Abstract
Introduction
Locoregional variation in the human colon is important in surgical practice; the length and mobility of different colonic regions impacts on laparoscopic and endoscopic colorectal procedures. The aim of this study was to refine anatomical understanding of the colon in terms of segmental length and mobility.
Methods
The colons of 35 cadavers were examined to determine lengths of caecum as well as ascending, transverse, descending and rectosigmoid colon, and to characterise colonic mobility at each location in terms of the mesenteric attachments. The presence of Jackson’s membrane (a congenital peritoneal band of the right colon) was also documented.
Results
The mean total colonic length was 131.2cm (standard deviation [SD]: 13.4cm). There was no correlation with height, age or sex; the best predictor of total colonic length was the length of the rectosigmoid segment. The mean height of the transverse mesocolon was 7.4cm (SD: 3.6cm) and that of the sigmoid mesocolon was 6.3cm (SD: 2.6cm). Two-thirds of the subjects had a mobile portion of the ascending colon and nearly one-third had a mobile descending colon. A mobile ascending colon was significantly more common in females. Jackson’s membrane was present in 66% of the subjects.
Conclusions
This cadaveric study suggests that rectosigmoid length accounts for most of the variability in total colonic length. The significant proportion of colons with mobility of the ascending and descending segments prompts revision of the traditional anatomical teaching of these segments as fixed and retroperitoneal. Mobility of the ascending colon may account for the anecdotal finding that colonoscopy is more challenging in female patients. Jackson’s membrane was identified in most colons.
Keywords: Colon, Mesocolon, Ascending, Descending, Transverse, Sigmoid
Locoregional variation in the human colon is increasingly important in surgical practice. Adenoma incidence,1,2 molecular protein expression3 and APC gene inactivation4 have all been found to vary by colonic location. More practically, the difficulty and duration of clinical procedures, including laparoscopic resections and colonic endoscopy, are often affected by the length and mobility of colonic segments.5 Increased length and mobility of the sigmoid colon and caecum may also predispose a patient to volvulus.
Overall colonic length has been investigated previously in both live patients6–9 and non-fixed cadavers10–12 with varying results. Some of these studies also measured the length of individual colonic segments (ascending, transverse, descending and sigmoid).7–9,12 However, comparison between the studies is hampered by differing definitions of the colonic segments. No previous study has analysed how the length of each segment correlates with total colonic length.
In clinical practice, the mobility and length of individual colonic segments is arguably as important as overall colonic length. The traditional anatomical view is that the ascending and descending colon are fixed and retroperitoneal. Embryologically, the human midgut (including the right colon and two-thirds of the proximal transverse colon) returns to the abdomen in the tenth week. Thereafter, the dorsal mesenteries of the ascending and descending colon degenerate while the posterior body wall lengthens. Consequently, the ascending and descending colon become ‘secondarily’ retroperitoneal while the caecum becomes fixed by a shortened mesentery, and the transverse and sigmoid colon remain suspended.13 A congenital peritoneal band, ‘Jackson’s membrane’, has been described as a broad layer of peritoneum or peritoneum-like membrane arising from the posterolateral wall of the abdominal cavity on the right side, emerging above from beneath the liver and at times extending downward to the outer side of the ascending colon as far as the caecum.14,15
Gray’s Anatomy notes that ‘the caecum and ascending colon may possess a mesentery to a variable degree’ while the descending colon ‘is a retroperitoneal structure covered anteriorly and on both sides by peritoneum’ although it also goes on to state that it is ‘more frequently covered posteriorly by peritoneum, than the ascending colon’.16 In contrast, significant mobility of the right and left colon is commonly noted clinically during resection. Variation in anatomical description and absence in the published literature of the frequency with which individual colonic segments are found to be mobile emphasises that clarification is needed on this subject.
The aim of this cadaveric study was to refine our present understanding of colonic anatomy, not only in terms of total length but also in terms of the length of clinically relevant colonic segments, and to identify any factors predictive of colonic length. We also sought to further characterise the mobility of these colonic segments with regard to their mesenteric attachments. Lastly, the incidence of Jackson’s membrane was investigated.
Methods
Forty-eight human cadavers were examined. All cadavers were fixed using a mixture of formaldehyde, ethanol and methanol. The medical history for each subject was reviewed. Exclusion criteria included a previous diagnosis of bowel malignancy, inflammatory bowel disease, bowel resection (not including appendicectomy) or intra-abdominal pathology that could potentially distort the normal anatomy of the colon. Demographic details of the subjects were recorded, including age at death, height and sex.
Colonic measurements were performed with a flexible tape measure by four researchers working in pairs to ensure validity and consistency of measurement. Colonic length measurements were made along the course of the anterior taenia coli using predetermined reference points (Table 1, Fig 1).
Table 1.
Reference points for measurements in this study. (See Fig 1.)
| Measurement | Start point | End point |
| a) Length of ascending colon (plus caecum) | Base of appendix | Hepatic flexure |
| b) Length of transverse colon | Hepatic flexure | Splenic flexure |
| c) Length of descending colon | Splenic flexure | End of descending colon |
| d) Length of rectosigmoid colon | Genu of descending colon at pelvic brim | Point of passage through pelvic peritoneum |
| e) Total colonic length | = a + b + c + d | |
| f) Height of transverse mesenterya | Root of transverse mesentery at posterior abdominal wall | Mesenteric wall of transverse colon |
| g) Height of sigmoid mesenterya | Root of sigmoid mesentery | Mesenteric wall of sigmoid colon |
| h) Length of mobileb ascending colon | Base of appendix | Most proximal point fixedc to abdominal wall |
| i) Length of mobile descending colon | Distal end of descending colon (genu) | Most proximal point fixed to abdominal wall |
| j) Peritoneal band (if present) | Base of appendix | Ascending colon parallel to band adhesion to lateral abdominal wall |
Greatest perpendicular distance between the two points with posteroanterior traction applied to the colon segment
A ‘mobile’ segment of colon has a visceral layer of peritoneum covering the colonic serosa such that it separates it from the parietal peritoneum, demonstrable by the presence of a distinct, palpable edge to the colon, which is connected to the parietal peritoneum via a ‘mesentery’.
Complete adherence of the visceral peritoneum covering the colonic serosa to the parietal peritoneum of the posterior abdominal wall; two layers that are inseparable under normal conditions
Figure 1.
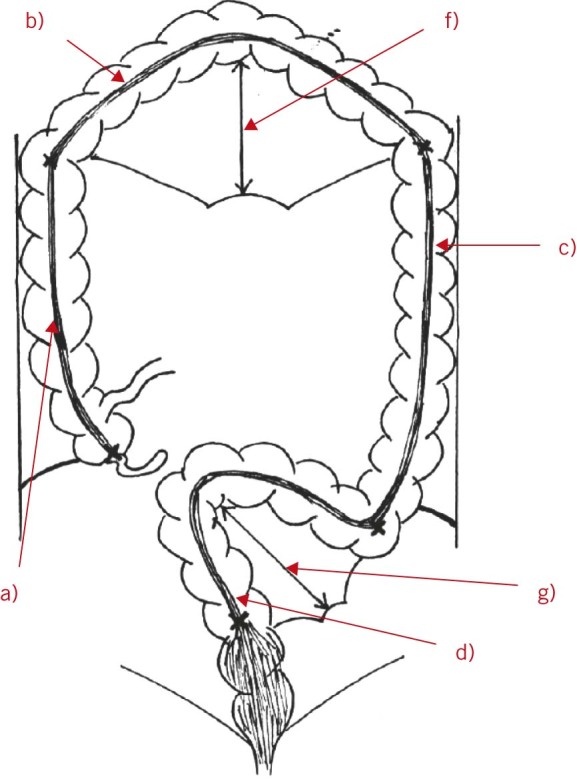
Diagram showing measurements for this study. (See Table 1.)
The transition from the ascending colon to the transverse colon was the point where the most superior point flexes medially, the transition from the transverse colon to the descending colon was the point where the most lateral point flexes inferiorly and the transition from the descending colon to the sigmoid colon was the genu of the descending colon at the pelvic brim. Owing to the difficulty in defining the point at which the caecum meets the ascending colon, these two sections of the colon were measured together. Furthermore, because of difficulty in defining the transition point from sigmoid colon to rectum, particularly when observing only the gross anatomy of the external surface of the colon, a portion of the rectum (intraperitoneal) was included in the section of the colon referred to in this paper as ‘rectosigmoid’.
Two surrogate measures of ‘bowel mobility’ were recorded. The first was the height of the mesentery from the root of the mesentery on the posterior abdominal wall to the mesenteric border of the transverse and sigmoid colon (those parts of the bowel considered traditionally to be suspended on a mesentery). This was measured by gently elevating the bowel segments and measuring the resulting distance. The length of ‘mobile’ ascending and descending colon was considered to be the segmental length that was suspended on a mesentery as opposed to lying in a (secondarily) retroperitoneal position.
The mesentery was considered present in cases where two layers of visceral peritoneum met, having completely enveloped the full circumference of the colon, forming a palpable ridge that is connected subsequently to the parietal peritoneum (Fig 2). The mesentery of the descending colon was distinct from the mesentery of the sigmoid colon as where present, it formed a ridge of variable length in the superior to inferior direction, as opposed to the inverted-V nature of the sigmoid mesentery.
Figure 2.
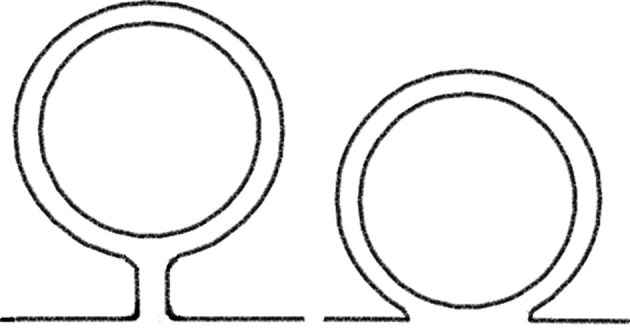
Diagram demonstrating colon with mesentery (left) and retroperitoneal mesentery (right)
Statistical analysis
The data were analysed using Prism® (GraphPad Software, La Jolla, CA, US). Distributions were tested for normality using the Kolmogorov–Smirnov test and D’Agostino–Pearson omnibus test. Group comparison analysis was performed using the paired t-test and Mann–Whitney U test. Linear regression was used to test correlation, with a p-value of &8804;0.05 considered statistically significant.
Results
Thirteen cadavers were excluded from the study owing to a history of colonic malignancy and resection or existing colonic malignancy at time of death. Thirty-five (18 male and 17 female) cadavers remained in the study. The mean age was 84 years (standard deviation [SD]: 13.2 years) and the mean height at time of death was 174.3cm (SD: 9.1cm).
Colonic length
The mean total length of colon and intraperitoneal rectum was 131.2cm (SD: 13.4cm). The lengths of the colonic segments are shown in Table 2 and Figure 3. It was identified that the length of the rectosigmoid segment strongly predicted total colonic length (R2=0.40, p<0.0001) (Fig 4). This had not been hypothesised prior to the study. The length of each of the other segments did not correlate with the total colonic length.
Table 2.
Segmental colonic length
| Mean | |
| Ascending colon (plus caecum) | 20.9cm (SD: 4.7cm) |
| Transverse colon | 50.2cm (SD: 9.5cm) |
| Descending colon | 21.8cm (SD: 5.4cm) |
| Rectosigmoid colon | 38.3cm (SD: 10.5cm) |
Figure 3.
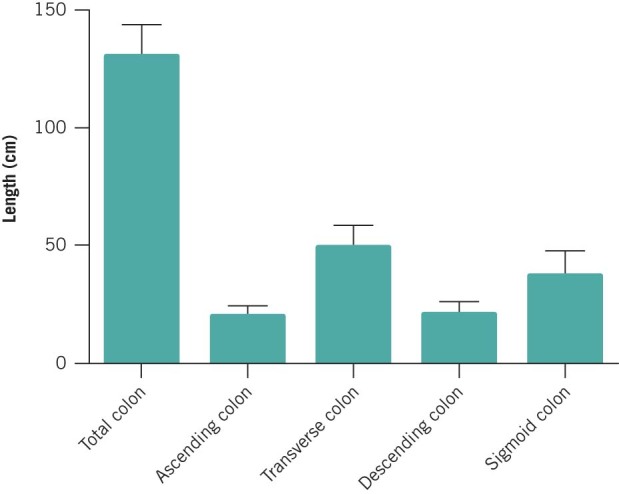
Mean total colonic length and segmental lengths
Figure 4.
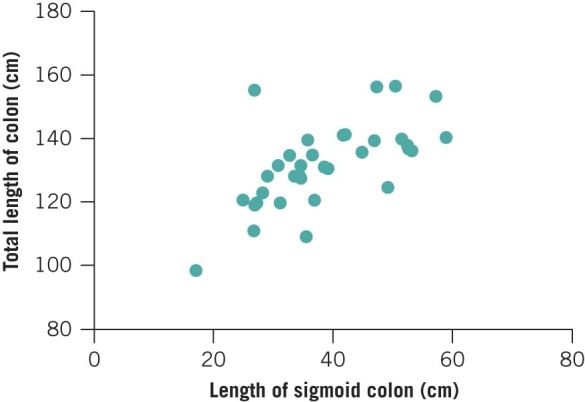
Relationship between length of sigmoid colon and total colonic length
There was no significant difference in colonic length between male and female subjects (133.8cm [SD: 3.7cm] vs 128.9cm [SD: 2.7cm]). Similarly, age at death and height were not predictive of total colonic length (although the range of ages was narrow in this study).
Colonic mobility
A proximal portion of ascending colon was mobile (defined by the presence of a distinct, palpable edge to the colon, which is connected to the parietal peritoneum via a mesentery) in 23 subjects (66%). In these cases, 53.9% (SD: 27.0 percentage points) of the length of the ascending colon was suspended on a mesentery. There were mobile portions of the descending colon in 11 cases (31%), with 44.4% (SD: 29.0 percentage points) of the length of the descending colon suspended on a mesentery. The transverse colon and rectosigmoid colon were invariably mobile. The perpendicular heights of the transverse colon and rectosigmoid colon mesenteries were 7.4cm (SD: 3.6cm) and 6.3cm (SD: 2.6cm) respectively. The maximal perpendicular height of the transverse colon mesentery correlated with the total length of transverse colon (R2=0.34, p=0.0002). There was a similar relationship for the rectosigmoid colon (R2=0.40, p=0.0001).
Female subjects were significantly more likely to have a mobile portion of the ascending colon (62.7% [95% confidence interval: 38.0–74.9%] vs 0.0% [95% confidence interval: 0.0–13.2%]; Mann–Whitney U test, p=0.0003) (Fig 5). However, there was no significant sex difference in descending colonic mobility.
Figure 5.
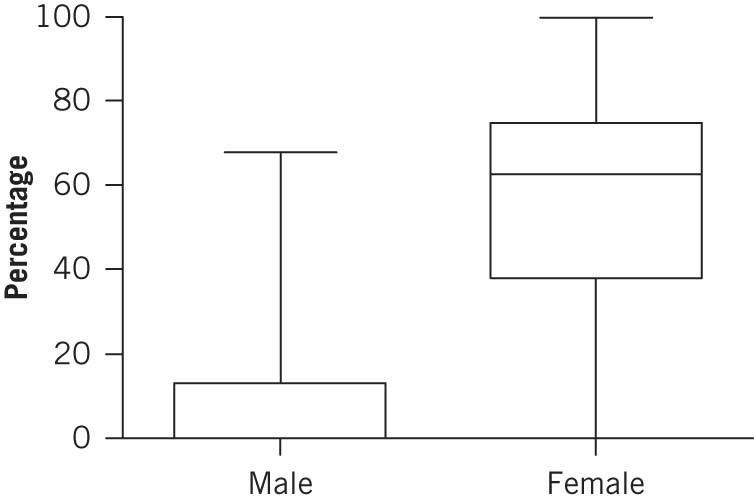
Percentage of male and female cadavers with mobile ascending colon
The presence of a mobile ascending colon did not predict mobility in the descending segment and vice versa. Similarly, the height of transverse colon mesentery did not correlate with height of sigmoid colon mesentery.
Jackson’s membrane
The incidence of Jackson’s membrane was 65.7%. In the cases where Jackson’s membrane was present, the mean distance from the base of the appendix was 6.6cm (SD: 5.51cm).
Discussion
There is considerable variation in the literature regarding the total length of the colon. Previous studies have used a variety of methods, on live patients6–9 or non-fixed cadavers.10–12 These are summarised in Table 3. The reported length of the colon ranges from 139.7cm10 to 169.0cm11 for cadaveric studies and from 109.0cm6 to 167.0cm9 for in vivo studies.
Table 3.
Published studies of colonic length
| Study | Technique | n | Sex | Large bowel length |
| Ahrens6 | Swallowed polyvinyl tube | 6 4 10 |
Male Female Total |
107.8cm 109.8cm 109.0cm |
| Sadahiro7 | Barium enema | 434 486 920 |
Male Female Total |
125.9cm 132.8cm 129.5cm |
| Saunders8 | Laparotomy | 66 52 118 |
Male Female Total |
113.5cm 114.9cm 114.1cm |
| Eickoff9 | Computed tomography colonography | 60 40 100 |
Male Female Total |
– – 167.0cm |
| Treves10 | Cadavers | 100 | Male Female Total |
142.2cm 137.2cm – |
| Underhill11 | Cadavers | 65 35 100 |
Male Female Total |
180.3cm 157.5cm 169.0cm |
| Hounnou12 | Cadavers | 100 100 200 |
Male Female Total |
166.0cm 155.0cm 160.5cm |
| Present study | Cadavers (fixed) | 18 17 35 |
Male Female Total |
133.8cm 128.9cm 131.2cm |
These varied findings may be partly attributable to the different methods employed to determine colonic length (swallowed polyvinyl tube,6 barium enema examination,7 laparotomy8 and CT colonography).9 In addition, the exact parts of the colon being measured has varied. The measurement used for the total colonic length in the present study is most similar to that used by Treves10 and Saunders et al 8 as it includes the caecum and the intraperitoneal rectum. Other studies have used slightly different definitions. Ahrens et al,6 Sadahiro et al 7 and Underhill11 did not include the caecum but did measure as far as the anal canal. Hounnou et al included the caecum but finished at the colorectal junction.12 Eickhoff et al measured from the base of the caecum to the anal canal.9
Interestingly, the results in this study are most closely comparable with those obtained in vivo in a large barium enema study involving 920 Japanese patients.7 Previous cadaveric studies have shown longer mean lengths.10–12 The variation may be due to the fixation of cadavers in the present study. Using cadavers is more practical, and allows for greater exposure and repeat measurements than in live patients. On the other hand, Ahrens et al suggested that intestinal length shortens immediately after death and then lengthens as autolysis proceeds.6 Fixation arrests the process of autolysis. However, this is unlikely to have decreased the accuracy when comparing measurements between the cadavers in the present study, particularly when recording and comparing mesentery incidence.
There is also conflicting evidence for the relationship between colonic length and age, sex and height.7,10–12
This is the first study to analyse how the length of each colonic segment correlates with total colonic length. The finding that the rectosigmoid segment was the single best predictor of overall colonic length agrees with the results of Saunders et al.5 In their study, the rectosigmoid and total colonic lengths (as measured using barium enema) were greater in patients who had been technically difficult to intubate on colonoscopy. This suggests that it may be the length of the rectosigmoid section that has clinical procedural impact with regard to colonoscopy. This highlights the clinical endoscopic experience that ‘shortening the scope’ (withdrawal of the endoscope while keeping its tip fixed) after negotiating the sigmoid colon facilitates further progress.
Despite the ascending and descending segments generally being regarded as secondarily retroperitoneal, our study found that approximately two-thirds of subjects had a mobile portion of ascending colon and that one-third had a mobile portion of descending colon although the length of this portion was highly variable. Saunders et al published the single previous notable study in the literature that examined large bowel length and mobility, which was performed in vivo at laparotomy.8 They described the mean length of the ascending, transverse, descending and sigmoid mesocolons but not the incidence with which they occur.
In contrast to the study by Saunders et al,8 we observed a shorter colonic length, and longer average heights of the mesenteries of the transverse and sigmoid colon (Table 4). The discrepancy in the findings may be due to technique; it is possible that the fixation process affected measurements in our study or there may have been technical difficulties encountered in vivo, including taking measurements through laparotomy incisions and using traction on the bowel segments by 23 different observers. It is particularly interesting that Saunders et al did not treat the ascending and descending colon differently to the transverse and sigmoid colon when discussing mesentery, thereby recognising their tendency to be mobile as opposed to retroperitoneal.8
Table 4.
Comparison of findings for mean height of mesenteries
| Ascending colon mesentery | Transverse colon mesentery | Descending colon mesentery | Sigmoid colon mesentery | |
| Saunders8 | 4.5cm | 14.3cm | 4.8cm | 11.4cm |
| Present study | – | 7.4cm | – | 6.3cm |
It should be emphasised that even if the process of fixation in our study did affect bowel length and/or mesenteric height, it is likely to have affected all parts equally. The overall findings of the study should therefore remain valid in terms of the relative lengths and mobility of colonic segments, and the correlation of mobility with sex.
Finally, despite there being much in the anatomical literature about the nature of Jackson’s membrane, little has been said recently about its incidence and length. Oliver suggested that it occurs in 60–75% of cases although he did not make it clear how this figure was obtained.17 Carslaw reported an incidence of 32%.18 However, this was in a series of 242 patients who underwent colopexy for ‘right-sided visceroptosis’; it can therefore be assumed that they had abnormal colonic anatomy. Carslaw also found extensive variation in the appearance of the membrane. Perhaps owing to a lack of contemporary literature on Jackson’s membrane, it may not be noted consciously during colonic resection. Its high incidence (65.7%) was therefore surprising. Surgeons wishing to identify the membrane intraoperatively may note that the mean extent from the base of the appendix was 6.6cm.
Conclusions
This study has investigated the relationship between total colonic length, segmental lengths and mobility. It has demonstrated that overall colonic length is dependent on the length of the rectosigmoid segment but independent of sex and height. In contrast to traditional anatomical teaching, the ascending and descending colon are frequently mobile, and women are more likely to have a mobile ascending colon. These findings have applications in colonic surgery and endoscopy.
Acknowledgements
The authors would like to thank the technical staff of the Human Anatomy Centre at the Department of Physiology, Development and Neuroscience, University of Cambridge, Mrs Maria Wright and Mr Laurence Fisher, for their assistance with this project as well as the donors, who made this study possible.
References
- 1.Patel K, Hoffman NE. The anatomical distribution of colorectal polyps at colonoscopy. J Clin Gastroenterol 2001; 33: 222–225. [DOI] [PubMed] [Google Scholar]
- 2.Yamaji Y, Mitsushima T, Yoshida H, et al. Right-side shift of metachronous colorectal adenomas after polypectomy. Scand J Gastroenterol 2007; 42: 1,466–1,472. [DOI] [PubMed] [Google Scholar]
- 3.Kushiyama Y, Fukuda R, Suetsugu H, et al. Site-dependent production of transforming growth factor beta1 in colonic mucosa: its possible role in tumorigenesis of the colon. J Lab Clin Med 2000; 136: 201–208. [DOI] [PubMed] [Google Scholar]
- 4.Will OC, Leedham SJ, Elia G, et al. Location in the large bowel influences the APC mutations observed in FAP adenomas. Fam Cancer 2010; 9: 389–393. [DOI] [PubMed] [Google Scholar]
- 5.Saunders BP, Halligan S, Jobling C, et al. Can barium enema indicate when colonoscopy will be difficult? Clin Radiol 1995; 50: 318–321. [DOI] [PubMed] [Google Scholar]
- 6.Ahrens EH, Blankenhorn DH, Hirsch J. Measurement of the human intestinal length in vivo and some causes of variation. Gastroenterology 1956; 31: 274–284. [PubMed] [Google Scholar]
- 7.Sadahiro S, Ohmura T, Yamada Y, et al. Analysis of length and surface area of each segment of the large intestine according to age, sex and physique. Surg Radiol Anat 1992; 14: 251–257. [DOI] [PubMed] [Google Scholar]
- 8.Saunders BP, Phillips RK, Williams CB. Intraoperative measurement of colonic anatomy and attachments with relevance to colonoscopy. Br J Surg 1995; 82: 1,491–1,493. [DOI] [PubMed] [Google Scholar]
- 9.Eickhoff A, Pickhardt PJ, Hartmann D, Riemann JF. Colon anatomy based on CT colonography and fluoroscopy: impact on looping, straightening and ancillary manoeuvres in colonoscopy. Dig Liver Dis 2010; 42: 291–296. [DOI] [PubMed] [Google Scholar]
- 10.Treves F. Lectures on the anatomy of the intestinal canal and peritoneum in man. BMJ 1885; 1: 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underhill B. Intestinal length in man. BMJ 1955; 2: 1,243–1,246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hounnou G, Destrieux C, Desmé J, et al. Anatomical study of the length of the human intestine. Surg Radiol Anat 2002; 24: 290–294. [DOI] [PubMed] [Google Scholar]
- 13.Schoenwolf GC, Bleyl SB, Brauer PR, et al. Larsen’s Human Embryology. 4th edn. London: Churchill Livingstone; 2008. [Google Scholar]
- 14.Williams RB. Pericolic membranes and Lane’s kink. Ann Surg 1914; 59: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oelhafen K, Shayota BJ, Muhleman M, et al. Peritoneal bands: a review of anatomical distribution and clinical implications. Am Surg 2012; 78: 377–384. [PubMed] [Google Scholar]
- 16.Borley NR. Gastrointestinal Tract – Large Intestine. : Standring S. Gray’s Anatomy. 39th edn. London: Churchill Livingstone; 2004. pp1,187–1,188, 1,197–1,198. [Google Scholar]
- 17.Oliver RK. Membranes of the right iliac region. Anat Rec 1917; 13: 281–287. [Google Scholar]
- 18.Carslaw RB. Right-sided visceroptosis. Br J Surg 1928; 15: 545–604. [Google Scholar]


