Abstract
Objective(s):
Pesticides has wide range of infertility in female reproductive. This study was done to evaluate the effect of movento pesticide on development of granulosa cells and ovarian follicles and FoxO1 and Vnn1 gene expression in BALB/c mice.
Materials and Methods:
In this study 40 healthy BALB/c mice 5-6 weeks age were used. Animals were randomly allocated into four groups. Control (without any intervention), three experimental groups received 25, 50 and 100 mg/kg movento dissolved in PBS by gavage for 21 days. Animals scarified after three weeks. For determining the effects of movento on granulosa cells in culture, treatments were conducted to movento (125, 250, 500 μg/ml) for 24 hr. We surveyed the expression of the FoxO1 and Vnn1 in granulosa cells in vitro, and its relation to cell death by flowcytometer and DAPI. Levels of FoxO1 and Vnn1 were analyzed by real-time PCR.
Results:
Exposure to movento significantly decreased ovarian weight and the number of primary, secondary and antral follicles. Further, treatment with different concentration of movento induced apoptosis on granulosa cells. Gene expression analysis showed the transcriptional expression of FoxO1 and vnn1 in granulosa cells. Level of Vnn1 mRNA in granulosa cells was decreased in granulosa cells and expression of FoxO1 significantly increased in treated groups in compare to controls (P-value <0.05).
Conclusion:
Exposure to movento significantly reduced the number of follicles and increased apoptosis of granulosa cells leading disruption of the reproductive system. Also movento reduced expression of Vnn1 and increased FoxO1 genes in a dose dependent manner.
Keywords: Apoptosis, FoxO1, Granulosa cell, Movento, Ovary, Vnn1
Introduction
Insecticides are an important part of our farming system and have significant role among animals and human from insecticide exposure (1, 2). Pesticides may interfere with the female hormonal function, dysfunction and female infertility thereby cause negative effects on the reproductive system such as impaired gametogenesis, ovulation and menstrual disturbances, infertility, spontaneous abortions, ovarian disorders and developmental anomalies (2, 3).
The exposure to pesticides may induce alterations in survival granulosa cell (GCs) (4). Few data have been reported concerning the effects of these pesticides on oocytes, granulosa, maturation and fertilization. Effect of organochlorine pesticides on maturation of starfish and mouse oocytes showed that many of the tested pesticides have been found to be more toxic to females than males (5).
Movento as a pesticide is innovative, containing active substances such as spirotetramat that derived from tetramic acid (6). Spirotetramat, cis-4-(thoxy-carbonyloxy)-8-methoxy-3-(2,5-xylyl)-1 azaspiro [4.5]dec-3-en-2-one, an insecticide is used against a wide range of insect pests in a variety of crops, in grapevine orchards and other vegetables for the control of sucking pests and domestic practices (6-8). The movento is an inhibitor of lipid biosynthesis and metabolism against sucking insect pests (9). The overall acute toxicity of spirotetramat as well as long term, reproductive, neuro and geno-toxicity substance are low (10). Movento induces liver, kidney, pancreas and spleen toxicity in male Wistar rats (7). Also a study showed that movento toxicity effects on the growth and skeletal disorders as well as has a neurotoxicity (6).
Vanin-1 (Vnn1) is a member of a protein family, consisting of at least three members in humans (Vanin-1, -2, and -3) and two members in mice (Vanin-1 and -3) (11). It is one of main signs of follicle integrity. Forkhead O (FoxO), a subfamily of transcription factors, are able to regulate diverse cellular processes that include cell cycle arrest, DNA damage repair, apoptosis, proliferation, differentiation, stress response, senescence, longevity and metabolism (12-15). The importance of FoxO transcription factors have been demonstrated in mammalian ovaries.
This study was done to evaluate the effect of movento on development of granulosa cells and ovarian follicles and FoxO1 and Vnn1 gene expression in BALB/c mice.
Materials and Methods
Chemicals
DMEM medium 1X, FBS (fetal bovein serum), PBS (phosphate buffer salin), Trypsin-EDTA, antibiotic (penicillin-streptomycin) and ITS (insulin transferin selenium) were purchased from Gibco (USA). DAPI solution prepared from Applichem (USA), and AnnexinV-FITC kit was purchased from Abcam (England), FSH (Follicle Stimulating Hormone) was purchased from BSV (Germany), and methanol from Merck (Germany). The RNA isolation kit and C-DNA synthesis kit were purchased from Thermo Scientific (EU), SYBR Green Real-time PCR mastermix kit was purchased from Pars Tous (Iran), Xylenand and Bouin Fixative solution were purchased from Sigma (Germany), Bouins Fixative solution was from Sigma (Germany). Movento pesticide was provided from Bayer (Germany). Homozygous female BALB/c mice were purchased from Pasteur institute of Tehran, Iran.
Experimental animals
In this study, 40 healthy female BALB/c mice 5–6 week’s age, weighing 25–30 g were used. Temperature was maintained at 22±1 °C, and animals were subjected to 12 hr light-dark cycles. Animals were acclimatized for one week in laboratory conditions. Animals were randomly allocated into four groups. Control (without any intervention), and to the remaining three groups, movento was dissolved in PBS, administered as: experimental group 1 (25 mg/kg), experimental group 2 (50 mg/kg), and experimental group 3 (100 mg/kg), through oral intubation (by gavage) for 21 days. All of these animals were euthanized after three weeks. Following dissection, the ovaries were fixed in Bouin’s solution for 24 hr and then embedded in paraffin wax (melting point 58–60 °C). The 5 µm thick serial sections were stained with hematoxylin and eosin (H&E), and slides were observed by Olympus microscope (Japan).
Ovary weight and total number of normal primary, secondary and antral follicles were counted by Kaur and Guraya method (16).
Cell culture
Female BALB/c mice were euthanized and their ovaries were removed. Granulosa cells were isolated mechanically from the ovary. The granulosa cells were put into culture plate containing DMEM medium 1X(1:1) with 10% (v/v) FBS, ITS 10U, FSH 100U and 1% antibiotic and incubated at 37 °C with 5% CO2 for 5 days. For examining the effects of movento on granulosa cells in culture, treatment were conducted using different concentrations of movento (125, 250, 500 μg/ml) for 24 hr.
DAPI staining
DAPI (4’, 6-diamidino-2-phenylindole dihydro-chloride) staining was used to evaluate the changes morphology of the nuclei. For the nuclear analysis, after treatment of the granulosa cells with different concentrations of movento as previously explained, the cells were washed using PBS and fixed with methanol at room temperature for 10 min. Then, the fixed cells were incubated with 0.5 μg/ml of DAPI for 5 min. The apoptotic nuclei were examined under fluorescent microscope.
Flow cytometry analysis of apoptosis
After exposure of granulosa cells to movento, granulosa cells were washed in PBS. Then, the cells were trypsinized and resuspended in 500 μl of 1X binding buffer and 5 µl Annexin V- FITC and 5 µl PI were added and incubated at 37 °C for 30 min in dark. Finally, were analyzed the cells by flow cytometry with WinMDI software. Positioning of quadrants Annexin V/PI on dot plots was performed and living cells (Annexin V−/PI−), early apoptotic cells (Annexin V+/PI−), late apoptotic (Annexin V+/PI+) and necrotic cells (Annexin V−/PI+) were distinguished.
Evaluation of gene expression by real-time PCR
The expression of FoxO1 and Vnn1 mRNA were evaluated by real-time PCR. The total RNA was extracted from untreated and treated granulosa cells using the high pure RNA isolation kit (Thermo Scientific, USA) according to the manufacturer’s instructions and then stored at -80°C. Then DNA was synthesized using a revertaid first strand cDNA synthesis kit (Thermo Scientific, USA) according to the manufacturer’s protocol. Real-time experiments were conducted on a real-time PCR detection system (Bio-Rad CFX96) using SYBR Green real-time PCR master mix (Pars Tous, Iran).
The reaction PCR was performed in a final volume of 20 μl containing: 10 μl SYBR Green real-time PCR master mix, 2 μl cDNA, 4 μl water and 2 μl primer (forward and reverse). The sequences of the primers used are as follows:
FoxO1 Forward 5’TACGCCGACCTCATCACC 3’
Reverse 5’CACGCTCTTCACCATCCACT 3’
Vnn1 Forward 5’AAGTGTTGCTGAGTGAGG 3’
Reverse 5’TGTGCTATGAAGTCTGAGG 3’
GAPDH Forward 5’CAAGGTCATCCATGACAACTTTG 3’
Reverse 5’GTCCACCACCCTGTTGCTGTAG 3’, which was used as reference gene.
Statistical analysis
Data were expressed as mean±standard error. Data were analyzed by one-way ANOVA followed by the Tukey test, and the differences were considered statistically significant at P-value < 0.05.
Results
Histological studies
In the phases of the follicular development, primary, secondary and antral observed in control and movento exposed-treated groups. The total number of primary, secondary and antral follicles were reduced significantly in movento exposed groups (25 µg/ml, 50 µg/ml and 100 µg/ml) in compare to the controls (**P-value<0.01, ***P-value < 0.001) in (Table 1 and Figure 1).
Table 1.
The number of primary, secondary and antral follicle in control group and treated groups at 21 days after administrating movento with different concentrations
| Parameters | Control | 25 mov µg/ml | 50 mov µg/ml | 100 mov µg/ml |
|---|---|---|---|---|
| Primary | 5.17± 0.75 | 2.83± 0.75** | 2.17± 0.41*** | 1.33± 0.52*** |
| Secondary | 4.17± 1.17 | 1.83± 0.41*** | 1.33± 0.52*** | 0.83± 0.41*** |
| Antral | 2.67± 0.82 | 1.17± 0.41*** | 0.83± 0.41*** | 0.67± 0.52*** |
The values are expressed as mean±SD of 6 animals in each group. Significant difference
P-value<0.01,
P-value<0.001) as compare to control
Figure 1.
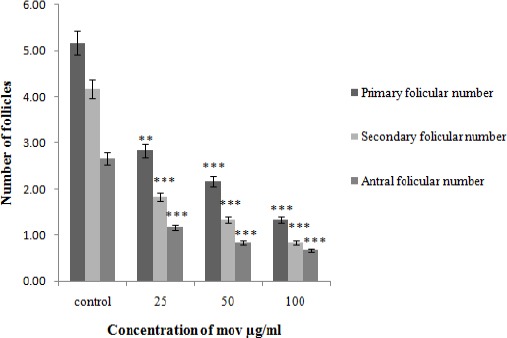
Number of follicular primary, secondary and antral in different test groups (25, 50, 100 µg/ml) in compare to the control group at 21 days after administrating movento with different concentrations. Data are expressed as mean±SD. The values are significantly different (**P-value<0.01, ***P-value< 0.001)
Ovary weight
Ovary weight significantly reduced in exposed movento experimental groups in compare to the control group (***P-value<0.001) (Figure 2).
Figure 2.
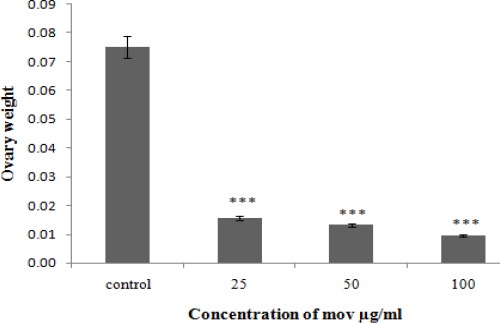
Weight of ovarian in control group and treated groups at 21 days after administrating movento with different concentrations. Data are expressed as mean±SD. The values were significantly different (***P-value<0.001)
In vitro studies
Cell shrinkage, reducing cell volume, plasma membrane distortion, nuclear condensation and apoptotic bodies of granulosa cells which are all known as the apoptotic characteristics were observed in exposed movento experimental groups in compare to the control group (Figure 3A).
Figure 3.
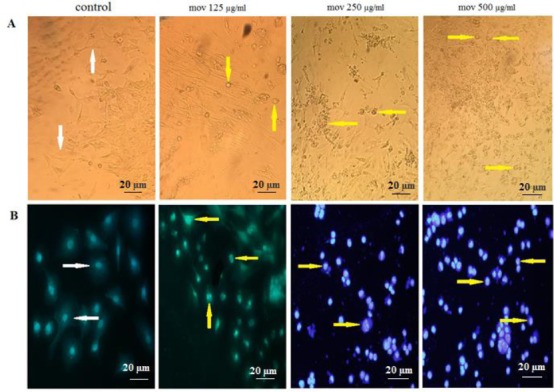
A) Cytomorphological effects of movento on granulosa cells. Granulosa cells were treated with different concentrations of movento (125, 250, 500 μg/ml). The morphology of granulosa cells 24 hr after treatment. B) The fluorescence micrograph of granulosa cells treated with different concentrations of movento (125, 250, 500 μg/ml) after 24 hr treatment with DAPI assay (20X magnification)
Detecting apoptosis by DAPI staining
Based on the output of DAPI staining, DNA fragmentation occurred in 125 µg/ml (particularly in 250 and 500 µg/ml) of granulosa cells of movento treated groups in compare to the untreated cells (Figure 3B).
In DAPI staining, the nuclei are perfectly round in the untreated cells while the treated cells with movento showed some features of apoptosis such as condensed chromatin and fragmented nuclear.
Flow cytometry analysis for apoptosis detection
Annexin V-FITC is a recombinant protein that specifically binds to phosphatidylserine and remains with high affinity for distinguishing apoptosis from necrosis. Studying the effects of different movento pesticide concentrations on granulosa cells, using Annexin V-FITC/PI kit, indicated that in 125 µg/ml, 250 µg/ml and 500 µg/ml concentrations of movento the apoptotic ratio increased in treated granulosa cells compared to untreated cells. These results demonstrated that movento pesticide, like other chemical pesticide, inhibited granulosa cells proliferation by inducing apoptosis (Figure 4).
Figure 4.
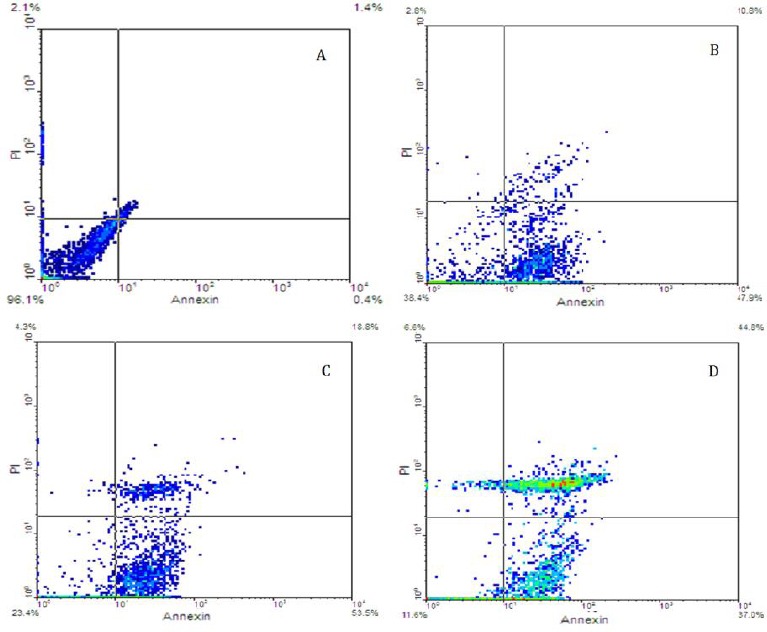
Flow cytometry results. Demonestrating apoptosis of granulosa cells, which were treated using different concentrations of movento, after 24 hr based on Annexin-v/PI assay. A) Control, B) 125 µg/ml, C) 250 µg/ml and D) 500 µg/ml of movento
Effect of movento on FoxO1 and Vnn1 expression in granulosa cells
Gene expression analysis involved in granulosa cells treated with various concentrations of movento was evaluated by real- time PCR. Our data exhibited increasing concentrations of movento elevated the transcription of FOXO1 and attenuated the expression of VNN1 in granulose cells as compared with the control group (Figure 5).
Figure 5.
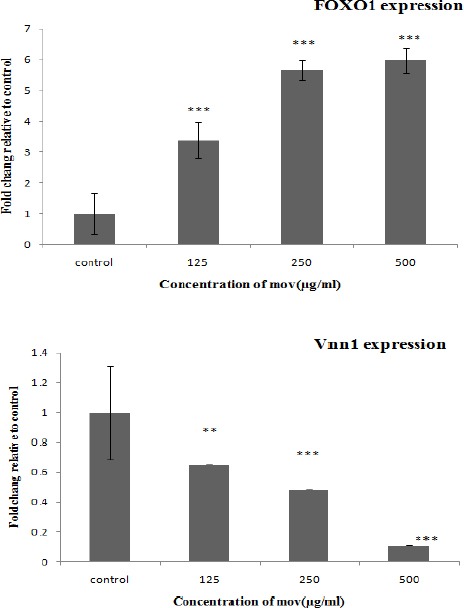
FoxO1 and Vnn1 genes expression in granulosa cells treated with various concentrations of movento (125, 250, 500 μg/ml). Data are expressed as mean±SD. The values are significantly different (**P-value < 0.01, *** P-value < 0.001) in comparison with the controls
Discussion
Commonly pesticides are used in agriculture, industry and health against insects and pests. Pesticides and insecticides have a wide range of infertility in men and women but relatively little is known about their possible adverse health effects, especially their female reproductive effects (5). Exposure to pesticide during development could cause molecular and morphological alterations in the ovary, possibly leading to multiple reproductive abnormalities, including accelerated puberty, irregular reproductive cyclicity, and early reproductive senescence. Also, exposure directly affects the ovary, leads to adult ovarian dysfunction and female infertility (17).
The present study is the first study to show the effect of movento pesticide on expression of folliculogenic genes in granulosa cells of mice. Our finding showed that exposure to movento significantly decreased ovarian weight in female mice. Also movento reduced the number of primary, secondary and antral follicles. Further, treatment with different concentration of movento induced apoptosis on granulosa cells, which might disrupt the reproductive system.
Mokhtar et al in 2013 showed that pesticides disrupt hormonal regulation, change the levels of estrogen and progesterone hormones, induce endocrine disorders, reduced ovulation and fertility, increased follicular atresia in rats (2).
Also, exposed male rats to movento pesticide increased abnormal sperm cell count, caused hypospermya in epididymis, testicular weight loss and degeneration of the testes (6).
Our results showed that treatment of mice with movento significantly decreased the number of ovarian follicles (primary and secondary and antral), which is similar to a study carried out by Saleh et al in 2014 (18). They demonstrated the reduction in ovarian follicles, reduction of the corpus luteumas well as increase in the number of atretic follicles in rats treated with the carbamate insecticides.
Several studies display that the weight of the mice female reproductive system significantly decreased with thiodicarb treatment. Degeneration of the ovarian follicles and increase of atretic follicles induced by thiodicarb may result from the inhibition of RNA synthesis in the follicular cells (19, 20).
Previous studies, evaluated malathion-induced reproductive stress, damage and changes in apoptosis frequency in ovarian granulosa cells of antral follicles showed a positive correlation between malathion-induced lipid peroxidation and granulosa cell apoptosis. Bhardwaj and Saraf demonstrated that there is a significantly increased percentage of granulosa cell apoptosis in antral follicles at flow cytometry. Increased levels of apoptosis account for a large-scale loss of healthy follicles responsible for follicular atresia (21).
This is in accordance with studies on other organophosphore substance increased level of atretic granulosa cells due to pesticide exposure (22, 23). Apoptosis in granulosa cells could be the consequence of several factors and in the influence of multiple pathways. Biochemical changes in the cells play a pivotal role in bringing down its apoptosis (24).
Oxidative stress in the cell may cause cell death because ROS are cell permeable and can lead to activation of proapoptotic signaling molecules.
Malathion exposure induces cytogenetic damage in the granulosa cells that leads to condensation of chromatin, loss of nuclear membrane integrity, damage to the structure of mitochondria, and lipid droplets with increased vacuolization (21). Sharma et al in 2015 showed that apoptosis of ovary granulosa cells can be an indicator of the apoptotic behavior of the cell against Triazophos -induced oxidative stress. As more atresia due to TZ intoxication, more granulosa cells were programmed to die through apoptosis (5).
In the present study, movento has a moderate oxidative activity in different antioxidant systems and could induce apoptosis and inhibit the growth of granulosa cells in a dose dependent manner. Some genes are activated during development and are specific to a particular transformation during follicular development. Expression of these genes observed in atretic follicles is consistent with the reduced proliferation that characterizes this stage, and suggests that they can be used as biomarkers to identify follicles committed to atresia (25, 26).
Expression of some genes such as Vnn1 increased as a function of time as well as follicle diameter. These genes are potential indicators of follicular growth and/or differentiation. They are necessary to reach the maximal competence status (27). Vnn1 (vanin1) is known to act as a regulator of tissue response to oxidative stress by modulating the GSH store in mouse (28, 29). Girard et al found that Vnn1 responded to oxidative stress and decreased expression of this gene is related to cell proliferation and its disruption caused atresia of granulosa cells and induced oxidative stress (30). The previous studies showed that expression of Vnn1 (Vanin-1) in the development of mouse gonad is required to provide an appropriate environment for male germ cell development (31).
Our findings showed that with increasing concentrations of movento, Vnn1expression levels decreased which is an indicative of atresia of granulosa cells.
FoxO transcription factors are known as critical mediators in the regulation of oxidative stress and apoptosis. FOXO1, FOXO3 and FOXO4 are expressed in rodent ovaries granulosa cells. Matsuda et al study indicated that the level of FOXO1 is elevated in granulosa cells of developing follicles (32).
Shen et al in in vivo and in vitro models, demonstrated the involvement of FoxO1 in oxidative stress-induced apoptosis of mouse follicular granulosa cells (MGCs). Shen observations showed that increased apoptotic signals correlated with elevated expression of FoxO1 in MGCs when mice were treated with the oxidant (33). Also, overexpression of wild-type FoxO1 or a constitutively active FoxO1 mutant apparently enhanced MGC apoptosis rates. In contrast, when the FoxO1 expression is knocking down, the MGCs could be prevented from the oxidant-induced apoptosis (33). Evidence from various types of mammal cells indicated that FoxO1 induces apoptosis by activating apoptosis-related gene expression (34).
Also, our real-time PCR analysis showed that FoxO1 expression significantly increased in granullosa cells, exposed to movento pesticide.
FoxO1 is expressed in mice ovarian follicles especially in granullosa cells and induces apoptosis in granullosa cells, suggesting that it is a candidate to identify follicular atresia.
Conclusion
This study showed that exposure to movento significantly reduced ovarian weight, number of primary, secondary and antral follicles and apoptosis of granulosa cells which can lead to disruption the reproductive system. Also, this study showed that movento pesticide reduced expression of Vnn1 and increased FoxO1 genes in a dose dependent manner.
Acknowledgment
The authors have special thanks to the Science and Research Branch, Tehran Islamic Azad University, Tehran, Iran; Animal Development and Applied Biology Research Center of Mashhad Branch, Islamic Azad University, Mashhad, Iran for their support of this work as a PhD dissertation.
References
- 1.Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- 2.Mokhtar HI, Abdel HA, Elmazoudy RH, Abdelwahab WM, Saad MI. Effect of methomyl on fertility, embryotoxicity and physiological parameters in female rats. J App Pharm Sci. 2013;3:109–119. [Google Scholar]
- 3.Saleh AMT, Hijji AM, Elel SJ, Al Yacoub AN. Effect of carbamate insecticide, Lannate, on the gonads of mice. J Adv. Lab. Res. Biol. 2014;5:140–145. [Google Scholar]
- 4.Sargazi Z, Nikravesh MR, Jalali M, Sadeghnia HR, RahimiAnbarkeh F, Mohammadzadeh L. Gender-related differences in sensitivity to diazinonin gonads of adult rats and the protective effect of vitamin E. IJWHR Sci. 2015;3:40–47. [Google Scholar]
- 5.Sharma D, Sangha GK, Khera KS. Triazophos-induced oxidative stress and histomorphological changes in ovary of female wistar rats. pestic Biochem physiol. 2015;117:9–18. doi: 10.1016/j.pestbp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Sverdrup LE, Bjorge C, Eklo OM, Grung M, Kallqvist T, Klingen I, et al. Risk assessment of the insecticide Movento 100 SC with the active substance spirotetramat. Bayer Crop Sci. 2012;13:1–25. [Google Scholar]
- 7.Etchechury FM, Gramont S, Sanchez R, Romero C, Enriquez R, Cervantes L, et al. Spirotetramat induces histological and biochemical changes in wistar rats. Rev Toxicol. 2015;30:215–217. [Google Scholar]
- 8.Roffeni S, Arangeli G, Boebel A, Gollo M, Gualco A, Venturini V, et al. Spirotetramat (MOVENTO®): A novel systemic insecticide to control main harmful sucking pests of cultivated crops. ATTI Giornate Fitopatolo. 2010;1:3–10. [Google Scholar]
- 9.Marcic D, Mutavdzic S, Medjo I, Prijovic M, Peric P. Spirotetramat toxicity to immatures and sublethal effects on fecundity of female adults of Tetranychus urticae Koch. J Zoosymposia. 2011;6:99–103. [Google Scholar]
- 10.Bruck E, Elbert A, Fischer R, Krueger S, Kuhnhold J, Klueken AM, et al. Movento® an innovative ambimobile insecticide for sucking insect pest control in agriculture: Biological profile and field performance. Crop prot. 2009;10:838–844. [Google Scholar]
- 11.Martin F, Malergue F, Pitari G, Philippe JM, Philips S, Chabret C, et al. Vanin genes are clustered (human 6q22–24 and mouse 10A2B1) and encode isoforms of pantetheinase ectoenzymes. Immunogenetics. 2001;53:296–306. doi: 10.1007/s002510100327. [DOI] [PubMed] [Google Scholar]
- 12.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 14.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 16.Kaur P, Guraya SS. Follicular growth and kinetics during the estrous cycle, pregnancy and postpartum in the Indian mole rat (Bandicota bengalensis) Am J Anat. 1983;166:469–482. doi: 10.1002/aja.1001660407. [DOI] [PubMed] [Google Scholar]
- 17.Armenti AME, Mahakali ZA, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian: folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh AMT, Hijji AM, Elel SJ, Al Yacoub AN. Effect of carbamate insecticide, lannate, on the gonads of mice. J Adv. Lab. Res. Biol. 2014:140–145. [Google Scholar]
- 19.El-Sayyad HI. Histological effects of thiosicarb on the reproductive system of the adult female mouse. J Egypt Germ. Soc. Zool. 1994;13:47–65. [Google Scholar]
- 20.Matter FE, Farid NM, Rageb AF. A histochemical study on the ovaries of rat treated with an insecticide. “Carbaryl”. Egypt J Histol. 2000;4:87–92. [Google Scholar]
- 21.Bhardwaj JK, Saraf P. Malathion-induced granulosa cell apoptosis in caprine nntral follicles: an ultrastructural and flow cytometric analysis. Microsc Microanal. 2014;20:1861–1868. doi: 10.1017/S1431927614013452. [DOI] [PubMed] [Google Scholar]
- 22.Wojtowicz AK, Gregoraszczuk EL, Ptak A, Falandysz J. Effect of single and repeated in vitro exposure of ovarian follicles to o, p’-DDT and p, p’-DDT and their metabolites. Pol J Pharmacol. 2004;56:465–472. [PubMed] [Google Scholar]
- 23.Rocket JC, Narotsky MG, Thomson KE, Thilladarajah I, Blystone CB, Goetza A, et al. Effect of conozole fungicides on reproductive development in female rats. J Reprod Toxicol. 2006;22:647–648. doi: 10.1016/j.reprotox.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Carbone MC, Tatone C, Delle MS, Marci R, Caserta D, Colonna R, et al. Antioxidant enzymatic defenses in human follicular fluid: Characterization and agedependent changes. Mol Hum Reprod. 2003;9:639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- 25.Li HJ, Liu DJ, Cang M, Wang LM, Jin MZ, Ma YZ, Shorgan B. Early apoptosis is associated with improved developmental potential in bovine oocytes. Anim Reprod Sci. 2009;114:89–98. doi: 10.1016/j.anireprosci.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Bunel A, Nivet A, Blondin P, Vigneault C, Richard F, Sirard M. Cumulus cell gene expression associated with pre-ovulatory acquisition of developmental competence in bovine oocytes. Reprod Fertil Dev. 2014;26:855–865. doi: 10.1071/RD13061. [DOI] [PubMed] [Google Scholar]
- 27.Nivet AL, Vigneault C, Blondin P, Sirard MA. Changes in granulosa cells’ gene expression associated with increased oocyte competence in bovine. Reproduction. 2013;145:555–565. doi: 10.1530/REP-13-0032. [DOI] [PubMed] [Google Scholar]
- 28.Grimmond S, Van Hateren N, Siggers P, Arkell R, Larder R, Soares MB, et al. Sexually dimorphic expression of protease nexin-1 and vanin-1 in the developing mouse gonad prior to overt differentiation suggests a role in mammalian sexual development. Hum Mol Genet. 2000;12:1553–1560. doi: 10.1093/hmg/9.10.1553. [DOI] [PubMed] [Google Scholar]
- 29.Berruyer C, Martin FM, Castellano R, Macone A, Malergue F, Garrido-Urbani S, et al. Vanin-1K/K mice exhibit a glutathione-mediated tissue resistanceto oxidative stress. Mol Cell Biol. 2004;24:7214–7224. doi: 10.1128/MCB.24.16.7214-7224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girard A, Dufort I, Douville G, Sirard MA. Global gene expression in granulosa cells of growing, plateau and atretic dominant follicles in cattle. J Reprod Biol Endocrinol. 2015;13:1–15. doi: 10.1186/s12958-015-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megan JW, Jeyasuria P, Keith LP, Koopman P. The transcription factors steroidogenic factor-1 and SOX9 regulate expression of Vanin-1 during mouse testis development. J Biol chem. 2005;18:5917–5923. doi: 10.1074/jbc.M412806200. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda F, Inoue N, Maeda A, Cheng Y, Sai T, Gonda H, et al. Expression and function of apoptosis initiator FOXO3 in granulosa cells during follicular atresia in pig ovaries. J Reprod Dev. 2011;57:151–158. doi: 10.1262/jrd.10-124h. [DOI] [PubMed] [Google Scholar]
- 33.Shen M, Lin F, Zhang J, Tang Y, Chen WK, Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J Biol chem. 2012;31:25727–25740. doi: 10.1074/jbc.M112.349902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgering BM, Medema RH. Decisions on life and death: FOXO forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]


