Abstract
Objective(s):
Menopause and diabetes obviously increase the risk of cardiovascular disease in women. The aims of the present study were to evaluate the effects of ovariectomy in type 2 diabetes on the histology and expression of miRNA-29, miRNA-133, IGF-1 and Bcl-2 genes and Bcl-2 protein and caspase 3 activity in the hearts of female rats.
Materials and Methods:
Forty Female Wistar rats were divided into four groups: control, sham, ovariectomized (OVX), and ovariectomized with type 2 diabetes (OVX.D). After the 8-week experiment, the histological evaluation of the heart tissue was performed using H&E staining and PAS analysis, and cardiac expression of miRNA-29, miRNA-133, IGF-1, and Bcl-2 were evaluated using real-time PCR, and Bcl-2 protein and caspase 3 activity were evaluated using Western blot and ELISA.
Results:
Ovariectomy significantly decreased miRNA-29, miRNA-133, IGF-1, and BCL-2 expression and Bcl-2 protein and increased caspase 3 activity in the heart compared to sham animals group (P<0.05). Type 2 diabetes in ovariectomized rats markedly decreased expression of miRNA-29, miRNA-133, IGF-1, BCL-2 genes, and Bcl-2 protein, and increased caspase 3 activity and reduced collagen and fibroblast tissue and glycogen granule deposition in relation to OVX group (P<0.05).
Conclusion:
Our findings suggest that type 2 diabetes and menopause synergically could enhance the cardiac fibrosis through dysregulation of miRNA-29, miRNA-133, IGF-1, and Bcl-2 genes expression and Bcl-2 protein and upregulation of caspase 3 activity.
Keywords: Cardiac fibrosis, Diabetes, Menopause, MicroRNA
Introduction
People who have diabetes are more susceptible to the cardiovascular diseases which lead to death (1). Some prevalent complications of diabetes include hypertrophy, fibrosis, and apoptosis since they damage the function of the cardiac muscle (2). Moreover, the commonness and development of diabetes have increased tremendously in postmenopausal women (3).
It has been established that estrogen has a cardioprotective effect on the women with functional ovaries and can preserve cardiovascular function (4). Cardiovascular tissues can be influenced by the major forms of estrogen; so that one of the main causes of apoptosis and fibrosis of cardiac myofilament in rats is the lasting deficiency of ovarian sex hormones, and cardiovascular diseases are more prevalent in postmenopausal women (5). Nonetheless, the mechanism of the reciprocated influences of ovarian hormone deficiency and diabetes on cardiac failure is not well identified. Numerous investigations have found that microRNAs (miRNAs) have some essential roles in coordinating cardiac development, gene expression, and heart functions (6). In effect, microRNAs, single-stranded RNA, and 20-24 nucleotides encoded by the genome of cells modify gene expression by constraining mRNA translation and stimulating mRNA degradation (7-9).
MiRNA-133a and miRNA-29 are two kinds of important controllers in cardiac physiology which regulate the cardiac hypertrophy, apoptosis, and fibrosis (10, 11). Mitochondrial pathway of cell death are regulated by the upstream of Bcl-2 proteins and decreased caspase 3 proteins (12) expression of Bcl-2 is regulated by miR-133a (13). Contemporary investigations have established that abnormal down-regulation of miRNA-133 expression contributes to the pathogenesis procedures mediating diabetic cardiomyopathy. In order to explicate the key role of microRNAs in cardiac heart failure, hypertrophy, and myocardial fibrosis, it has been established that miRNA-133a has a significant role in diabetes-induced cardiomyocytes hypertrophy and miRNA-29 plays an important role in regulating cardiac fibrosis (11, 14). Some studies have revealed that miRNA-29 plays a significant role in the reduction of collagen expression and development of ventricular com-pliance (15). However, miRNA-29 is defined as the significant regulator of IGF-1 gene expression in the rat heart (16). Certainly, IGF-1 up-regulation can constrain the cardiac fibrosis (17) and hypertrophy (18). Consequently, it is no surprise that the endogenous miRNA-29 can diminish numerous fibrotic events, particularly in the heart and the addition of exogenous miRNA-29 shrinks fibroids. Generally, metabolic changes and lipid profiles are the main causes of cardiovascular diseases among diabetic and postmenopausal women. However, the deregulation of genes expression and their role in these disturbances have not been studied yet. The main objective of this study is to examine the cause of fibrosis and apoptosis in the heart and their influence on the total death of diabetic female rats experiencing ovarian hormone deprivation. Some fundamental studies are required to provide a deep understanding of diabetes-related changes in cardio-myocytes gene expression related to surgical ovariectomy. In fact, the impact of microRNAs on controlling cardiovascular and autonomic dysfunc-tion of women with diabetes after ovarian hormone deficiency is not known yet. Owing to the vital role of miRNAs in the problems of diabetes and pathology in the hearts of postmenopausal women, the main objective of the current study is to examine the influence of ovariectomy with type 2 diabetes on expression changes of miRNA-133, miRNA-29, and IGF-1 and Bcl-2 genes, Bcl-2 protein, and caspase 3 activity in the heart of rats.
Materials and Methods
Animal care
This study used 40 female Wistar rats (weighing 180–220 g, approximately 10 weeks old) obtained from the Experimental Animal Research Center, Medical Faculty, Tabriz University, Tabriz, Iran. All rats were preserved under controlled conditions (temperature 22 °C-24 °C with 12:12 hr light/dark cycle) and received standard chow diet and water ad libitum for 1 week. The University Ethics Committee accepted and verified this study. After 1 week, rats were separated arbitrarily into 4 groups (n=10) as follows; 1. Control, 2. Sham: surgery without ovariectomy, 3. OVX (bilateral ovariectomy), and 4. OVX.D (OVX+Diabetes). Two weeks before beginning the experiment, animals in OVX and OVX.D groups were bilaterally ovariectomized (19). However, the rats in the sham group received the sham ovariectomy and control groups did not receive this treatment. Ovaries were excised and oviducts replaced with minimum disruption to surrounding soft tissues.
Induction of type 2 diabetes
Ten days after ovariectomy (20), rats in the diabetic groups were fed HFD (58% fat, 17% carbohydrate, and 25% protein) ad libitum for the initial period of 8 weeks, and then a low dose of STZ (35 mg/kg) was injected intraperitoneally (IP) in a 0.1 M citrate buffer (pH 4.5) (21). Blood glucose level was measured with a glucometer in all rats after induction of diabetes. Animals with blood glucose levels more than 200 mg dl−1 were selected as diabetic rats (22).
Biochemical measurements
After retro-orbital blood collection, the level of serum glucose was assessed by using an Accu-Chek Active glucometer. Serum insulin level was deter-mined using a sandwich ELISA kit (Millipore) according to the manufacturer’s instructions.
Lipid profile was assessed by using a commercial diagnostic kit (Randox (UK)) in accordance with the manufacturer’s instructions.
Molecular analysis
RNA isolation and cDNA synthesis
At the end of 8 weeks, rats were sacrificed and their hearts were harvested. Total RNA including messenger RNA (mRNA) and microRNA was extracted from the left heart ventricles using RNX-Plus solution kit (Fermentase, Cinagen Co, Iran) and miR-amp kit (parsgenome Co, Iran), respectively in accordance with the manufacturer’s instructions (using chloroform layer separation followed by treatment with isopropanol and ethanol). RNA quantity and A260/280 ratio were measured using the NanoDrop 1000 (Thermo Scientific, Waltham, and Mass) and gel electrophoresis with GelRed (Biotium, Hayward, California) was used to evaluate the integrity of the samples. The IGF-1, Bcl-2, miR-29, and miR-133 genes expression were quantitatively assessed by real-time polymerase chain reaction (qRT-PCR). Primers’ sequences for each gene were mentioned in Table 1. The amount of PCR products was normalized to that of the housekeeping gene -3-phosphate dehydrogenase (GAPDH) mRNA samples and miR-191 for microRNA samples (internal control). For synthesis of cDNA in the mRNA sample, 1 μl of total RNA was reverse transcribed by means of Revert Aid M-MuLV reverse transcriptase (1 μl), DNase I (1 μl) and random hexamer primers (1 μl), dNTPS (2 μl), and RiboLock RNase-inhibitor (0.25 μl), for 10 min at 25 °C, followed by 60 min at 42 °C in a final volume of 20 μl. The reaction was terminated by heating at 70 °C for 5 min. In addition, synthesis of cDNA in microRNA sample was performed according to the miR-amp kit (parsgenome Co, Iran).
Table 1.
The primer sequences for each of the genes
| Genes | Accession number | Primers Sequence a |
|---|---|---|
| IGF-1 | NM- 001082477 | F: AAG CCT ACA AAG TCA GCT CG R: GGT CTT GTT TCC TGC ACT TC |
| Bcl-2 | NM_016993.1 | F: F: CGGGAGAACAGGGTATGA R: CAGGCTGGAAGGAGAAGAT |
| GAPDH | NM_017008.4 | F: TGCCGCCTGGAGAAACCTGC R: TGAGAGCAATGCCAGCCCCA Target sequenceb |
| miR-29 | MIMAT0004718 | ACUGAUUUCUUUUGGUGUUCAG |
| miR-133 | MIMAT0017124 | AGCUGGUAAAAUGGAACCAAAU |
| miR-191a | MIMAT0000866 | CAACGGAAUCCCAAAAGCAGCUG |
Sequences were derived from NCBI (www.ncbi.nlm.nih.gov);
Sequences were derived from miRBase (www.mirbase.org)
Real-time quantitative PCR
A master mix of 25 μl containing 12.5 μl SYBR Green PCR Master Mix (Jena Bioscience, Germany), 1 μl forward primer, 1 μl reverse primer, and 8.5 μl water was prepared to carry out real-time PCR. Two microliters of reverse transcribed cDNA were then added to the PCR master mix to achieve a final volume of 25 μl. Furthermore, to check the accuracy of amplifications, we included a negative control in each run by eliminating the cDNA sample in the tube.
The PCR protocol was used on the real-time PCR machine (Rotor-Gene 3000) in three steps including: 1- initial denaturation (10 min at 95 °C); 2- a three-step amplification program (15 sec at 95 °C followed by 30 sec at 60 °C for miR-29, miR-133, and IGF-1 genes and 30 sec at 58°C for Bcl-2 gene, and 30 sec at 72 °C) repeated 40 times; and 3- melting curve analysis (1 cycle: 72 °C to 95 °C with temperature transition rate 1 °C/sec for 5 sec). All runs were performed in duplicates. Real-time quantification was monitored by measuring the increase in fluorescence caused by binding of the SYBR Green dye to double-stranded DNA at the end of each amplification cycle. The relative amount of mRNA for each target gene was calculated based on its threshold cycle (Ct) compared to the Ct of the housekeeping (reference) gene (GAPDH). The relative quantification was performed using the 2^(DDCt) method.
The specificity of the PCR reactions was verified by generation of a melting curve analysis followed by gel electrophoresis, stained with GelRed (Biotium, Hayward, California).
Western blot analysis
The level of Bcl-2 was measured by Western blotting. In brief, after electrophoresis of samples, proteins in SDS-gels were transferred to a polyvin-ylidene fluoride (PVDF) membrane (Millipore). The transfer was carried out at about 90 mA for 2 hr. Thereafter, the membranes were blocked in 3% skim milk buffer containing 0.1% Tween- 20 for 2 hr and then incubated with primary antibodies against the Bcl-2 and β-actin (all antibodies from Santa Cruz, USA) overnight at 4°C on a shaker. After 4 x 5 min washes with tris buffer saline containing 0.1% Tween-20, membranes were incubated with horseradish peroxidase-conjugated (HRP) secondary antibody (Santa Cruz, USA) for 1 hr at RT on a shaker. The membranes were then rinsed at least 3 x 5 min with wash buffer before detection. Blots were then developed using the enhanced chemiluminescence (ECL) method. Following incubation with the ECL reagents, the membranes were exposed to an X-ray hyperfilm inside a hypercassette in the darkroom and then the chemiluminescence of antibody binding was visualized by a visualizing machine. The intensity of protein bands in the blots was digitally quantified using densitometric analysis. To analyze the amount of Bcl-2 to β-actin was calculated and expressed in arbitrary unit [w1](AU).
ElISA
Caspase-3 activity, a marker of apoptosis, was determined by enzyme-linked immunosorbent assay ELISA method using Apotarget Apoptosis kit (Camarillo, California 93012, USA). Data were normalized to the levels of protein evaluated by the Lowery method (23) using the bovine serum albumin as a slandered.
Histological analysis
The Cardiac tissues were fixed in 10% buffered formalin solution, dehydrated in ascending grades of alcohol and embedded in paraffin. Sections of 5 μm were taken, stained with H&E and periodic acid-Schiff (PAS), and examined under a light microscope (Olympus BH-2, Tokyo, Japan) in a blinded manner by a pathologist. Cardiac tissue was examined for changes of histology and glycogen granules in the sarcoplasm of cardiomyocytes.
Data analysis and statistics
Data analysis was performed using one-way ANOVA. The Tukey test was applied to make a comparison between groups. P-values of less than 0.05 were considered as statistically significant.
Results
Because of absence of statistically significant differences among control and sham animal groups, we only discussed the sham group.
Body and heart weight
Body and heart weight as analyzed in Table 2, showed the OVX rats had a higher body weight 8 weeks after experiment than the sham rats (not significantly). However, a significantly decreased body weight (BW) was found in OVX.D in compassion to sham and OVX groups (P<0.05). In addition, heart weight (HW) in the OVX rats was increased insignificantly compared to the sham, but HW was decreased in OVX.D compared to OVX animal group significantly (P<0.05). Table 2 also shows that the HW to BW ratio was significantly higher in OVX.D compared with the OVX group (P<0.05).
Table 2.
Effects of ovariectomy and diabetes on body and heart weight
| Sham | OVX | OVX.D | |
|---|---|---|---|
| BW final (g) | 261.87 ± 4.87 | 273.40±2.55 | 196.80±4.03 a |
| HW (mg) | 896 ± 20.28 | 967±25.36 | 862±29.64 b |
| HW/ BW (mg/g) | 3.54 ± 0.133 | 3.39±0.116 | 4.04±0.235b |
BW, body weight; HW, heart weight and HW/BW, heart to body weight ratio. Data are expressed as mean±SEM
P<0.05 vs sham & OVX;
P<0.05 vs OVX
Biochemical results
Fasting blood glucose (FBG) and serum insulin levels were measured in all animals after eight weeks. One-way ANOVA results showed that fasting blood glucose (FBG) levels were significantly higher in OVX.D compared to sham and OVX rat groups (P<0.05) (Table 3). Moreover, FBG was increased in the OVX rats, but this elevation was not significantly different from the sham group. Insulin levels were significantly increased in OVX.D rats in contrast to sham and OVX rat groups after eight weeks (P<0.05). Furthermore, OVX rats showed a significant increase in insulin levels when compared to sham groups (P<0.05). Also, Plasma glycosylated hemoglobin levels showed an elevation in the OVX.D rats compared to sham and OVX groups, also OVX rats compared to sham rats, although this elevation was not statistically significant.
Table 3.
Parameters of glucose homeostasis of different studied groups at the end of the study
| Sham | OVX | OVX.D | |
|---|---|---|---|
| Fasting blood glucose(mg/dl) | 98.20±2.13 | 109.00±1.87 | 269.00±13.26a |
| Serum insulin(µIU/ml) | 4.3±1.8 | 13.2± 1.1b | 28.9±1.5a |
| Plasma glycosylated hemoglobin (%) | 4.380±0.57 | 4.920±0.63 | 5.960± 0.60 |
OVX: ovariectomized group, OVX.D: ovariectomized with 8-week diabetes group. Data are expressed as mean±SEM
P<0.05 vs sham & OVX;
P<0.05 vs sham & OVX.D
The plasma lipid profiles are been shown in Figure 1. Cholesterol, triglyceride, and LDL levels were markedly higher in the OVX.D than in the OVX and sham animal groups and in the OVX group they were higher than in the sham group (P<0.05). However, HDL in the OVX.D group was decreased compared to OVX and sham groups and in the OVX group compared with sham animal groups, significantly (P<0.05).
Figure 1.
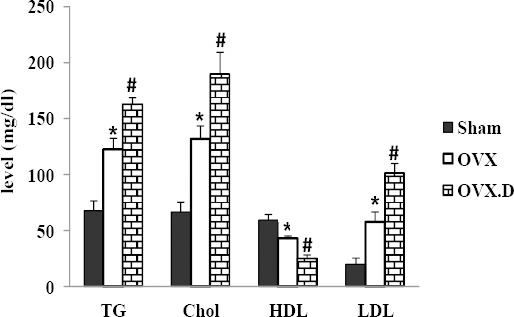
Biochemical measurements after the 8-week experiment
OVX: ovariectomized group, OVX.D: ovariectomized with 8-weeks diabetic group. Data are expressed as mean±SEM
* Significant difference compared with the sham group (P<0.05)
# Significant difference compared with sham and OVX groups (P<0.05)
MiRNA-29 and miRNA-133 expression
Cardiac miRNA-29 and miRNA-133 expression levels were measured in all animals after eight weeks. As seen in Figure 2, one-way ANOVA results showed that miRNA-29 expression level was significantly decreased in the heart of OVX compared to that of the sham animal group (P<0.05). Also, in the heart of OVX.D group was found a low significant expression of miRNA-29 compared to OVX and sham animal groups (P<0.05). In addition, the miRNA-133 expression level was significantly decreased in the heart of OVX in comparison to the sham animal group (P<0.05). At the end of the experiment, expression of miRNA-133 down-regulated in the heart of OVX.D compared to OVX and sham animal groups (P<0.05).
Figure 2.
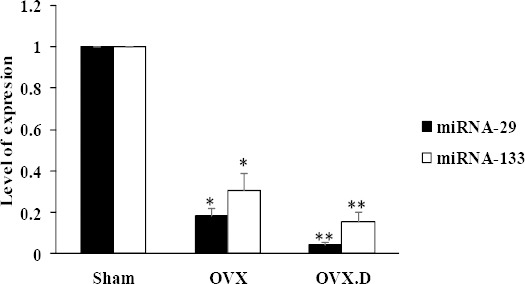
Relative expression levels of miR-29 and miR-133 in the heart of the studied groups
OVX: ovariectomized group, OVX.D: ovariectomized with 8-weeks diabetic group. Data are expressed as mean±SEM
*Significant difference compared with sham group (P<0.05) **Significant difference compared with sham & OVX groups (P<0.05)
IGF-1 and Bcl-2 expression:
The levels of expression of heart IGF-1 and Bcl-2 are presented in Figure 3. IGF-1 expression level was found to be significantly lower in the heart of OVX compared with the sham groups (P<0.05). Also, the results showed a decreased expression of IGF-1 in the heart of the OVX.D compared to OVX and sham groups (P<0.05). In addition, the BCL-2 expression level was found to be significantly lower in the heart of the OVX compared with the sham animal group (P<0.05). Expression of BCL-2 was decreased in the heart of the OVX.D compared to OVX and sham animal groups (P<0.05).
Figure 3.
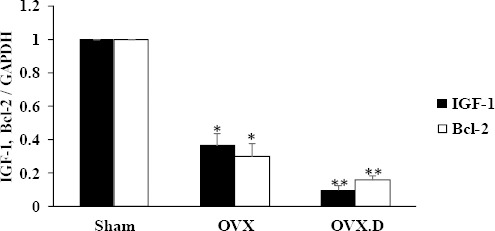
Levels of IGF-1 and Bcl-2 expression in the hearts of the studied groups
OVX: ovariectomized group, OVX.D: ovariectomized with 8-weeks diabetic group. Data are expressed as mean±SEM
* Significant difference compared with sham group (P<0.05)
** Significant difference compared with sham & OVX groups (P<0.05)
Levels of the anti-apoptotic protein Bcl-2 and caspase 3 activity in the heart
The levels of Bcl-2 and caspase 3 activity are presented in Figures 4 and 5. Bcl-2 levels showed significant decrease in heart myocytes of the OVX group compared to the sham group (P<0.05) and diabetes intensified it (P<0.05).
Figure 4.
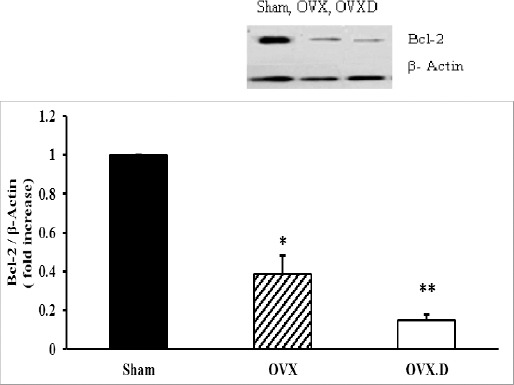
The levels of antiapoptotic Bcl-2 protein in the hearts of the studied groups
OVX: ovariectomized group, OVX.D: ovariectomized with 8-weeks diabetic group. Data are expressed as mean±SEM
* Significant difference compared with sham and OVX.D groups (P<0.05)
** Significant difference compared with the sham group (P<0.05)
Figure 5.
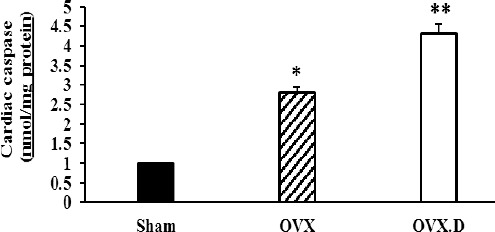
The levels of caspase 3 activity in the hearts of the studied groups
OVX: ovariectomized group, OVX.D: ovariectomized with the 8-week diabetic group. Data are expressed as mean±SEM
* Significant difference compared with sham and OVX.D groups (P<0.05)
** Significant difference compared with the sham group (P<0.05)
In addition, as seen in Figure 5, caspase 3 activity was significantly increased in the OVX group in comparison to the sham animal group (P<0.05). However, diabetes could reduce further the level of caspase 3 activity compared to the OVX animal group (P<0.05).
Histological results
The histopathological changes were graded and analyzed quantitatively. According to the histological assessment of cardiac tissue, diabetes resulted in obvious reduction of myocardial fibrosis, leukocytes infiltration, and disorganization myocardial infarction in OVX animals (Table 4) (Figures 6a-6f). The histological evaluation of the heart tissue in H & E staining revealed that the cardiac muscle cells were located in normal features in myocytes such as a cross-striated banding pattern, nucleus with oval, vesicular and centrally located pale staining appearance, and inserted disks established at the boundary between contiguous cardiac muscle cells in the sham animal group. Furthermore, myocyte sarcoplasms were acidophilus and barred and were organized on an unvarying basis (Figure 6a). It was concluded that, in the OVX animal group, a sequence of fluctuations such as protection structural integrity was analogous to the one observed in the sham animal group and other outcomes, such as the upsurge of thick fibrosis and necrotic cell compactness as well as outstanding prominence in the amount of connective tissue elements (Figure 6b). It was argued that diabetes in the OVX.D animal group defeated the sequences of changes such as a decreased tendency in a fibrotic area and necrotic cell density. Normalization in the amount of connective tissue is depicted in Figure 6c. However, the histological evaluations of the heart tissue in PAS analysis revealed that a continuous and homogeneous granule of glycogen in the sarcoplasm of cardio-myocytes was completely evident (Figure 6d) in the sham animal groups. Nevertheless, disintegration and uneven gathering of glycogen granules were observed in the OVX animal group compared with the sham animal group (Figure 6e). In comparison with OVX animal group in the sarcoplasm of cardiomyocyts (Figure 6f), diabetes amplified the disintegration of glycogen granules in the OVX.D group.
Table 4.
Histological changes of the hearts (H&E) in the studied groups
| Sham | OVX | OVX.D | |
|---|---|---|---|
| Cardiac fibers hypertrophy | 0 | 6.60 ± 0.50* | 9.20 ± 0.37** |
| Myocardial fibrosis | 0 | 5.20 ± 0.58* | 9.00 ± 0.31** |
| Leukocyte Infiltration | 0 | 5.00 ± 0.37* | 7.80 ± 0.24** |
| Disorganization myocardial fibers disarray | 0 | 5.40 ± 0.60* | 9.40 ± 0.40** |
| Cardiomyocyte vacuolation | 0 | 5.00 ± 0.31* | 7.80 ± 0.37** |
| Cardiomyocyte degeneration | 0 | 4.80 ± 0.37* | 8.60 ± 0.50** |
| Cardiac nucleus eccentric | 0 | 5.00 ± 0.44* | 9.00 ± 0.44** |
A minimum of ten fields for each heart slide was examined and assigned for severity of injury based on numbers of 10 microscopic fields (n=10 for each group). Data are expressed as mean ± SEM. OVX: ovariectomized group, OVX.D: ovariectomized with 8-week diabetes group
P<0.05 vs sham & OVX.D
P<0.05 vs sham
Figure 6.
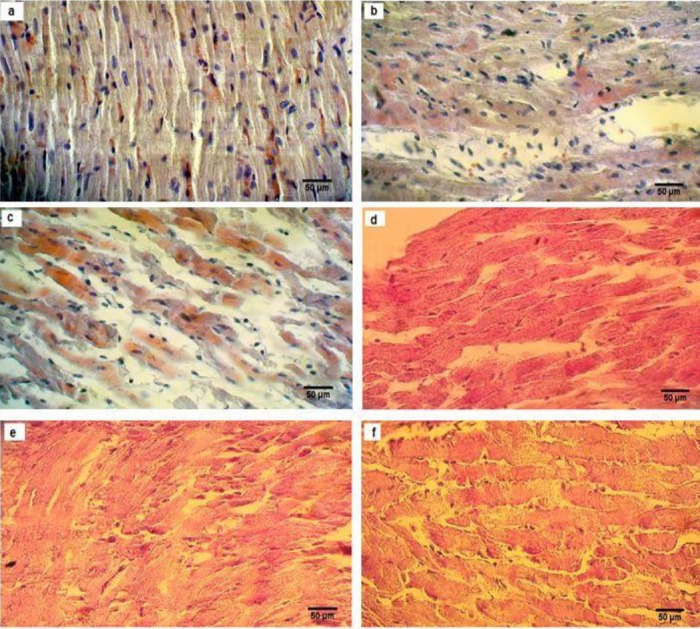
Histological evaluation of H&E staining (a, b, c) and periodic acid-Schiff (PAS) (d, e, f), examined and analyzed by a light microscope (magnification 40×) in the heart of sham-ovariectomized (OVX) and diabetic ovariectomized (OVX.D) rat groups. Results showed histological changes in sham (a), OVX (b), OVX.D (c), and PAS showed different storage of glycogen in sham (d), OVX (e), and OVX.D (f) animal groups
Discussion
This study seeks to investigate the important role of ovariectomization on the expression of changes in miRNA-133, miRNA-29, IGF-1, and BCL-2 genes, anti-apoptotic protein Bcl-2 and caspase 3 activity as an apoptotic marker in the heart of diabetic rats. Hence, the rat models of the surgical ovariectomy and the ovariectomized diabetic group are compared with the sham and intact control groups. In this study, the differences in gene expression are investigated between rats undergoing OVX and the sham rats by applying real-time PCR. Our main results were as follows: 1) ovariectomy decreased cardiac expression of miRNA-133 and miRNA-29, which may be associated with the amplified Bcl-2 and IGF-1 genes expression, anti-apoptotic protein Bcl-2 and increased caspase 3 activity as an apoptotic marker, 2) diabetes caused further reduction in anti-apoptotic and anti-fibrosis-associated genes expression in ovariectomized rats and 3) abnormal tissue construction, disintegration, and uneven build-up of glycogen granules were in the hearts of OVX and OVX.D groups not the sham animals group.
Menopausal females are more susceptible to developing diabetes, as it is well known that the occurrence of the disease rises with age. Estrogen deficiency during and after menopause are believed to increase a woman’s risk for cardiovascular disease. This risk is increased in women with diabetes. In fact, studies have shown that a menopausal woman with diabetes has a higher chance of death from cardiovascular disease than a menopausal woman without diabetes (24, 25).
Several studies have shown that increased total lipids, as well as cholesterol and LDL, are the high-risk factors for promoting cardiovascular diseases (26). Consistently, the changes in risk factors were seen in OVX and OVX.D groups in the present study. Some investigators presented a brief review on menopause or bilateral ovariectomy with diabetes or without diabetes, showing that cardiovascular disease is related to cardiac apoptosis and fibrosis (27). In the present study, histological changes including abnormal features in myocytes as well as the disintegration and uneven gathering of glycogen granules were observed in the heart of the studied OVX and OVX.D groups in contrast with the sham group. Recently, investigations have concentrated more on the analysis of variant miRNA expression in heart diseases (28). Commonly, miRNAs reduce the expression of their mRNA targets through translational suppression or improved mRNA deterioration (29). Numerous miRNAs including miRNA-133 and miRNA-29 have been augmented in the human heart (30, 31). Moreover, miRNAs have been determined as a key node in the post-transcriptional control of apoptosis, growth (32), as well as cardiac fibrosis (33) and as one of the most indispensable controllers and one of the main regulators in the human physiology and pathology (34). Previously, it has been recognized that miRNAs can control compound biological procedures such as cardiomyocytes apoptosis via multiple mechanisms (32). The signs of cardiovascular problems are related to the increased OVX-induced tBid, Bad, Bax, Bak, cytochrome c overexpression, and caspase-9 and caspase-3 activation (35) and also decreased BCL-2 expression (36). It has been shown that miR-133a suppresses the expression of apoptotic proteins including caspase activity and improves expression of Bcl-2 gene (13, 37). Also, miRNA-29 has been recognized as the main controller of IGF-1 gene expression in the hearts of rats (38) and its downregulation can cause cardiac fibrosis and hypertrophy (39).
In this study, in comparison with the sham surgery rats, ovariectomized rats down-regulated the cardiac expression of miR-133 and mir-29 genes related to anti-apoptosis and anti-fibrosis, which were probably associated with the increased Bcl-2 and IGF-1 expressions. Also, diabetes led to more reduction in anti-apoptotic and anti-fibrosis-associated genes expression in ovariectomized rats. Our results are in agreement with other studies that reported cardiac fibrosis and apoptosis were related to decreased IGF-1 and Bcl-2 genes expression in OVX or diabetes-induced cardiac myopathy (40, 41).
The implications from humans and models of estrogen deficiency induced cardiomyopathy have revealed that cardiac fibrosis and apoptosis result in heart failure, which leads to both systolic and, in particular, diastolic dysfunction (41, 42).
Effect of estrogen and diabetes on microRNAs expression is investigated in some tissues (43, 44). However, to our knowledge, the role of microRNAs in controlling cardiovascular dysfunction of women with or without diabetes after ovarian hormone deficiency is not known yet. Therefore, in the present study, we focused on changes of miRNA-133, miRNA-29, IGF-1 and Bcl-2 genes, Bcl-2 protein, and caspase 3 activity in the heart of an animal model of ovariectomy with or without diabetes.
Conclusion
This study, for the first time, suggests that downregulation of miRNA-133 and miRNA-29 expressions was involved in the ovariectomy-induced cardiac injury. Also, our results demonstrated that diabetes worsens cardiac apoptosis and fibrosis through decreased miRNA-133, miRNA-29, Bcl-2 and IGF-1 genes expression, anti-apoptotic protein Bcl-2, and increased caspase 3 activity as an apoptotic marker in the heart of OVX.D rats compared with OVX rats.
Acknowledgment
The results reported in this paper were part of the PhD thesis of Parisa Habibi (serial number: 92/1- 6/7) that was supported by Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes A call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006;113:2943–2946. doi: 10.1161/CIRCULATIONAHA.106.176583. [DOI] [PubMed] [Google Scholar]
- 2.Aneja A, Tang WW, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Bhupathy P, Haines CD, Leinwand LA. Influence of sex hormones and phytoestrogens on heart disease in men and women. Women’s Health. 2010;6:77–95. doi: 10.2217/whe.09.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X-P, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:133. doi: 10.1097/MNH.0b013e3283431921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazal L, Azibani F, Vodovar N, Cohen Solal A, Delcayre C, Samuel JL. Effects of biological sex on the pathophysiology of the heart. Br J Pharmacol. 2014;171:555–566. doi: 10.1111/bph.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gama-Carvalho M, Andrade J, Brás-Rosário L. Regulation of cardiac cell fate by microRNAs: implications for heart regeneration. Cells. 2014;3:996–1026. doi: 10.3390/cells3040996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceman S, Saugstad J. MicroRNAs: Meta-controllers of gene expression in synaptic activity emerge as genetic and diagnostic markers of human disease. Pharmacol Ther. 2011;130:26–37. doi: 10.1016/j.pharmthera.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guleria P, Goswami D, Yadav KS. Computational identification of miRNAs and their targets from Crocus sativus L. Arch Biol Sci. 2012;64:65–70. [Google Scholar]
- 9.Abhari A, Zarghami N, Shahnazi V, Barzegar A, Farzadi L, Karami H, et al. Significance of microRNA targeted estrogen receptor in male fertility. Iran J Basic Med Sci. 2014;17:81. [PMC free article] [PubMed] [Google Scholar]
- 10.Da Costa Martins PA, De Windt LJ. MicroRNAs in control of cardiac hypertrophy. Cardiovasc Res. 2012;93:563–572. doi: 10.1093/cvr/cvs013. [DOI] [PubMed] [Google Scholar]
- 11.Roncarati R, Anselmi CV, Losi MA, Papa L, Cavarretta E, Martins PDC, et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2014;63:920–927. doi: 10.1016/j.jacc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Skommer J, Rana I, Marques F, Zhu W, Du Z, Charchar F. Small molecules, big effects: the role of microRNAs in regulation of cardiomyocyte death. Cell Death Dis. 2014;5:e1325. doi: 10.1038/cddis.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A-y, Yang Q, Yang K. miR-133a mediates the hypoxia-induced apoptosis by inhibiting TAGLN2 expression in cardiac myocytes. Mol Cell Biochem. 2015;400:173–181. doi: 10.1007/s11010-014-2273-2. [DOI] [PubMed] [Google Scholar]
- 14.Chavali V, Tyagi SC, Mishra PK. MicroRNA-133a regulates DNA methylation in diabetic cardiomyocytes. Biochem Biophys Res Commun. 2012;425:668–672. doi: 10.1016/j.bbrc.2012.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melo SF, Fernandes T, Baraúna V, Matos KC, Santos AA, Tucci PJ, et al. Expression of microRNA-29 and collagen in cardiac muscle after swimming training in myocardial-infarcted rats. Cell Physiol Biochem. 2014;33:657–669. doi: 10.1159/000358642. [DOI] [PubMed] [Google Scholar]
- 16.Oberbauer A. The regulation of IGF-1 gene transcription and splicing during development and aging. Front Endocrinol. 2013:4. doi: 10.3389/fendo.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touvron M, Escoubet B, Mericskay M, Angelini A, Lamotte L, Santini MP, et al. Locally expressed IGF1 propeptide improves mouse heart function in induced dilated cardiomyopathy by blocking myocardial fibrosis and SRF-dependent CTGF induction. Dis Model Mech. 2012;5:481–491. doi: 10.1242/dmm.009456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musarò A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 19.Irigoyen M-C, Paulini J, Flores LJ, Flues K, Bertagnolli M, Moreira ED, et al. Exercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized rats. Hypertension. 2005;46:998–1003. doi: 10.1161/01.HYP.0000176238.90688.6b. [DOI] [PubMed] [Google Scholar]
- 20.Cai DJ, Zhao Y, Glasier J, Cullen D, Barnes S, Turner CH, et al. Comparative effect of soy protein, soy isoflavones, and 17β-estradiol on bone metabolism in adult ovariectomized rats. J Bone Miner Res. 2005;20:828–839. doi: 10.1359/JBMR.041236. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451. [PubMed] [Google Scholar]
- 22.Soni H, Patel S, Patel G, Paranjape A. Evaluation of anti-diabetic activity of Glucova Active Tablet on Type I and Type II diabetic model in rats. J Ayurveda Integr Med. 2014;5:97. doi: 10.4103/0975-9476.133806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Maric C. Risk factors for cardiovascular disease in women with diabetes. Gender Med. 2010;7:551. doi: 10.1016/j.genm.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 27.Liou CM, Yang AL, Kuo CH, Tin H, Huang CY, Lee SD. Effects of 17beta-estradiol on cardiac apoptosis in ovariectomized rats. Cell Biochem Funct. 2010;28:521–528. doi: 10.1002/cbf.1687. [DOI] [PubMed] [Google Scholar]
- 28.Hata A. Functions of microRNAs in cardiovascular biology and disease. Ann Rev Physiol. 2013;75:69–93. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson DW, Bracken CP, Goodall GJ. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–6853. doi: 10.1093/nar/gkr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boštjančič E, Zidar N, Štajer D, Glavač D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–169. doi: 10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 31.Dong D, Yang Bf. Role of microRNAs in cardiac hypertrophy, myocardial fibrosis and heart failure. Acta Pharm Sin B. 2011;1:1–7. [Google Scholar]
- 32.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277:2015–2021. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang T-C, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CC, Ou HC, Lee SD. Effects of exercise training on cardiac mitochondrial apoptosis in ovariectomized rats. FASEB J. 2010;24:601–5. [Google Scholar]
- 36.Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Deng H, Xu S, Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci. 2015;16:24895–24917. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han C, Chen X, Zhuang R, Xu M, Liu S, Li Q. miR-29a promotes myocardial cell apoptosis induced by high glucose through down-regulating IGF-1. Int J Clin Exp Med. 2015;8:14352. [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh SA, Davidoff AJ. Heart smart insulin-like growth factor 1. Hypertension. 2012;59:550–551. doi: 10.1161/HYPERTENSIONAHA.111.188441. [DOI] [PubMed] [Google Scholar]
- 40.Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4:575. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu CH, Liu JY, Wu JP, Hsieh YH, Liu CJ, Hwang JM, et al. 17β-Estradiol reduces cardiac hypertrophy mediated through the up-regulation of PI3K/Akt and the suppression of calcineurin/NF-AT3 signaling pathways in rats. Life Sci. 2005;78:347–356. doi: 10.1016/j.lfs.2005.04.077. [DOI] [PubMed] [Google Scholar]
- 42.Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens. 2011;2011:495349. doi: 10.4061/2011/495349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Zhang Y, Liang H. [Role of microRNAs in diabetes and diabetes-associated complications] Sheng li ke xue jin zhan [Progress in physiology] 2010;41:133–136. [PubMed] [Google Scholar]
- 44.Queirós AMGCC. Sex-and oestrogen-dependent regulation of miRNAs in cardiac hypertrophy: Humboldt-Universität zu. Berlin: Lebenswissenschaftliche Fakultät; 2015. [Google Scholar]


