Abstract
Early detection of cancer is the key to improving survival. Since most clinically used serum cancer biomarkers are either glycoproteins or glycan structures that can be recognized by specific monoclonal antibodies, developing glycan structure-based biomarkers from human serum/plasma glycoproteins through mass spectrometry (MS) analysis are active research field during the past decades. Numerous studies have shown that changes in serum/plasma glycan structures occur during cancer initiation, progression, and treatment. This review describes N- and O-linked glycan structures identified from serum/plasma glycoprotein (s) by MS analysis with focus on alterations associated with different types of human cancers. The global changes in serum N- and O-linked glycan structures, especially the glycans that are not made by cancer cells such as B lymphocyte-derived IgG and liver-synthesized haptoglobin and α1 acid glycoprotein, suggest that glycans might be the long sought diagnostic biomarkers associated with system malfunction in the blood circulation of cancer patients. Therefore, N- and O-linked glycan structures have great potential to serve as cancer diagnosis, prognosis, and treatment monitoring biomarkers to facilitate personalized medicine.
Keywords: N-glycans, O-glycans, serum, cancer, biomarker
Introduction
Glycans are most information-dense biomolecules in animal cells [1]. The chemical information contents residing in glycan structures are far greater than that of DNAs, RNAs and proteins combined [2]. Two major types (>95%) of glycans in serum are N-linked and O-linked glycans [3-5], which are composed of N-acetyl galactosamine, galactose, N-acetylglucosamine, fucose, mannose, sialic acid, and other monosaccharides and present in most proteins in human blood circulation. N-linked glycans are assembled on the core proteins initiated in the endoplasmic reticulum through nitrogen (N) in the side chain of asparagine with the sequon Asn-X-Ser or Asn-X-Thr, where X is any amino acid except proline, and accomplished in the Golgi through a complicated process. In contrast, O-linked glycans are assembled one monosaccharide at a time on a serine or threonine residue of proteins in the Golgi. Unlike N-linked glycans, there is no known consensus sequence for O-linked glycans. However, the placement of a proline residue at either -1 or +3 relative to the serine or threonine is favorable for O-linked glycosylation. The changes in glycan structures are observed in the glycoproteins of serum/plasma or tumor tissues from different types of cancer patients [6-11]. The conventional scheme of serum/plasma glycan structural analysis by mass spectrometry (MS) is shown in Figure 1.
Figure 1.
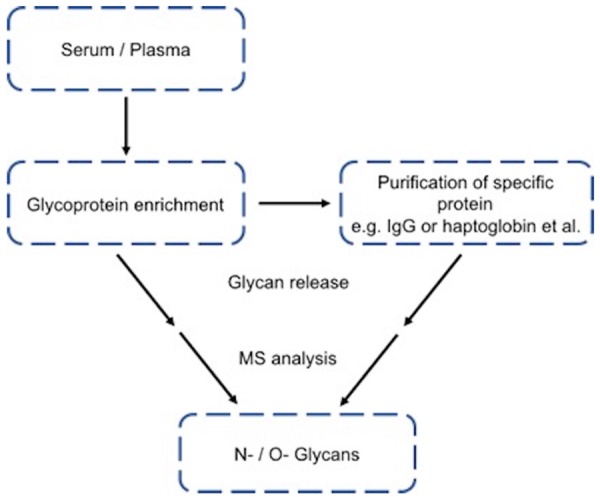
The conventional scheme used for MS analysis of N-/O-glycans from serum/plasma glycoproteins or specific serum/plasma glycoprotein.
Current clinically used antibody-based immunochemical tests that identify biomarkers for different types of cancers target either highly glycosylated proteins or specific glycan structures [12]. However, these tests lack the specificity and sensitivity required for cancer detection [13] due to the non-template nature of glycan biosynthesis, which is governed by roughly 1-2% of the entire genome [14] that encodes enzymes such as glycosyltransferases, glycosidases, and transporters as well as a variety of environmental factors that affect sugar nucleotide production and gene transcription.
Glycoproteins play important roles in various biological processes including intracellular transport, cell adhesion, cell-cell interactions, and many others [7,15-17]. N- and O-glycans are the most abundant and best studied glycans from serum/plasma glycoproteins. It is known that glycan structures are not only affected by pathological conditions, such as cancer, bacterial/viral infection, chronic/acute inflammation but also affected by physiological conditions such as cell differentiation, cell morphogenesis, and tissue development. Moreover, glycan structures are different at different development stages of the same cancer. Furthermore, glycan structures are affected by environmental factors such as nutrition status that has direct impact on sugar nucleotide production. Therefore, using glycan structures as biomarkers for cancer diagnosis, prognosis, and treatment monitoring has great clinical potentials. In this review we summarize all the reported N- and O-glycan structural changes associated with different types of cancers.
Serum/plasma N-glycans as putative cancer biomarkers
Changes in N-linked glycan structures from serum/plasma glycoproteins are observed and well documented during the development and progression of different types of cancers based on the studies of many research groups. N-glycans from serum/plasma glycoprotein (s) are released by several enzymes and many analytical methods have been developed for N-glycan structural characterization, such as HPLC [18], CE [19], LC-MS/MS [20], MALDI-QIT-MS [21], MALDI-TOF-MS [22], RP-LC-ESI-MS [23], DSA-FACE [24], LC/MS [25], GC-MS [26], NMR [27], etc.
N-glycans from serum glycoproteins of cancer patients
N-glycan structures from serum glycoproteins of breast, prostate, ovarian, pancreatic, liver, and lung cancer patients have been characterized; the published N-glycan structures are summarized in Table 1. In this table, a total of 92 N-glycan structures from 1-1 to 1-92 are plotted according to the published report [28] where sialic acid placed on the left side of N-glycans is α 2, 3-linked whereas those on the right side is α 2, 6-linked. Aberrant expression of sialic acids (Sia) that cap N- and O-glycan chains in different types of cancers and their potential for personalized medicine has been reviewed previously [29].
Table 1.
Summary of N-glycan structures identified in serum/plasma of cancer patients
Lung cancer leads in mortality among all cancers in the world. In the sera of lung cancer patients, the biantennary N-glycan chains that contain SLex are increased, but biantennary N-glycan chains that contain core fucose or both core fucose and sialic acid are decreased compared to those of normal control. Most of triantennary N-glycan chains are decreased, which contain only one sialic acid or both fucose and sialic acid. In contrast, the increased triantennary N-glycan chains contain two or three sialic acids and the SLex motif. Remarkably, all of tetraantennary N-glycan chains are increased.
Breast cancer leads in mortality in women. In the sera of breast cancer patients, most of biantennary N-glycan chains are decreased compared with those of normal control. Interestingly, α 2, 3 sialic acid-modified N-glycans are also decreased. In contrast, most of biantennary and triantennary N-glycan chains that contain sialic acid and core fucose in the sera of prostate cancer patients are increased. In the sera of pancreatic cancer patients, all N-glycans that are detected are decreased compared to the normal control. However, serum glycan 1-56 is only detected in pancreatic cancer patients. These data suggest that serum N-glycan profiles might be unique in pancreatic cancer patients. In the sera of liver cancer patients, biantennary N-glycan chains are decreased. Since most of serum glycoproteins are synthesized in liver, under-processed high mannose triantennary N-glycan chains observed only in the liver but not other cancer patients make sense.
Based on the data presented in Table 1, it can be summarized that all of tetraantennary N-glycans in cancer patient sera are increased except in that of pancreatic cancer, which indicates that increased tetraantennary N-glycans represent a common N-glycan structural feature in different types of cancer patient sera. However, no comparable pattern of N-glycan profiles is observed among other types of cancer patients, indicating each type of cancers is unique and aberrant N-glycan structures might be useful in distinguishing different types of cancer based on serum glycan analysis in future.
N-glycan structures from serum IgG of cancer patients
Both humoral and innate immunity plays a critical role in cancer biology. One of the key factors in humoral immunity is immunoglobulin G (IgG), which is the most prevalent serum immunoglobulin with concentrations of approximately 10-15 mg/mL. IgG consists of an antigen binding fragment (Fab) and a crystallizable fragment (Fc) region that contains an N-glycosylation site at asparagine 297 (Asn297). N-glycosylation of IgG Fc region is reported to be affected by sex, age, autoimmune disease, and cancers. Changes in N-glycan structures of IgG alter their respective functions not limited to complement fixation, complement-dependent cytotoxicity, elimination of antigens, and antibody-dependent cytotoxicity activity.
IgG is produced by B lymphocytes but not by cancer cells. A total 22 N-glycan structures from IgG purified from the sera of gastric, ovarian, breast, and lung cancer patients are listed in Table 2. The data shows that IgG in the sera of these cancer patients have an overall different N-glycan structures from that of normal cancer-free patients. A recent publication is demonstrated that N-glycan structures of IgG in the sera of breast cancer patients have better sensitivity and specificity compared to currently used clinically cancer biomarkers [30]. Most importantly, serum IgG N-glycan structures at stage 0 breast cancer patients are already different from that of normal controls, suggesting that cancer was accompanied by abnormal B lymphocyte-produced N-glycan structures of IgGs from the earliest stage of breast cancer, which also suggests that the malfunction of IgG Fc region N-glycosylation may play a role in tumor development and cancer is a system malfunction disease.
Table 2.
Summary of N-glycans purified from IgG in serum of cancer patients
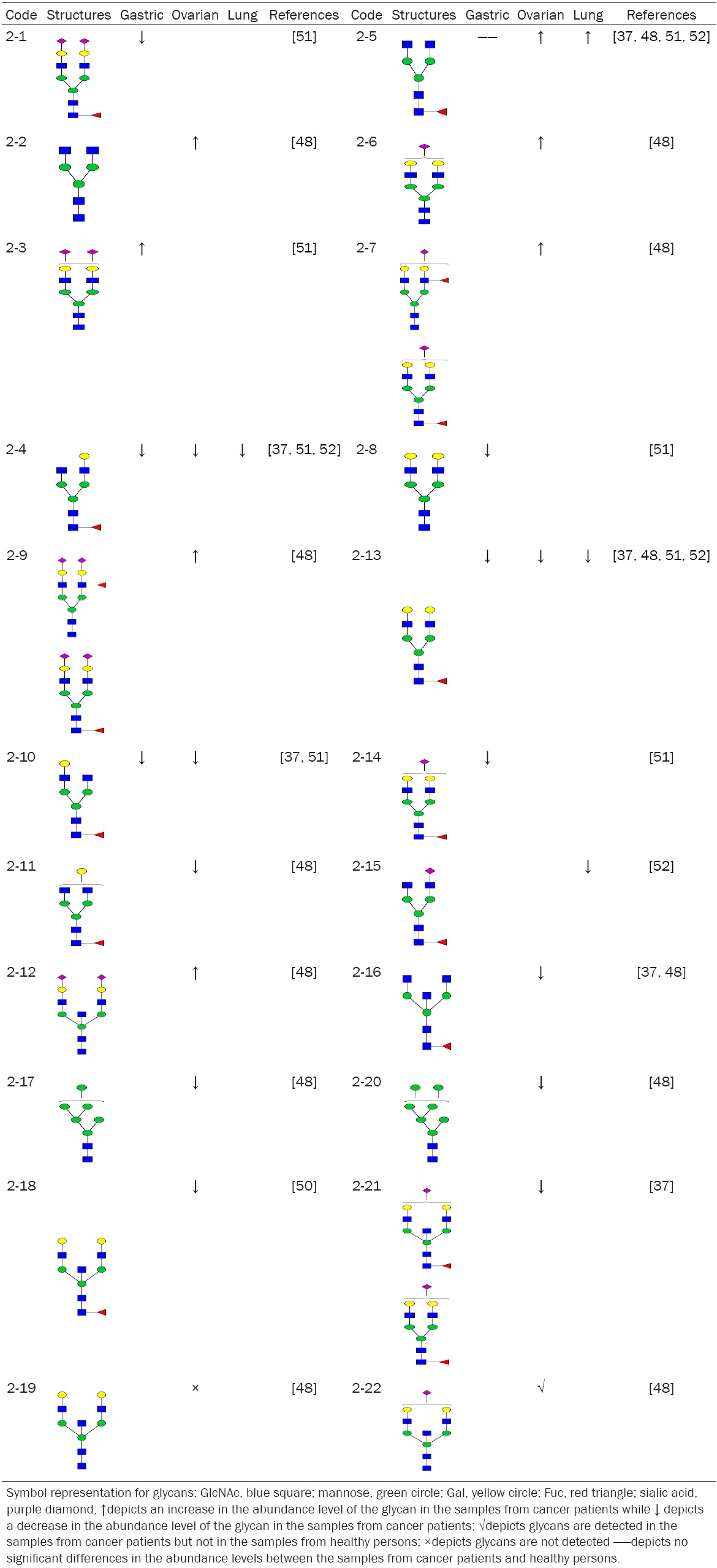
|
As shown in Table 1, tetraantennary N-glycans as well as SLex motif in all but pancreatic cancer patient sera are increased. In contrast, no such N-glycan structures are detected in the serum IgG of gastric, ovarian, breast, and lung cancer patients (Table 2). However, all biantennary N-glycan chains with core fucose-modifications are decreased in all four cancer sera tested compared to that of normal control (Table 2). In addition, biantennary N-glycan chains with fucose and galactose modifications are decreased in the sera of ovarian cancer patients and most of triantennary N-glycan chains that have high mannose structures are also decreased. Interestingly, Glycan 2-22 is only detected in the sera of ovarian cancer patients and 2-19 is only detected in the sera of normal control. Most of biantennary N-glycan chains are decreased in the sera of lung cancer patients. The N-glycans 2-4 and 2-13 are decreased in the sera of gastric, ovarian and lung cancer patients.
N-glycans from serum haptoglobin of cancer patients
Haptoglobin, an acute-phase protein produced in the liver, contains four glycosylation sites (Asn184, Asn207, Asn211, and Asn241). Haptoglobin has been found to be a biomarker for different types of cancers including esophageal, gastric, colon, gallbladder, pancreatic, prostate, and ovarian (Table 3). All of biantennary N-glycans are increased, triantennary N-glycans without core fucose are decreased, and core-fucosylated glycans are increased in the sera of gastric, ovarian, and pancreatic cancer patients. There are four N-glycans (Table 3, 3-19, 3-20, 3-22 and 3-28) that are only detected in the sera of pancreatic cancer patients but not in that of normal persons. The N-glycan 3-1 is increased in gastric, ovarian and pancreatic cancer patients (Table 3).
Table 3.
Summary of N-glycans obtained from haptoglobin in the sera of cancer patients
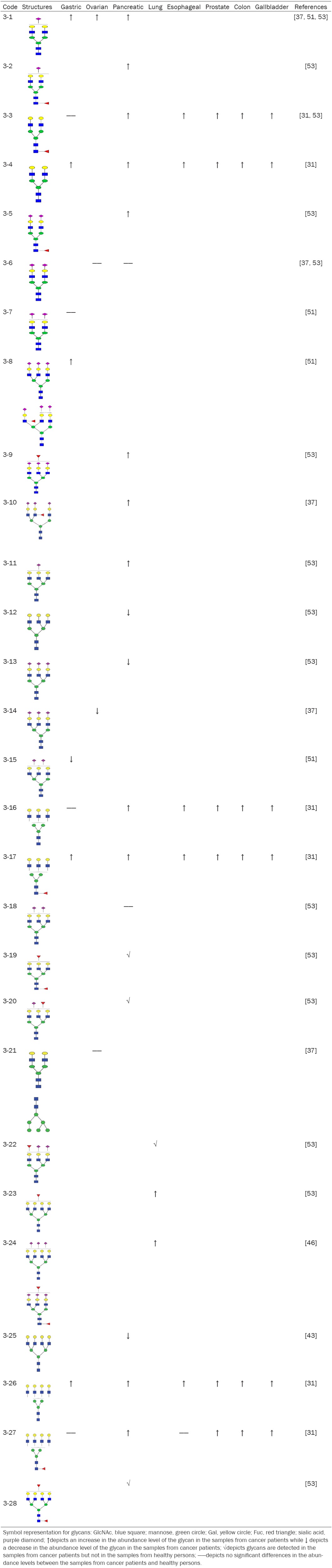
|
Because serum haptoglobin N-glycans could serve as biomarkers for different types of cancers, it was believed that haptoglobin must be synthesized by cancer cells and then secreted into the blood circulation. If this was the case, serum haptoglobin N-glycan structures from different type of cancers should be different since each type of cancer cells should have different N-glycan assembly line, thus different overall N-glycan structures. However, remarkable differences in the structure or fucosylation of N-glycan are not detected in esophageal, gastric, colon, gallbladder, pancreatic, and prostate cancers in a recent report [31]. It suggests that the haptoglobin in the sera of cancer patients might be produced in the liver. These results demonstrate again that cancer is a system malfunction disease.
Because abnormal N-glycan biosynthesis in the liver is common for different types of cancers studied thus far, haptoglobin N-glycan structures in the sera of cancer patients that are synthesized by hepatocytes but not cancer cells could serve as cancer diagnosis biomarkers.
Serum N-glycans containing SLex motif
Sialyl LewisX, also known as sialyl LeX, or SLeX, consists of a sialic acid α 2, 3 linked to galactose β1-4 linked to GlcNAc, to which a fucose is α 1, 3 linked (Table 4, 4-11). SLeX is usually located in the terminal of both N- and O-glycans. SLex was first described in a ganglioside fraction of human kidney [32]. Trace amounts of SLex were also found in human milk [33]. The SLeX, an E-selectin ligand, is constitutively expressed on monocytes and granulocytes. The interaction between E-selectin and SLeX partially mediates inflammatory extravasation of monocytes and granulocytes [34]. Resting T and B lymphocytes start to express SLeX upon activation. SLeX is expressed on hepatocytes during inflammation. SLeX is also over-expressed by different types of cancer cells and plays a critical role in cancer metastasis. Thus, it is not surprising that SLex-containing triantennary N-glycan (Table 4, 4-5) is increased in the sera of breast, prostate, ovarian, pancreatic, melanoma, peritoneal, endometrial cancer patients. Coincidently, the three most devastating cancers, i.e. pancreatic, lung, and gastric cancers, have the highest level of SLex (Table 4, 4-11), suggesting that SLex was expressed not only by cancer cells but also by liver and immune cells. Thus, SLex might be able to serve as a cancer prognostic cancer biomarker.
Table 4.
Summary of SLex and N-glycans contain SLex in serum of cancer patients
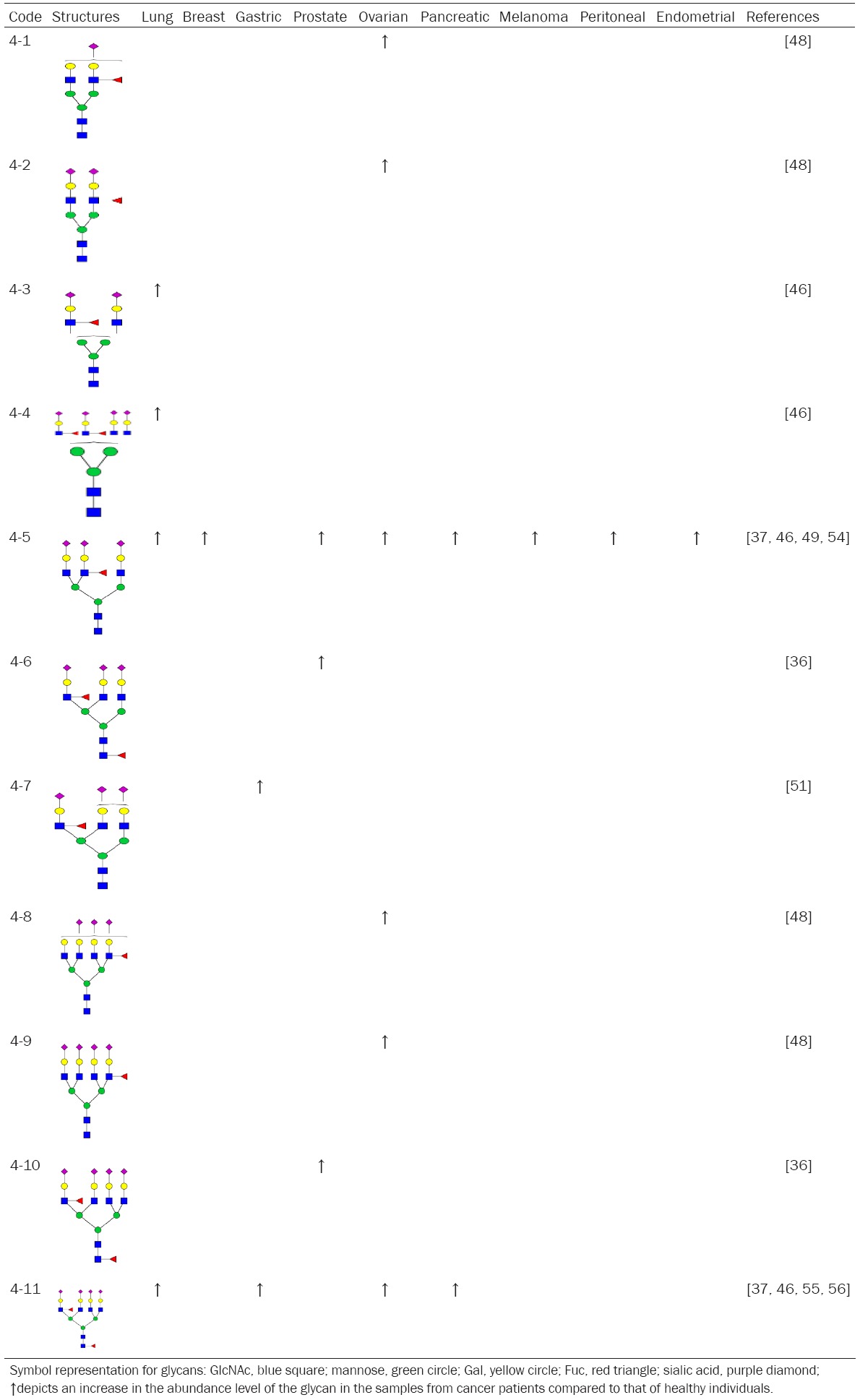
|
N-glycans from serum α1-acid glycoprotein
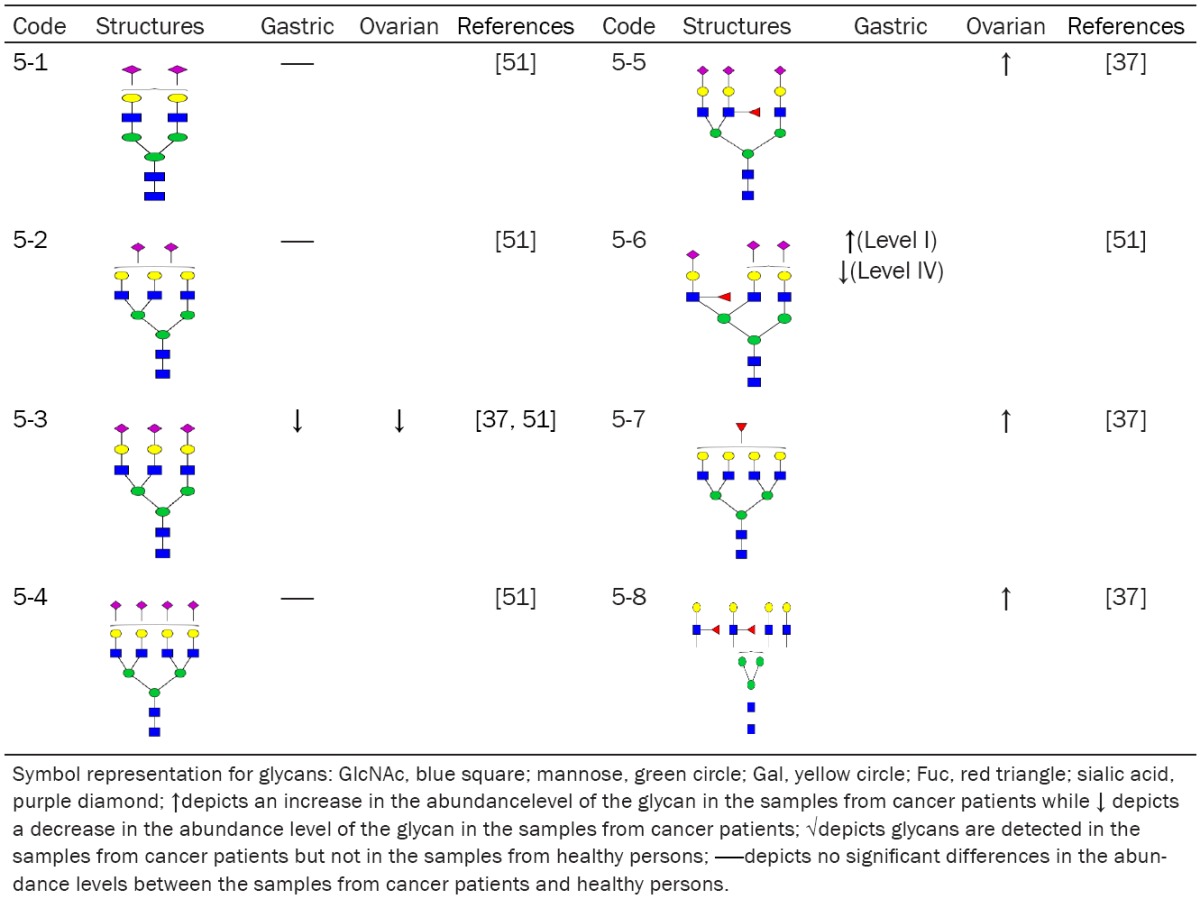
Alpha-1-acid glycoprotein is a 41-43 kDa glycoprotein with 5 N-linked glycans at Asn33, Asn56, Asn72, Asn93 and Asn103, respectively, resulting in a glycan content of 45% of its molecular mass. It has a normal plasma concentration between 0.6-1.2 mg/mL and represents 1-3% plasma protein. α1-acid glycoprotein is similar to haptoglobin in that it is an acute phase glycoprotein and synthesized primarily by hepatocytes. Most importantly, its N-glycans also serve as biomarkers for different types of cancers (Table 5). Moreover, α1-acid glycoprotein along with albumin, very low-density lipoprotein particle size, and citrate has been identified as one of four potentially useful circulating biomarkers for estimating the five-year risk of all-cause mortality [35].
Table 5.
Summary of N-glycans purified from α1-acid glycoprotein in the sera of cancer patients
|
The N-glycans identified from serum α1-acid glycoprotein of different cancer patients are shown in Table 5. The content of glycan 5-3 is decreased in the sera of both gastric and ovarian cancer patients. All N-glycans that contain SLex are increased, which have the same trend as that of N-glycans from serum haptoglobin in pancreatic cancer patients. However, the serum level of glycan 5-6 in α1-acid glycoprotein is increased in gastric cancer stage I and is decreased in gastric cancer stage IV, which is consistent with that α1-acid glycoprotein might be a useful biomarkers for estimating the five-year risk of all-cause mortality [35].
O-glycans as putative cancer biomarkers
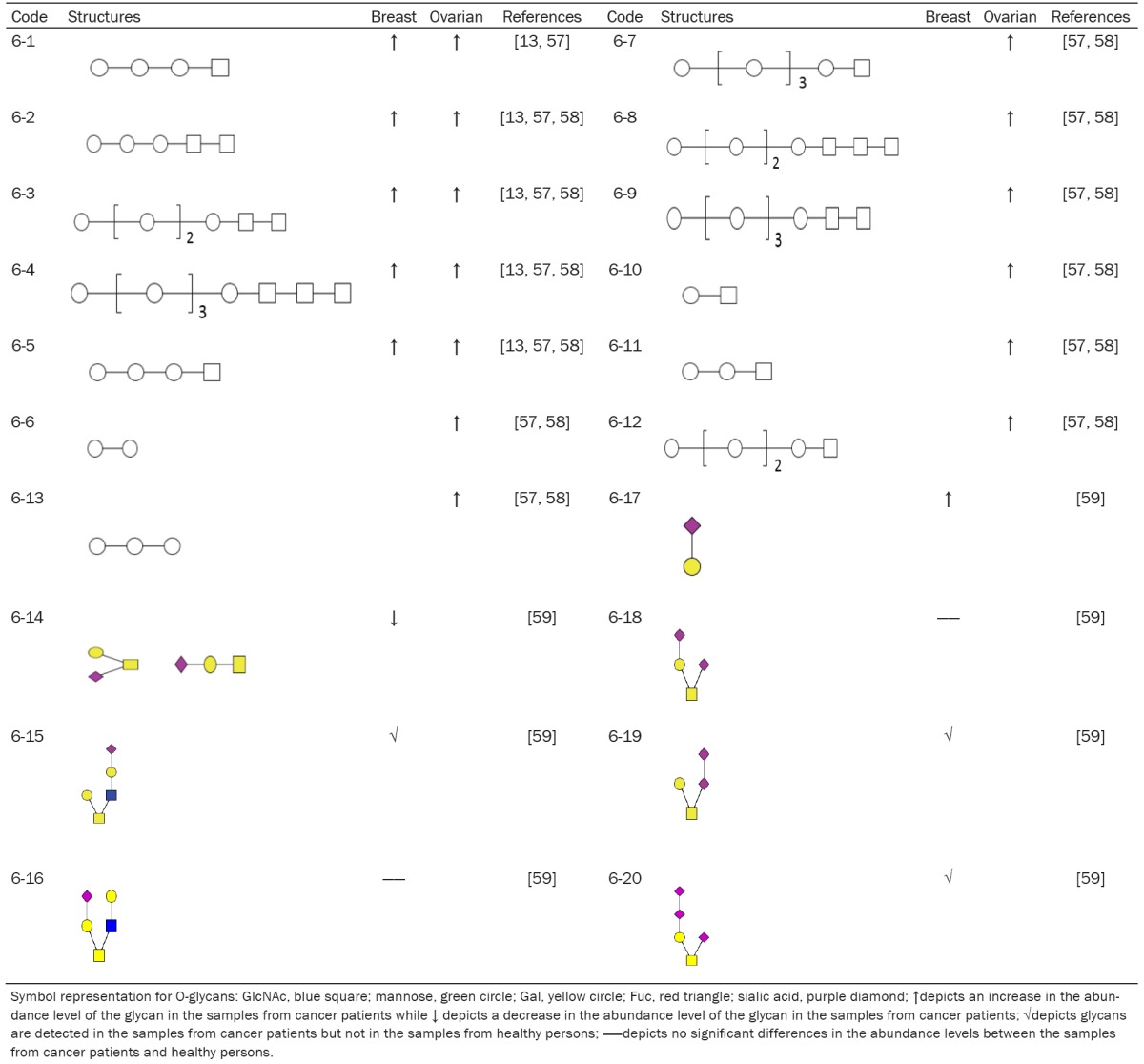
Because of the lack of proper enzymes, O-glycans cannot be released efficiently from most of core proteins. This technique difficulty makes O-glycan analysis difficult, insensitive and time consuming. O-glycans have diverse structures. In addition, multiple reactions and cleaning steps are needed to prepare O-glycans for structural analysis. Therefore, O-glycan profiling studies from the serum/plasma glycoproteins of cancer patients have been limited. So far there are a few references available where O-glycans are purified and characterized from the sera of breast and ovarian cancer patients (Table 6). Even though most of O-glycans identified are still lacking the linkage information, O-glycans 6-1, 6-2, 6-3, 6-4, and 6-5 are increased in the sera of both breast and ovarian cancer patients compared to that of normal persons. In addition, O-glycans 6-15, 6-19 and 6-20 are detected in the sera of breast cancer patients but not in that of healthy persons. Thus, serum O-glycans are also useful biomarkers for cancer diagnosis.
Table 6.
Summary of O-glycans in serum of cancer patients
|
Conclusion and future perspectives
Non-invasive glycan biomarkers from serum/plasma are promising cancer diagnostic, prognostic, and treatment-monitoring biomarkers. The global changes in serum N- and O-linked glycan structures, especially the glycans that are not made by cancer cells such as B lymphocyte-derived IgGs and liver-synthesized haptoglobin and α1 acid glycoprotein, suggest that glycans might be the long sought diagnostic biomarkers associated with system malfunction in the blood circulation of cancer patients. Such biomarkers would be very useful biomarkers not only for early diagnosis but also for prognosis and treatment monitoring.
The major limitation in serum/plasma glycan-based biomarker discovery is that all the methods involved require considerable expertise as well as expensive instrumentation. Despite the advances in glycan analysis, the technical difficulties associated with glycan release, labeling, detection, and quantification by MS are still the bottle neck in developing serum/plasma glycan-based cancer biomarkers. Further progress in analysis should be made to yield exact structures with extensive site specific heterogeneity in a quantitative manner. Thus, with improved technology, the glycomics has the greatest potential for cancer biomarker discovery.
In future, it will be possible to overlay serum/plasma data obtained from the approaches of genomics, proteomics, lipidomics, metabolomics and glycomics to perform multiple dimensional pathway analysis. Therefore, the next generation of serum/plasma biomarkers could be a combination of biomarkers including glycans, proteins/peptides, DNA, RNA, lipids, and metabolites, or any combinations of them.
Acknowledgements
The correspondence author would like to thank Professors Weitong Wang, Yixian Sha, Victor Ginsberg, Jeffrey D. Esko, and Robert Rosenberg for introducing me to the different aspects of glycobiology research and for their excellent mentorship since 1984. This work was financially supported by the Natural Science Foundation of China (Grant No. 91129706), NSFC-Shandong Joint Fund (U1406402), and the Taishan Scholar Followship of Shandong Province in China to L.Z.
Disclosure of conflict of interest
None.
References
- 1.Zhang L. Glycosaminoglycans in development, health and disease. Academic Press; 2010. [DOI] [PubMed] [Google Scholar]
- 2.Wells L, Hart GW Athens Guidelines for the Publication of Glycomics Data. Glycomics: Building Upon Proteomics to Advance Glycosciences. Mol Cell Proteomics. 2013;12:833–835. doi: 10.1074/mcp.E113.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol. 2011;21:576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Lu A, Zhang L. Tumor-Dependent and -Independent Serum/Plasma Biomarkers for Early Diagnosis of Lung Cancer. Transl Med. 2015;5:1–9. [Google Scholar]
- 6.Dube DH, Bertozzi CR. Glycans in cancer and inflammation-potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 7.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Arnold JN, Saldova R, Hamid UM, Rudd PM. Evaluation of the serum n-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2008;8:3284–3293. doi: 10.1002/pmic.200800163. [DOI] [PubMed] [Google Scholar]
- 10.Pan S, Chen R, Aebersold R, Brentnall TA. Mass spectrometry based glycoproteomics-from a proteomics perspective. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.R110.003251. R110.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis CA, Osorio H, Silva L, Gomes C, David L. Alterations in glycosylation as biomarkers for cancer detection. J Clin Pathol. 2010;63:322–329. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 12.Kailemia MJ, Park D, Lebrilla CB. Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem. 2016 doi: 10.1007/s00216-016-9880-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirmiz C, Li B, An HJ, Clowers BH, Chew HK, Lam KS, Ferrige A, Alecio R, Borowsky AD, Sulaimon S. A serum glycomics approach to breast cancer biomarkers. Mol Cell Proteomics. 2007;6:43–55. doi: 10.1074/mcp.M600171-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, Etzler M. Essentials of glycobiology. New York: Cold Spring Harbor; 2009. [PubMed] [Google Scholar]
- 15.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 16.Rudd PM, Woods RJ, Wormald MR, Opdenakker G, Downing AK, Campbell ID, Dwek RA. The effects of variable glycosylation on the functional activities of ribonuclease, plasminogen and tissue plasminogen activator. Biochim Biophys Acta. 1995;1248:1–10. doi: 10.1016/0167-4838(94)00230-e. [DOI] [PubMed] [Google Scholar]
- 17.Rudd PM, Wormald MR, Stanfield RL, Huang M, Mattsson N, Speir JA, DiGennaro JA, Fetrow JS, Dwek RA, Wilson IA. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J Mol Biol. 1999;293:351–366. doi: 10.1006/jmbi.1999.3104. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Li H, Feng X, Liu BF, Liu X. Purification of derivatized oligosaccharides by solid phase extraction for glycomic analysis. PLoS One. 2014;9:e94232. doi: 10.1371/journal.pone.0094232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamczyk B, Tharmalingam-Jaikaran T, Schomberg M, Szekrényes Á, Kelly RM, Karlsson NG, Guttman A, Rudd PM. Comparison of separation techniques for the elucidation of IgG N-glycans pooled from healthy mammalian species. Carbohydr Res. 2014;389:174–185. doi: 10.1016/j.carres.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Shah B, Jiang XG, Chen L, Zhang Z. LC-MS/MS peptide mapping with automated data processing for routine profiling of N-glycans in immunoglobulins. J Am Soc Mass Spectrom. 2014;25:999–1011. doi: 10.1007/s13361-014-0858-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Wu J, Yin H, Marrero J, Lubman DM. Mass spectrometric N-glycan analysis of haptoglobin from patient serum samples using a 96-well plate format. J Proteome Res. 2015;14:4932–4939. doi: 10.1021/acs.jproteome.5b00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren X, Bai H, Pan Y, Tong W, Qin P, Yan H, Deng S, Zhong R, Qin W, Qian X. A graphene oxide-based immobilized PNGase F reagent for highly efficient N-glycan release and MALDI-TOF MS profiling. Anal Methods-Uk. 2014;6:2518–2525. [Google Scholar]
- 23.Hu Y, Mechref Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis. 2012;33:1768–1777. doi: 10.1002/elps.201100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robajac D, Masnikosa R, Vanhooren V, Libert C, Miković Ž, Nedić O. The N-glycan profile of placental membrane glycoproteins alters during gestation and aging. Mech Ageing Dev. 2014;138:1–9. doi: 10.1016/j.mad.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Hashii N, Harazono A, Kuribayashi R, Takakura D, Kawasaki N. Characterization of N-glycan heterogeneities of erythropoietin products by liquid chromatography/mass spectrometry and multivariate analysis. Rapid Commun Mass Spectrom. 2014;28:921–932. doi: 10.1002/rcm.6858. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, Hanneman AJ, Chasteen ND, Reinhold VN. Anomalous N-glycan structures with an internal fucose branched to GlcA and GlcN residues isolated from a mollusk shell-forming fluid. J Proteome Res. 2013;12:4547–4555. doi: 10.1021/pr4006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagae M, Soga K, Morita-Matsumoto K, Hanashima S, Ikeda A, Yamamoto K, Yamaguchi Y. Phytohemagglutinin from Phaseolus vulgaris (PHA-E) displays a novel glycan recognition mode using a common legume lectin fold. Glycobiology. 2014;24:368–78. doi: 10.1093/glycob/cwu004. [DOI] [PubMed] [Google Scholar]
- 28.Alley WR Jr, Novotny MV. Glycomic analysis of sialic acid linkages in glycans derived from blood serum glycoproteins. J Proteome Res. 2010;9:3062–3072. doi: 10.1021/pr901210r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padler-Karavani V. Aiming at the sweet side of cancer: Aberrant glycosylation as possible target for personalized-medicine. Cancer Lett. 2014;352:102–112. doi: 10.1016/j.canlet.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi-Sakita N, Kaneshiro-Nakagawa K, Kawashima M, Sugimoto M, Tokiwa M, Suzuki E, Kajihara S, Fujita Y, Iwamoto S, Tanaka K. Serum immunoglobulin G Fc region N-glycosylation profiling by matrix-assisted laser desorption/ionization mass spectrometry can distinguish breast cancer patients from cancer-free controls. Biochem Biophys Res Commun. 2015;469:1140–1145. doi: 10.1016/j.bbrc.2015.12.114. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi S, Sugiyama T, Shimomura M, Kamada Y, Fujita K, Nonomura N, Miyoshi E, Nakano M. Site-specific and linkage analyses of fucosylated N-glycans on haptoglobin in sera of patients with various types of cancer: possible implication for the differential diagnosis of cancer. Glycoconj J. 2016;33:471–82. doi: 10.1007/s10719-016-9653-7. [DOI] [PubMed] [Google Scholar]
- 32.Rauvala H. Gangliosides of human kidney. J Biol Chem. 1976;251:7517–7520. [PubMed] [Google Scholar]
- 33.Wang W, Lundgren T, Lindh F, Nilsson B, Grönberg G, Brown JP, Mentzer-Dibert H, Zopf D. Isolation of two novel sialyl-Lewis X-active oligosaccharides by high-performance liquid affinity chromatography using monoclonal antibody Onc-M26. Arch Biochem Biophys. 1992;292:433–441. doi: 10.1016/0003-9861(92)90013-m. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda M, Spooncer E, Oates J, Dell A, Klock J. Structure of sialylated fucosyl lactosaminoglycan isolated from human granulocytes. J Biol Chem. 1984;259:10925–10935. [PubMed] [Google Scholar]
- 35.Fischer K, Kettunen J, Würtz P, Haller T, Havulinna AS, Kangas AJ, Soininen P, Esko T, Tammesoo ML, Mägi R. Biomarker Profiling by Nuclear Magnetic Resonance Spectroscopy for the Prediction of All-Cause Mortality: An Observational Study of 17,345 Persons. PLoS Med. 2014;11:e1001606. doi: 10.1371/journal.pmed.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyselova Z, Mechref Y, Al Bataineh MM, Dobrolecki LE, Hickey RJ, Vinson J, Sweeney CJ, Novotny MV. Alterations in the serum glycome due to metastatic prostate cancer. J Proteome Res. 2007;6:1822–1832. doi: 10.1021/pr060664t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, Petrescu SM, Dwek RA, Rudd PM. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17:1344–1356. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 38.Kyselova Z, Mechref Y, Kang P, Goetz JA, Dobrolecki LE, Sledge GW, Schnaper L, Hickey RJ, Malkas LH, Novotny MV. Breast cancer diagnosis and prognosis through quantitative measurements of serum glycan profiles. Clin Chem. 2008;54:1166–1175. doi: 10.1373/clinchem.2007.087148. [DOI] [PubMed] [Google Scholar]
- 39.Bartling B, Vanhooren V, Dewaele S, Libert C, Hofmann HS, Haerting J, Nuding S, Silber RE, Simm A, Chen CC. Altered desialylated plasma N-glycan profile in patients with non-small cell lung carcinoma. Cancer Biomarkers. 2011;10:145–154. doi: 10.3233/CBM-2012-0239. [DOI] [PubMed] [Google Scholar]
- 40.Isailovic D, Kurulugama R, Plasencia M, Stokes S, Kyselova Z, Goldman R, Mechref Y, Novotny M, Clemmer D. Profiling of human serum glycans associated with liver cancer and cirrhosis by IMS-MS. J Proteome Res. 2008;7:1109–1117. doi: 10.1021/pr700702r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J, Qiu W, Simeone DM, Lubman DM. N-linked glycosylation profiling of pancreatic cancer serum using capillary liquid phase separation coupled with mass spectrometric analysis. J Proteome Res. 2007;6:1126–1138. doi: 10.1021/pr0604458. [DOI] [PubMed] [Google Scholar]
- 42.Alley WR Jr, Madera M, Mechref Y, Novotny MV. Chip-based reversed-phase liquid chromatography-mass spectrometry of permethylated N-linked glycans: a potential methodology for cancer-biomarker discovery. Anal Chem. 2010;82:5095–5106. doi: 10.1021/ac100131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isailovic D, Plasencia MD, Gaye MM, Stokes ST, Kurulugama RT, Pungpapong V, Zhang M, Kyselova Z, Goldman R, Mechref Y. Delineating diseases by IMS-MS profiling of serum N-linked glycans. J Proteome Res. 2011;11:576–585. doi: 10.1021/pr200777u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaye M, Valentine S, Hu Y, Mirjankar N, Hammoud Z, Mechref Y, Lavine B, Clemmer D. Ion mobility-mass spectrometry analysis of serum N-linked glycans from esophageal adenocarcinoma phenotypes. J Proteome Res. 2012;11:6102–6110. doi: 10.1021/pr300756e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierce A, Saldova R, Hamid UM, Abrahams JL, McDermott EW, Evoy D, Duffy MJ, Rudd PM. Levels of specific glycans significantly distinguish lymph node-positive from lymph node-negative breast cancer patients. Glycobiology. 2010;20:1283–1288. doi: 10.1093/glycob/cwq090. [DOI] [PubMed] [Google Scholar]
- 46.Arnold JN, Saldova R, Galligan MC, Murphy TB, Mimura-Kimura Y, Telford JE, Godwin AK, Rudd PM. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 2011;10:1755–1764. doi: 10.1021/pr101034t. [DOI] [PubMed] [Google Scholar]
- 47.Bereman MS, Williams TI, Muddiman DC. Development of a nanoLC LTQ orbitrap mass spectrometric method for profiling glycans derived from plasma from healthy, benign tumor control, and epithelial ovarian cancer patients. Anal Chem. 2008;81:1130–1136. doi: 10.1021/ac802262w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alley WR Jr, Vasseur JA, Goetz JA, Svoboda M, Mann BF, Matei DE, Menning N, Hussein A, Mechref Y, Novotny MV. N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J Proteome Res. 2012;11:2282–2300. doi: 10.1021/pr201070k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamid UM, Royle L, Saldova R, Radcliffe CM, Harvey DJ, Storr SJ, Pardo M, Antrobus R, Chapman CJ, Zitzmann N. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology. 2008;18:1105–1118. doi: 10.1093/glycob/cwn095. [DOI] [PubMed] [Google Scholar]
- 50.Ruhaak LR, Kim K, Stroble C, Taylor SL, Hong Q, Miyamoto S, Lebrilla CB, Leiserowitz G. Protein-specific differential Glycosylation of Immunoglobulins in serum of Ovarian Cancer Patients. J Proteome Res. 2016;15:1002–1010. doi: 10.1021/acs.jproteome.5b01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bones J, Mittermayr S, O’Donoghue N, Guttman As, Rudd PM. Ultra performance liquid chromatographic profiling of serum N-glycans for fast and efficient identification of cancer associated alterations in glycosylation. Anal Chem. 2010;82:10208–10215. doi: 10.1021/ac102860w. [DOI] [PubMed] [Google Scholar]
- 52.Kanoh Y, Mashiko T, Danbara M, Takayama Y, Ohtani S, Imasaki T, Abe T, Akahoshi T. Analysis of the oligosaccharide chain of human serum immunoglobulin g in patients with localized or metastatic cancer. Oncology. 2004;66:365–370. doi: 10.1159/000079484. [DOI] [PubMed] [Google Scholar]
- 53.Lin Z, Simeone DM, Anderson MA, Brand RE, Xie X, Shedden KA, Ruffin MT, Lubman DM. Mass spectrometric assay for analysis of haptoglobin fucosylation in pancreatic cancer. J Proteome Res. 2011;10:2602–2611. doi: 10.1021/pr200102h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saldova R, Wormald MR, Dwek RA, Rudd PM. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis Markers. 2008;25:219–232. doi: 10.1155/2008/601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bones J, Byrne JC, O’Donoghue N, McManus C, Scaife C, Boissin H, Nastase A, Rudd PM. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J Proteome Res. 2010;10:1246–1265. doi: 10.1021/pr101036b. [DOI] [PubMed] [Google Scholar]
- 56.Balmaña M, Sarrats A, Llop E, Barrabés S, Saldova R, Ferri MJ, Figueras J, Fort E, de Llorens R, Rudd PM. Identification of potential pancreatic cancer serum markers: Increased sialyl-Lewis X on ceruloplasmin. Clin Chim Acta. 2015;442:56–62. doi: 10.1016/j.cca.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Mechref Y, Hu Y, Garcia A, Zhou S, Desantos-Garcia JL, Hussein A. Defining putative glycan cancer biomarkers by MS. Bioanalysis. 2012;4:2457–2469. doi: 10.4155/bio.12.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An HJ, Miyamoto S, Lancaster KS, Kirmiz C, Li B, Lam KS, Leiserowitz GS, Lebrilla CB. Profiling of glycans in serum for the discovery of potential biomarkers for ovarian cancer. J Proteome Res. 2006;5:1626–1635. doi: 10.1021/pr060010k. [DOI] [PubMed] [Google Scholar]
- 59.Storr SJ, Royle L, Chapman CJ, Hamid UM, Robertson JF, Murray A, Dwek RA, Rudd PM. The O-linked glycosylation of secretory/shed MUC1 from an advanced breast cancer patient’s serum. Glycobiology. 2008;18:456–462. doi: 10.1093/glycob/cwn022. [DOI] [PubMed] [Google Scholar]



