Abstract
Introduction
Spondylodiscitis refers to the infection of the intervertebral disc and osteomyelitis of the adjacent endplates, and it is uncommon in the developed world. Broad consensus indicates its incidence is on the rise.
Methods
The aim of this retrospective study was twofold. First, we sought to give an up-to-date incidence estimate by comparing case presentation over two time periods (1995–1999 and 2008–2011). Data from the England and Wales census in 2001 and 2011 were used for incidence estimation. The second part of this study aimed to generate management guidance from data from medical and radiographic records of the 2008–2011 patient cohort.
Results
The incidence of adult spontaneous spondylodiscitis in the local region between 2008 and 2011 was 3.67/100,000 per year, representing a 150% increase from the incidence in 1995–1999. Our data demonstrate that methicillin sensitive Staphylococcus aureus remains the most common offending pathogen of spontaneous spondylodiscitis. The mean C-reactive protein (CRP) level remained at >30mg/l after a month of starting antibiotic treatment in both medically and surgically managed groups.
Conclusions
Evidence suggests that the incidence of spondylodiscitis is on the rise. A review of our case series has demonstrated the effectiveness of intravenous antibiotic therapy. While no official guidance exists for when to switch from intravenous to oral antibiotics, our study shows that CRP at 1 month is >30mg/l and we recommend 6 weeks of intravenous therapy, followed by 6 further weeks of oral therapy.
Keywords: Discitis, Incidence, Antibiotic, Disease management
Spondylodiscitis refers to the infection of the intervertebral disc and osteomyelitis of the adjacent endplates.1 This does not usually involve infection of the spinal cord. In daily practice, it is often termed discitis for simplicity.
The management of spondylodiscitis poses a challenge. Delays in diagnosis are common owing to indolent initial onset.2–4 This is coupled with serious complications including paralysis and even death in severe cases. The management of this condition requires multidisciplinary input.
Spondylodiscitis is uncommon in the developed world. Its reported incidence varies from 0.2 to 2.4/100,000 per annum.2–4 It accounts for around 3–5% of all osteomyelitis cases.1 Risk factors include diabetes mellitus, immunosuppression, end-stage renal failure and old age.5,6 Broad consensus indicates the incidence of spinal infection, including spontaneous spondylodiscitis, is on the rise.7,8
Diagnostic criteria and treatment strategies are well established. Some questions still remain unanswered, such as the duration of antibiotic treatment.
The aim of this study was two-fold. First, we sought to give an up-to-date incidence estimate by comparing the caseload over two time periods (1995–1999 and 2008–2011). The second part of this study sought to gather data from medical and radiographic records, including clinical presentation, co-morbidities, causative organisms, spinal location of infection, treatment decisions and outcomes for the 2008–2011 patient cohort.
Methods
The inclusion criteria for this study were clinical symptoms suggestive of spondylodiscitis (back pain unrelieved by rest and irradiated pain with/without neurological deficits with/without pyrexia) and laboratory abnormalities for C-reactive protein (CRP)9 as well as abnormal radiological features compatible with infection of the spine.10 Cases secondary to previous spinal surgery, recurrent spinal infection, transfers from secondary centres and paediatric cases (age <17 years) were excluded. Spondylodiscitis cases were identified from the departmental admission database at the University Hospital North Staffordshire (UHNS). The UHNS is a tertiary centre with a primary catchment area covering the North Staffordshire region of Stoke-on-Trent, Newcastle-under-Lyme and the Staffordshire Moorlands. The use of radiological investigations was noted.
Antibiotics were withheld from suspected cases until a microbiology sample was obtained, except in cases of clinical sepsis.
Computed tomography (CT) guided biopsy was the preferred mode of specimen sampling when immediate surgery was not required. A second biopsy, either CT guided or open, was attempted if the first was inconclusive. If the first two attempts failed to yield the causative pathogen, a broad spectrum, first-line antibiotic treatment was commenced.
Multiple disciplines were involved in the management. These included spinal surgery, musculoskeletal radiology, infectious diseases, therapy and rehabilitation services. The infectious diseases team provided advice on antibiotic management on a case-by-case basis.
Progress of treatment was monitored by regular CRP measurements and plain radiography. Imaging was repeated if there was no improvement despite treatment. The minimum follow-up period was six months.
Results
A total of 55 cases (20 female and 35 male patients, mean age: 63.3 years, range: 26–96 years) from between 2008 and 2011 were included. Based on data from the 2011 population census, the adult population of the trust catchment area consisted of approximately 500,000 individuals. The incidence of adult spontaneous spondylodiscitis over this time period was therefore calculated as 3.67/100,000 per year.
Twenty-eight cases were included in the 1995–1999 case series. Based on data from the 2001 census, it was estimated that approximately 475,000 adults were registered in the local region and so the incidence for 1995–1999 was calculated as 1.47/100,000 per year, demonstrating a 150% increase over the two cohorts.
The use of magnetic resonance imaging (MRI) and CT in disease diagnosis increased from 67% in the 1995–1999 series to 100% in 2008–2011.
Presentation
All the patients had back pain on admission. Of the 55 patients in the 2008–2011 cohort, 11 (20%) had documented sepsis on admission and 12 (21%) had documented neurological deficit. Six (10%) had significant bony destruction demonstrated on the diagnostic MRI (Table 1).
Table 1.
Presenting features of patients according to treatment modality
| Medical | Surgical | Total | |
|---|---|---|---|
| Documented sepsis | 7 | 4 | 11 |
| Documented neurological deficit | 2 | 10 | 12 |
| Destructive changes on magnetic resonance imaging | 0 | 6 | 6 |
Management
Seventeen cases required surgical intervention while thirty-eight cases were managed medically. Our indications for surgery included development of neurological deficit, development of any epidural abscess at cord level, cord compromise, significant bony destruction, progressive deformity and intolerable pain not responding to medical control. The mean age of the group requiring surgery was 58.8 years (range: 27–83 years) with a female-to-male ratio of 1:2.4. The mean age of the medical treatment group was 65.3 years (range: 26–96 years) with a female-to-male ratio of 1:1.5.
Co-morbidity
The most significant risk factor was age. Three-quarters of the patients (n=42, 76%) were older than 50 years at diagnosis. Diabetes mellitus was present in eight cases (15%). Malignancy coexisted in six patients (11%). Immunodeficiency was noted in seven (13%). Known intravenous drug abuse was not common and was noted in two (4%). Recent systemic infection was recorded in 7 patients (13%) while other medical illnesses were documented in 27 cases (50%). The majority of patients (n=39, 71%) had more than one risk factor.
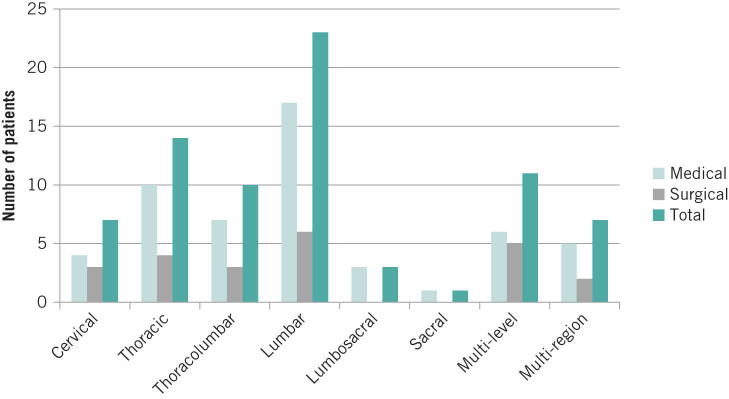
Distribution of infection
This was diagnosed on MRI. Figure 1 demonstrates the regions affected.
Figure 1.
Distribution of the number of cases of spondylodiscitis according to vertebral spinal level
Microbiology
The microbiology results were based on blood culture, CT guided biopsy or open biopsy. The most common offending organism was methicillin sensitive Staphylococcus aureus (MSSA) (n=15), followed by methicillin resistant S aureus (MRSA) (n=5), Streptococcus (n=5) and Escherichia coli (n=4) (Table 2). Nine patients (16%) had no growth and multiple organisms were found in six cases (11%). MSSA was the most common organism found in both the medical and surgical treatment groups. Streptococcus spp was the second most common group of organisms in the surgical group. There were no anaerobic organisms found in this series.
Table 2.
Organisms identified according to treatment modality
| Organisms | Medical | Surgical | Total |
|---|---|---|---|
| Gram positive aerobic cocci | |||
| MSSA | 8 | 7 | 15 |
| MRSA | 5 | 0 | 5 |
| Coagulase negative Staphylococcus | 3 | 0 | 3 |
| Other Staphylococcus | 3 | 1 | 4 |
| Streptococcus | 1 | 4 | 5 |
| Enterococcus faecium | 3 | 0 | 3 |
| Actinomyces | 0 | 1 | 1 |
| Corynebacterium | 1 | 0 | 1 |
| Micrococcus luteus | 1 | 0 | 1 |
| Unspecified | 1 | 0 | 1 |
| Gram negative aerobic bacilli | |||
| Escherichia coli | 2 | 2 | 4 |
| Pseudomonas aeruginosa | 2 | 0 | 2 |
| Enterobacter | 1 | 1 | 2 |
| Klebsiella | 0 | 1 | 1 |
| Proteus | 0 | 1 | 1 |
| Unspecified | 1 | 0 | 1 |
| Non-pyogenic bacteria | |||
| Mycobacterium tuberculosis | 0 | 2 | 2 |
| Brucella | 1 | 0 | 1 |
| No growth | 8 | 1 | 9 |
| Multiple growth | 3 | 3 | 6 |
Use of antibiotics
The choice of antibiotics was governed by the sensitivities provided by the samples. Table 3 shows the choice of antibiotics according to treatment modality. It is vital to acquire an antibiotic free period before microbiology sample collection, except in clinically septic patients. Multiple antibiotics were used in 50% of all cases.
Table 3.
Choice of antibiotics according to treatment modality
| Antibiotics | Medical | Surgical | Total |
|---|---|---|---|
| Intravenous flucloxacillin | 15 | 7 | 22 |
| Intravenous vancomycin | 10 | 3 | 13 |
| Intravenous piperacillin/tazobactam | 3 | 2 | 5 |
| Intravenous meropenem | 2 | 2 | 4 |
| Intravenous gentamicin | 1 | 0 | 1 |
| Intravenous teicoplanin | 1 | 0 | 1 |
| Intravenous benzylpenicillin | 0 | 1 | 1 |
| Oral rifampicin | 10 | 5 | 15 |
| Oral fusidic acid | 9 | 2 | 11 |
| Oral flucloxacillin | 4 | 0 | 4 |
| Oral co-trimoxazole | 1 | 0 | 1 |
| Oral doxycycline | 1 | 0 | 1 |
| Oral clindamycin | 0 | 1 | 1 |
| Isoniazid, rifampicin and ethambutol | 0 | 2 | 2 |
| None | 1 | 0 | 1 |
| Pure oral | 6 | 0 | 6 |
| Multiple antibiotics | 19 | 9 | 28 |
Trend of C-reactive protein
CRP was the inflammatory marker measured to monitor response to treatment. The trend of the change between CRP on admission and that a month after treatment is shown in Table 4. The mean CRP remained at >30mg/l after a month of starting treatment in both the medical and surgical groups. While there were exceptions, this probably indicates it is not suitable to end intravenous antibiotics in four weeks.
Table 4.
C-reactive protein (CRP) trends in both patient cohorts for one month following admission
| Cohort | CRP on admission | CRP at 1 week | CRP at 1 month |
|---|---|---|---|
| Medical | 125.4mg/l | 67.4mg/l | 38.9mg/l |
| Surgical | 144.7mg/l | 85.8mg/l | 33.9mg/l |
Length of stay
Although outpatient intravenous antibiotic services are available in our hospital trust, many patients are not well enough to be managed in the community. Consequently, the majority of patients were managed in acute hospital beds. The overall mean length of stay (LOS) was 48 days (range: 5–209 days). In the surgical group, the mean LOS was 59 days (range: 9–209 days) while the medical group had a mean LOS of 42 days (range: 5–149 days). The difference was not statistically significant (t-test, p=0.27).
Discussion
Our data demonstrate that MSSA remains the most common offending pathogen of spontaneous spondylodiscitis. The microbiology flora presented here is similar to the spectrum reported in the literature.
No randomised controlled trials have been conducted on antibiotic treatment for spondylodiscitis. There is a lack of consensus over the duration of total antibiotic treatment and when to switch from intravenous to oral antibiotics.2–4 It is generally agreed that the duration of treatment would be dependent on patients’ response, measured biochemically as a decrease in CRP. There is so far no consensus on what CRP level would be deemed safe to switch from intravenous to oral antibiotics.
From the CRP trend recorded in our series, it is clear that the majority of infections are not fully managed a month after the commencement of treatment in both surgical and medical groups. The mean CRP value stays at around 30mg/l. We therefore recommend a six-week duration of intravenous antibiotics.
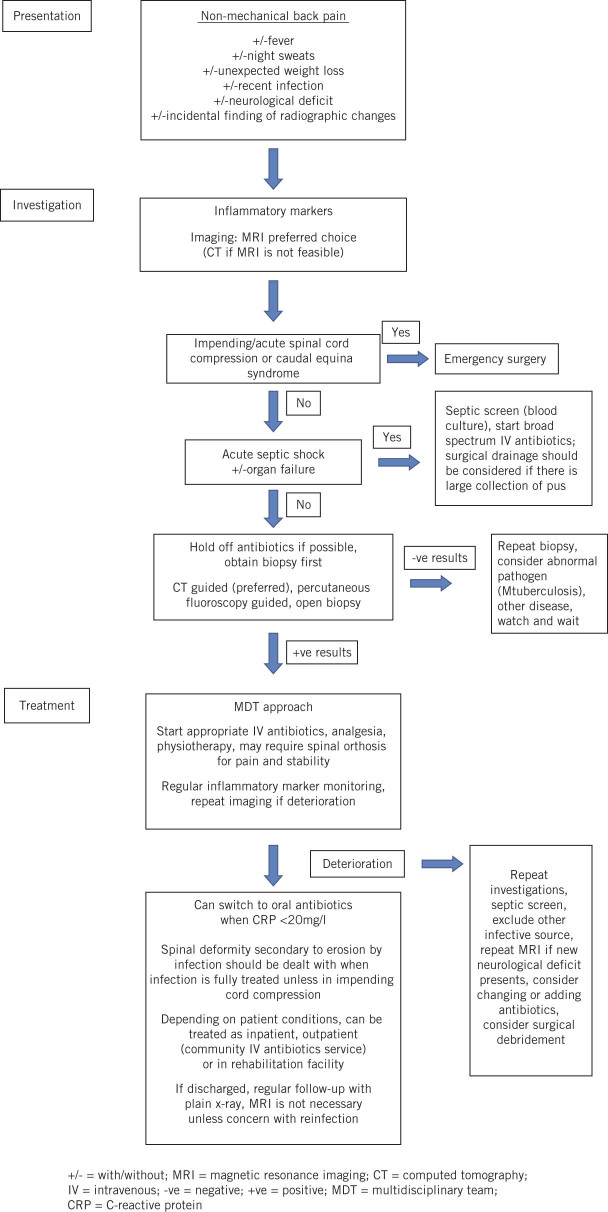
As part of our review of our caseload, departmental guidelines for the management of spondylodiscitis have been devised, which can be used in a centre with multidisciplinary capabilities. These are illustrated in Figure 2.
Figure 2.
Departmental management guidance for spondylodiscitis
There is not much evidence in the literature on LOS in hospital. Bhavan et al reported a median LOS of two weeks in acute hospital beds in the US.11 Patients in that series were mainly discharged to a step-down facility rather than going home directly. In our institution, it is not possible to administer intravenous antibiotics in step-down facilities. Even when outpatient antibiotics services are available, patients are not often able to be discharged to the community, resulting in a prolonged LOS in acute hospital beds. It is worth stating that some patients had very long stays due to multiple complications from their sepsis. If the incidence of spondylodiscitis continues to rise, it is important to factor this in when planning resources.
Conclusions
Evidence suggests the incidence of spondylodiscitis is on the rise. This is partly due to patient factors and improved radiological diagnosis rather than a change in pathogens. Our case series has recorded a 150% increase over 15 years.
Once established, spondylodiscitis can cause significant morbidity. Diagnosis is complicated because back pain is common in the elderly population. It is important to recognise red flag symptoms such as non-mechanical back pain, fever, night sweats and weight loss in those patients with risk factors.
Mortality for spondylodiscitis was as high as 25% before the era of antibiotics.5 This has since come down to between 2% and 11%.2
Review of our case series has demonstrated the effectiveness of intravenous antibiotic therapy. While no official guidance exists for when to switch from intravenous to oral antibiotics, our study shows that CRP at 1 month is >30mg/l and so we recommend six weeks of intravenous therapy followed by 6 further weeks of oral therapy.
References
- 1.Zarghooni K, Röllinghoff M, Sobottke R, Eysel P. Treatment of spondylodiscitis. Int Orthop 2012; : 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect 2008; : 401–412. [DOI] [PubMed] [Google Scholar]
- 3.Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health 2010; : 5–16. [DOI] [PubMed] [Google Scholar]
- 4.Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop 2012; : 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997; : 874–880. [DOI] [PubMed] [Google Scholar]
- 6.Dimar JR, Carreon LY, Glassman SD, et al. Treatment of pyogenic vertebral osteomyelitis with anterior debridement and fusion followed by delayed posterior spinal fusion. Spine 2004; : 326–332. [DOI] [PubMed] [Google Scholar]
- 7.Acosta FL, Galvez LF, Aryan HE, Ames CP. Recent advances: infections of the spine. Curr Infect Dis Rep 2006; : 390–393. [DOI] [PubMed] [Google Scholar]
- 8.Jensen AG, Espersen F, Skinhøj P, et al. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980–1990. J Infect 1997; : 113–118. [DOI] [PubMed] [Google Scholar]
- 9.Aagaard T, Roed C, Dragsted C, Skinhøj P. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006–2011. Scand J Infect Dis 2013; : 417–424. [DOI] [PubMed] [Google Scholar]
- 10.Tay BK, Deckey J, Hu SS. Spinal infections. J Am Acad Orthop Surg 2002; : 188–197. [DOI] [PubMed] [Google Scholar]
- 11.Bhavan KP, Marschall J, Olsen MA, et al. The epidemiology of hematogenous vertebral osteomyelitis: a cohort study in a tertiary care hospital. BMC Infect Dis 2010; : 158. [DOI] [PMC free article] [PubMed] [Google Scholar]