shh and Res (25, 50, 100 μM) were added into the HCT116 cells at the same time.
Flow cytometric assay
Cells were harvested after treatment with Shh and different concentrations of Res (25, 50, 100 μM) for 24 hr. Then, cells were washed with PBS (4 °C) and transferred to a tube in which annexin V and propidium iodide were added. The cells were then allowed to incubate at room temperature for 20 Min and analyzed using an FC500 flow cytometer (Beckman Coulter). Specific procedures were carried out according to manufacturer’s instructions.
Data were analyzed with FCS Express 4. P1, dead cells; P2, dead/late apoptotic cells; P3, normal cells; P4, early apoptotic cells; P2+P4, total apoptotic cells.
Scratch test
For a scratch test, HCT116 cells were monolayer cultured in 12-well plates and grown to 70% to 80% confluence. A scratch track was made using a 200-µl pipette tip, and the medium and any cell debris were removed. The plates were washed with PBS, and the cells were grown in fresh medium containing Res for a further 24 hr. Cell migration was observed under a bright-field microscope (Olympus, Tokyo, Japan) at a magnification of 100×. The relative migration distance was acquired by the following formula: the relative migration distance (%)= 100 (A-B)/A, where A represents the width of cell scratches before incubation, and B indicates the width of cell scratches after incubation. The migration distance was calculated independently in 3 experiments.
Western blot analysis
Each group of HCT116 cells were washed with PBS three times, then lysed in cell lysis buffer with 1 mM PMSF at 4 °C for 30 min on ice, vortexing every 10 min, followed by centrifugation at 12,000 rpm for 10 min at 4 °C, and the supernatants obtained were diluted to 4 mg protein/ml and kept frozen at -80 °C until use. A total of 50 μg of denatured protein was separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (PVDF membrane, Millipore, USA) and then incubated with primary antibodies of Ptch, Smo, Gli-1, and β-actin (1:1000) at 4 °C overnight. After washing with 0.05% Tween 20-PBS, membranes were incubated with secondary antibodies (1:40 000) conjugated with HRP for 2 hr at 37 °C. Then, the membranes were washed, and detection was achieved by measuring the chemiluminescence of the blotting agent after exposure of the filters on films. Finally, the densities of the bands were quantified with a computerized densitometer (ImageJ Launcher, Broken Symmetry Software). Equivalent protein loading and transfer efficiency were verified by staining for β-actin.
Statistical analysis
Data are expressed as mean and standard deviation (SD). Unpaired 2-tailed Student’s t-test was used to determine the statistical significance of the difference between two groups. A value of P<0.05 was considered statistically significant. Calculations were performed using the SPSS version 13.0 statistical package.
Results
Resveratrol inhibited the cell viability of HCT116 stimulated by Shh
HCT116 cells were stimulated with Shh at different concentrations (0.25, 0.50, 1.0 µg/ml) and different times (12 hr, 24 hr, 36 hr, 48 hr). The cell viability significantly increased at the concentration (0.50, 1.0 µg/ml) on 24 hr. According to the cell viability results, we chose 0.50 µg/ml and 24 hr as the stimulus condition of Shh (Figure 1A). In the next experiment, Shh (0.50 µg/ml) and Res (25, 50, 100 μM) were added into the HCT116 cells at the same time for 24 hr.
Figure 1.
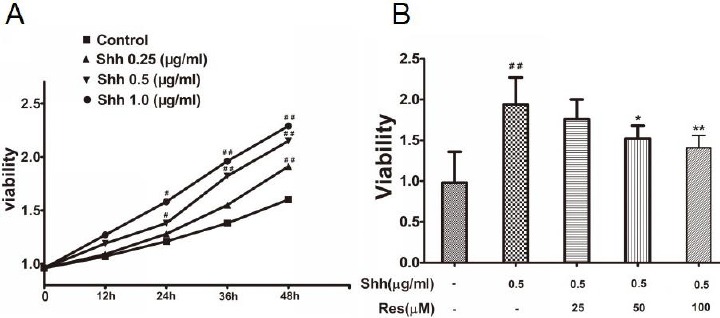
The effects of sonic hedgehog (Shh) and resveratrol (Res) on HCT116 cell viability
A: The effects of Shh on HCT116 cell viability. The cell viability significantly increased at the concentration (0.50, 1.0 µg/ml) on 24 hr B: The effects of Res on HCT116 cell viability stimulated by Shh. Res (50, 100 μM) obviously inhibited the cell viability stimulated by Shh Data are expressed as mean±SD. Six replicates for each group. #P<0.05, ##P<0.01 versus Control; *P<0.05, **P<0.01versus Shh group
The Shh group significantly increased the cell viability compared to the control group, while Res (50, 100 μM) obviously inhibited the cell viability stimulated by the Shh signaling pathway (Figure 1B).
Resveratrol promoted HCT116 cells apoptosis stimulated by Shh
P2 (dead/late apoptotic cells) +P4 (early apoptotic cells) represented the percentage of apoptotic cells. Flow cytometric assay analysis showed that the Shh group significantly inhibited the cell apoptosis compared to the control group, while Res (50, 100 μM) obviously increased the percent of apoptotic cells stimulated by the Shh signaling pathway (Figure 2).
Figure 2.
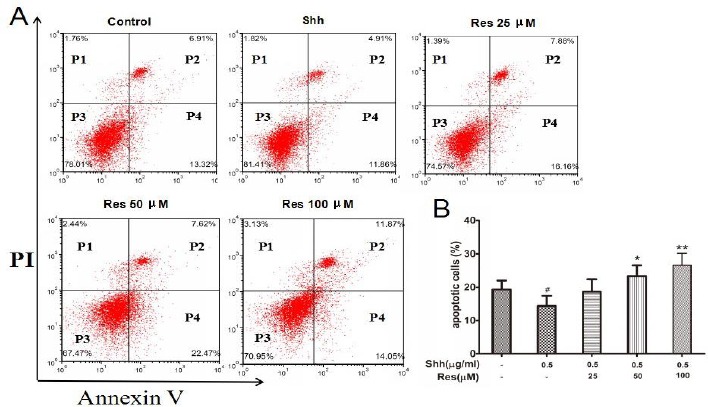
The effects of resveratrol (Res) on HCT116 cell apoptosis
HCT116 cells were harvested, then stained with antibodies to annexin V and propidium iodide, and analyzed by flow cytometry. Res (50, 100 μM) obviously increased the percent of apoptotic cells stimulated by Shh. A: Dot plot stained with annexin V and propidium iodide. B: Histogram presentation of apoptotic cell percentages. Data are shown as the means±SD from 3 replicate experiments. #P<0.05 versus Control; *P<0.05, **P<0.01 versus Shh group. P1, dead cells; P2, dead/late apoptotic cells; P3, normal cells; P4, early apoptotic cells; P2+P4, total apoptotic cells
Resveratrol inhibited HCT116 cells migration stimulated by Shh
Scratch test experiments were performed to assess the effect of Shh and Res on the cell migration ability of HCT116 cells. Confluent cells were scraped across center of the well, and the remaining cells were incubated to migrate into the gap in the absence or presence of Shh and Res. After 24 hr incubation, the scratch gap was narrower in the Shh groups compared to the control group, indicating that Shh significantly promoted cell migration, while the scratch gap was wider in the Res groups, indicating that Res obviously inhibited the cell migration stimulated by the Shh signaling pathway (Figure 3).
Figure 3.
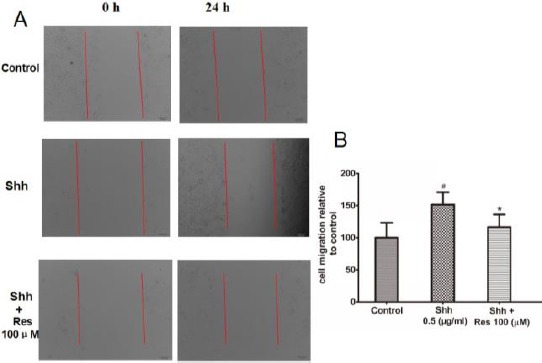
Resveratrol (Res) inhibited the migration of HCT116 cells stimulated by sonic hedgehog (Shh)
Res obviously inhibited the cell migration stimulated by Shh. A: Scratch-induced migration of cells into the scratched area was monitored after 24 hr. B: Quantification of cell migration. Data are shown as the means±SD from 3 replicate experiments. #P<0.05 versus Control; *P<0.05 versus Shh group
Resveratrol inhibited the hedgehog/Gli-1 signaling pathway in HCT116 cells
To discuss the mechanism of Res on HCT116 cells, we detected the hedgehog/Gli-1 signaling pathway, including Ptch, Smo, and Gli-1. The results showed that in the Shh group the expression of Ptch, Smo, and Gli-1 was significantly increased, while Res inhibited the expression of Ptch, Smo, and Gli-1 stimulated by Shh (Figure 4).
Figure 4.
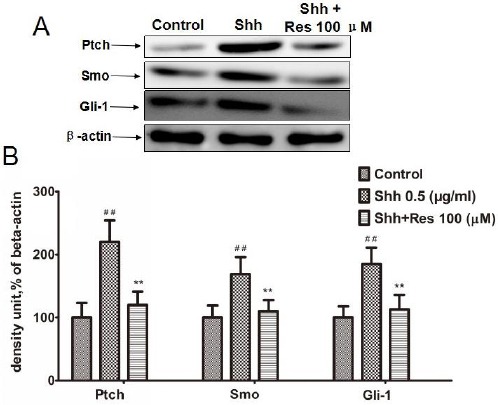
Resveratrol (Res) inhibited hedgehog/gli-1 signaling on hct116 cells
Res inhibited the expression of Ptch, Smo, and Gli-1 stimulated by Shh. A: The effects of Res on the expression of Ptch, Smo, and Gli-1. The expression was measured by Western blot. β-actin was measured as an internal control
B: Densitometric analysis from the above immunoblots is shown as bar chart. The results are representative of 3 independent experiments. ##P<0.01 versus control; **P<0.01 versus Shh group
Discussion
The Hh pathway of morphogenic proteins is known to participate in several physiologic cellular processes. Hh signaling has been associated with embryogenesis, adult tissue homeostasis and repair, regulation of the epithelial-to-mesenchymal transi-tion, and the control of cell survival and proliferation (13). The Shh signaling pathway, first identified in Drosophila melanogaster, may be a candidate for the mechanism behind surviving tumor cell growth. Moreover, many cancers are associated with Shh signaling, such as basal cell carcinoma, esophageal and stomach cancer, small-cell lung cancer, and pancreatic adenocarcinoma (14). Shh pathway activation is initiated by binding of the secreted and lipid-modified ligand Shh to the Ptch transmembrane receptor. As a consequence, Ptch inhibition of Smo, a 7-pass transmembrane-spanning protein, is relieved, and the Shh signaling cascade is initiated, which in turn activates the Gli transcription factors. In the presence of Shh, Gli-1 is transcriptionally activated and then phosphorylated and proteolytic processing of Gli-2 and Gli-3 to their truncated repressor forms is inhibited, thus leading to the activation of specific Shh signaling pathway target genes, such as Gli-1 and Ptch (15).
First, we detected the effects of Hh signaling on cell proliferation. The direct regulation of cell proli-feration by Hh signaling is shown in cultured cerebellar cells in the presence of recombinant Shh protein (16). There is extensive in vivo evidence showing regulation of Hh signaling on cell proliferation. In this study, we also showed that the Shh group significantly increased the cell viability compared to the control group. The exact molecular mechanism of underlying cell proliferation regulation varies by cell type, but it is known that Hh signaling regulates the gene expression of cell cycle-related molecules, such as cyclin D2 and N-myc (7). Therefore, in the next apoptosis study, we found that the Shh group significantly inhibited cell apoptosis compared to the control group, while Res (50, 100 μM) obviously inhibited cell viability and increased the percentage of apoptotic cells stimulated by the Shh signaling pathway. Furthermore, the effects of Res and Shh on human colorectal cancer HCT116 cells were in a dose- and time-dependent manner.
Hh signaling plays distinct roles in different types of cancer. Based on recent publications, there are three major roles of Hh signaling during cancer development: as a tumor development driver, a tumor promoter, or a regulator for residual cancer cells after therapy (17). What are the effects of Hh signaling on cell migration? Increasing evidence indicates that Hh signaling plays an important role during tumor metastasis in several types of cancer, such as pancreatic and breast cancers (18, 19). Studies from many groups indicate activation of Hh signaling in the stromal cells as well as tumor compartments in metastatic pancreatic cancer (20). The study showed that the inhibitors of Hh signaling could inhibited pancreatic cancer metastases. Hh signaling activation plays an important role in tumor metastasis, which was found both in the stroma and in the tumor compartment. The molecules that mediate Hh’s metastatic functions remain largely untested, but there are reports to indicate the following molecules: snail, TGFβ, and Wnt (21, 22). In this study, we also showed that the Shh group significantly promoted the HCT116 cell migration compared to the control group, while Res obviously inhibited the HCT116 cells migration stimulated by the Shh signaling pathway.
We further explored the mechanisms by which Res obviously inhibited the cell viability and migration, and increased the percentage of apoptotic cells stimulated by the Shh signaling pathway. It was reported that Res suppresses the proliferation of a wide variety of tumor cells, including breast, colon, pancreas, stomach, prostate, ovary, liver, lung, and melanoma (23-25). In our experiment, we found that Res obviously inhibited the viability in HCT116 cells. Besides inhibiting proliferation, Res also induces apoptosis (26). In this study, we found that Res inhibited HCT116 cell viability and migration and induced the HCT116 cell apoptosis stimulated by Shh signaling. We further detected the expression of the Hh signaling pathway and found that Res inhibited the expression of the Ptch, Smo, and Gli-1 Shh signaling pathways. The above results showed that the hedgehog/Gli-1 signaling pathways were involved in the inhibitory effect of Res on human colorectal cancer HCT116 cells.
Conclusion
The current study demonstrated that Res inhibited HCT116 cell viability and migration and induced the HCT116 cells apoptosis stimulated by Shh signaling. The inhibitory effect of Res on hct116 cells may be mediated by hedgehog/Gli-1 signaling pathways. Thus, our results provided the experimental basis that Res can be used as a treatment option for colorectal cancer.
Acknowledgment
Funded by the Natural Science Foundation of Zhejiang province (No.Y15H160192).
Conflict of interest
The authors alone are responsible for the content and writing of the article. The authors report no conflict of interest.
References
- 1.Pallag A, Rosca E, ţiŢ DM, MuŢiu G, Bungău SG, Pop OL. Monitoring the effects of treatment in colon cancer cells using immunohistochemical and histoenzymatic techniques. Rom J Morphol Embryol. 2015;56:1103–1109. [PubMed] [Google Scholar]
- 2.Wong MC, Wong SH, Ng SC, Wu JC, Chan FK, Sung JJ. Targeted screening for colorectal cancer in high-risk individuals. Best Pract Res Clin Gastroenterol. 2015;29:941–951. doi: 10.1016/j.bpg.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SA, Tomeh N, Theard S. Fluorouracil-induced hyperammonemia in a patient with colorectal cancer. Anticancer Res. 2015;35:6761–6763. [PubMed] [Google Scholar]
- 4.Li AM, Miao JH, Liu H, Ma YZ, Sun ZC. Drug-induced skin toxicity and clinical nursing of VitK cream on colorectal cancer patients. Pak J Pharm Sci. 2015;28:1499–1503. [PubMed] [Google Scholar]
- 5.Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. 2013;19:619–625. doi: 10.1038/nm.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;1:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 7.Jia Y, Wang Y, Xie J. The Hedgehog pathway: role in cell differentiation, polarity and proliferation. Arch Toxicol. 2015;89:179–191. doi: 10.1007/s00204-014-1433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stecca B, Ruiz I, Altaba A. Context-dependent regulation of the GLI code in cancer by Hedgehog and non-Hedgehog signals. J Mol Cell Biol. 2010;2:84–95. doi: 10.1093/jmcb/mjp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onishi H, Katano M. Hedgehog signaling pathway as a therapeutic target in various types of cancer. Cancer Sci. 2011;102:1756–1760. doi: 10.1111/j.1349-7006.2011.02010.x. [DOI] [PubMed] [Google Scholar]
- 10.Key TJ. Fruit and vegetables and cancer risk. Br J Cancer. 2011;104:6–11. doi: 10.1038/sj.bjc.6606032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 12.Frazzi R, Valli R, Tamagnini I, Casali B, Latruffe N, Merli F. Resveratrol- mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int J Cancer. 2013;132:1013–1021. doi: 10.1002/ijc.27748. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Bartels CM, Barton SW, Gu D. Targeting hedgehog signaling in cancer: research and clinical developments. Onco Targets Ther. 2013;6:1425–1435. doi: 10.2147/OTT.S34678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 18.Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, Hanenberg H, et al. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038–1048. doi: 10.1158/1535-7163.MCT-12-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller E, Hurchla MA, Xiang J, Su X, Chen S, Schneider J, et al. Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. Cancer Res. 2012;72:897–907. doi: 10.1158/0008-5472.CAN-11-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang RF, Moore TT, Hattersley MM, Scarpitti M, Yang B, Devereaux E, et al. Inhibition of the hedgehog pathway targets the tumor-associated stroma in pancreatic cancer. Mol Cancer Res. 2012;10:1147–1157. doi: 10.1158/1541-7786.MCR-12-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javelaud D, Pierrat MJ, Mauviel A. Crosstalk between TGF-βand hedgehog signaling in cancer. FEBS Lett. 2012;586:2016–2025. doi: 10.1016/j.febslet.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Joost S, Almada LL, Rohnalter V, Holz PS, Vrabel AM, Fernandez-Barrena MG, et al. GLI1 inhibition promotes epithelial-to-mesenchymal transition in pancreatic cancer cells. Cancer Res. 2012;72:88–99. doi: 10.1158/0008-5472.CAN-10-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- 25.Khan A, Aljarbou AN, Aldebasi YH, Faisal SM, Khan MA. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014;38:765–772. doi: 10.1016/j.canep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: Challenges for clinical translation. Biochim Biophys Acta. 2015;1852:1178–1185. doi: 10.1016/j.bbadis.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Empl MT, Albers M, Wang S, Steinberg P. The Resveratrol Tetramer r-Viniferin Induces a Cell Cycle Arrest Followed by Apoptosis in the Prostate Cancer Cell Line LNCaP. Phytother Res. 2015;29:1640–1645. doi: 10.1002/ptr.5443. [DOI] [PubMed] [Google Scholar]


