Abstract
Hepatocellular carcinoma (HCC) is the sixth most frequent malignant tumor with poor prognosis, and its clinical therapeutic outcome is poor. Volasertib, a potent small molecular inhibitor of polo-like kinase 1 (PLK1), is currently tested for treatment of multiple cancers in the clinical trials. However, the antitumor effect of volasertib on HCC is still unknown. In this study, our data show that volasertib is able to induce cell growth inhibition, cell cycle arrest at G2/M phase and apoptosis with the spindle abnormalities in human HCC cells. Furthermore, volasertib also increases the intracellular reactive oxidative species (ROS) levels, and pretreated with ROS scavenger N-acety-L-cysteine partly reverses volasertib-induced apoptosis. Moreover, volasertib markedly inhibits the subcutaneous xenograft growth of HCC in nude mice. Overall, our study provides new therapeutic potential of volasertib on hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma, volasertib, ROS, apoptosis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequent malignant tumor and the third leading cause of cancer-related mortality in the world [1]. Most cases of HCC are as a result of viral infection (hepatitis B or C) and metabolic toxins (alcohol or aflatoxin), and the patients with HCC are usually diagnosed when tumor is in an advanced stage [2]. Although treatment of HCC includes the combination of surgery, chemotherapy or radiotherapy, the 5-year survival rates of patients with HCC is poor [3]. Consequently, it is necessary to develop new therapeutic strategies against HCC.
The polo-like kinases (PLKs) are a family of conserved serine/threonine kinases and play the critical role in the regulation of multiple stages of cell cycle, including mitotic entry/exit, centrosome maturation, and maintenance of the bipolar spindle [4]. It has been reported that PLK1 is overexpressed in up to 80% of cancers and associated with poorer prognosis [5-7]. In human HCC, PLK1 expression is significantly upregulated and correlated with venous invasion, tumor nodules, Edmondson grade and survival rates [8,9]. Knockdown of PLK1 significantly inhibits the growth of HCC cells by inducing cell cycle arrest at G2/M phase and apoptosis [10,11]. Additionally, the HBV X protein can enhance the expression of PLK1, and inhibition of PLK1 suppresses the HBV X protein-mediated oncogenic transformation in HCC [12]. Therefore, PLK1 is a promising therapeutic target of HCC. Volasertib (BI 6727) is a potent small molecule inhibitor of PLK1 (IC50=0.87 nM) by competing with ATP for binding to the ATP binding pocket, and 6- and 65-fold greater activity against two closely related kinases PLK2 (IC50=5 nM) and PLK3 (IC50=56 nM) [13]. In the preclinical studies, volasertib shows broad antitumor activity in several cancer models including hematological malignancies, melanomas, cervical cancer and sarcomas [13-17]. However, the antitumor effect of volasertib on HCC is still unknown. In this study, we demonstrated that volasertib could potently suppress the growth of human HCC in vitro and in vivo.
Material and methods
Cell culture and reagents
Human hepatoma cancer cell lines BEL7402, HepG2, SMMC7721 and SK-Hep-1 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 ng/ml) in a humidified incubator at 37°C with 5% CO2. Volasertib was purchased from ApexBio Technology. N-acetly-L-cysteine (NAC) and dihydroethidium (DHE) were purchased from Sigma-Aldrich. Anti-PARP (9542), anti-β-tubulin (A11031) and Anti-GAPDH (KM9002) antibodies were from Cell Signaling Technologies, Ruiying Biological and Sungene Biotech, respectively.
Cell viability assays
Cells were firstly seeded into a 96-well plate at a density of 5~10×103 cells per well, and incubated with drugs in three parallel wells for 72 h. Then 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) was added to each well at a final concentration of 0.5 mg/ml. After incubation for 4 h, formazan crystals were dissolved in 100 μl of DMSO, and absorbance at 570 nm was measured by plate reader. The concentrations required to inhibit growth by 50% (IC50) were calculated from survival curves using the Bliss method [18,19]. For drug combinational experiments, cells were treated with the indicated concentrations of volasertib combined with cisplatin for 72 h. The data were analyzed by CompuSyn software with the results showed as combination index (CI) values according to the median-effect principle, where CI <1, =1, and >1 indicate synergism, additive effect and antagonism, respectively [20,21].
Cell cycle assays
Cells were harvested and washed twice with cold phosphate-buffered saline (PBS), then fixed with ice-cold 70% ethanol for 30 min at 4°C. After centrifugation at 200×g for 10 min, cells were washed twice with PBS and resuspended with 0.5 ml PBS containing PI (50 μg/ml), 0.1% Triton X-100, 0.1% sodium citrate, and DNase-free RNase (100 μg/ml), and detected by FCM after 15 min incubation at room temperature in the dark. Fluorescence was measured at an excitation wavelength of 480 nm through a FL-2 filter (585 nm). Data were analyzed using ModFit LT 3.0 software (Becton Dickinson) [22,23].
Apoptosis assays
Cell apoptosis was evaluated with flow cytometry (FCM) assay. Briefly, cells were harvested and washed twice with PBS, stained with Annexin V-FITC and propidium iodide (PI) in the binding buffer, and detected by FACS Calibur FCM (BD, CA, USA) after 15 min incubation at room temperature in the dark. Fluorescence was measured at an excitation wave length of 480 nm through FL-1 (530 nm) and FL-2 filters (585 nm). The early apoptotic cells (Annexin V positive only) and late apoptotic cells (Annexin V and PI positive) were quantified [24,25].
Reactive oxygen species (ROS) assay
Cells were incubated with 10 μM of DHE for 30 min at 37°C, and observed under fluorescence microscope (Olympus, Japan) immediately after washing twice with PBS. Five fields were taken randomly for each well [26,27].
Western blot analysis
Cells were harvested and washed twice with cold PBS, then resuspended and lysed in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 ng/ml PMSF, 0.03% aprotinin, 1 μM sodium orthovanadate) at 4°C for 30 min. Lysates were centrifuged for 10 min at 14,000×g and supernatants were stored at -80°C as whole cell extracts. Total protein concentrations were determined with Bradford assay. Proteins were separated on 12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% BSA and incubated with the indicated primary antibodies. Corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies were used against each primary antibody. Proteins were detected using the chemiluminescent detection reagents and films [28,29].
Immunofluorescence assay
BEL 7402 and Hep G2 cells grown on glass coverslips were washed thrice with PBS and fixed in 4% paraformaldehyde. The fixed cells were washed thrice with PBS containing 0.05% Tween 20, and then treated with PBS containing 0.2% Triton X-100 for 10 min at room temperature followed by washing, blocking and incubation with anti-β-tubulin for 1 hr at 37°C. The coverslips were washed, treated with Hoechst 33342 (5 μg/ml in PBS) for 5 min, washed again. The stained cells were analyzed by using the inverted Olympus microscope IX 70 FluoView system [30,31].
Nude mouse assays
Balb/c nude mice were obtained from the Balb/c nude mice were obtained from the Guangdong Medical Laboratory Animal Center and maintained with sterilized food and water. Six female nude mice with 5 weeks old and 20 g weight were used for each group. Each mouse was injected subcutaneously with BEL7402 and HepG2 cells (2×106 in 100 μl of medium) under the shoulder. When the subcutaneous tumors were approximately 0.3×0.3 cm2 (two perpendicular diameters) in size, mice were randomized into four groups, and were injected intraperitoneally with vehicle alone (0.5% carboxymethyl cellulose) and volasertib alone (15 mg/kg) every two days, respectively. The body weights of mice and the two perpendicular diameters (A and B) of tumors were recorded. The tumor volume (V) was calculated according to the formula:
v = (π/6)((A + B)/2)3
The mice were anaesthetized after experiment, and tumor tissues were excised from the mice and weighted. The rate of inhibition (IR) was calculated according to the formula [32,33]:
IR = 1 - (Mean tumor weight of experimental group/Mean tumor weight of control group) × 100%
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical analysis of the differences between two groups is performed with Student’s t-test. Values of P <0.05 were considered as significant differences.
Results
Volasertib inhibits the growth of HCC cells in vitro
To investigate the effect of volasertib on the growth of HCC, cell survival was detected by MTT assay. The four HCC cell lines BEL7402, HepG2, SMMC7721 and SK-Hep-1 were treated with various concentration of volasertib for 72 hr. As shown in Figure 1, the survivals of four cell lines were decreased in a dose-dependent manner after volasertib treatment. BEL7402 cells were most sensitive to volasertib with the IC50 values of 0.006 μM. The IC50 values of volasertib in other three HCC cell lines HepG2, SMMC7721 and SK-Hep1 were 2.854 μM, 3.970 μM and 7.025 μM, respectively.
Figure 1.
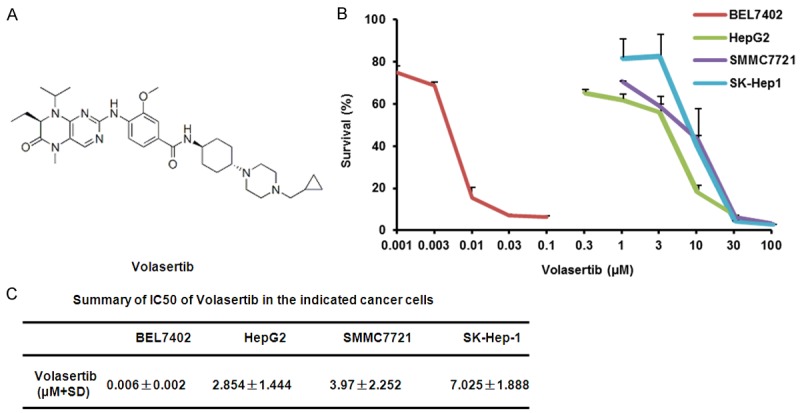
Volasertib inhibits the growth of HCC cells in vitro. A: Chemical structure of volasertib. B: The representative growth curves of cells treated with volasertib are shown. C: Summary of IC50 of volasertib in the indicated HCC cells is shown. Cells were treated with various concentrations of volasertib for 72 h and cell survival was determined by MTT assay. Datas were mean ± S.D. of three independent experiments.
Volasertib induces cell cycle arrest at G2/M phase in HCC cells
To determine whether the growth inhibition of volasertib on HCC cells is the results of cell cycle arrest, both BEL7402 and HepG2 cells were treated with volasertib at the indicated concentrations for 48 hr. The cell cycle distribution was assessed by FCM with PI staining, and analysed with ModFit LT 3.0 software. As shown in Figure 2, volasertib dose-dependently induced the accumulation in Sub G1 and G2/M phase and reduction in G0/G1 phase in both BEL7402 and HepG2 cells.
Figure 2.
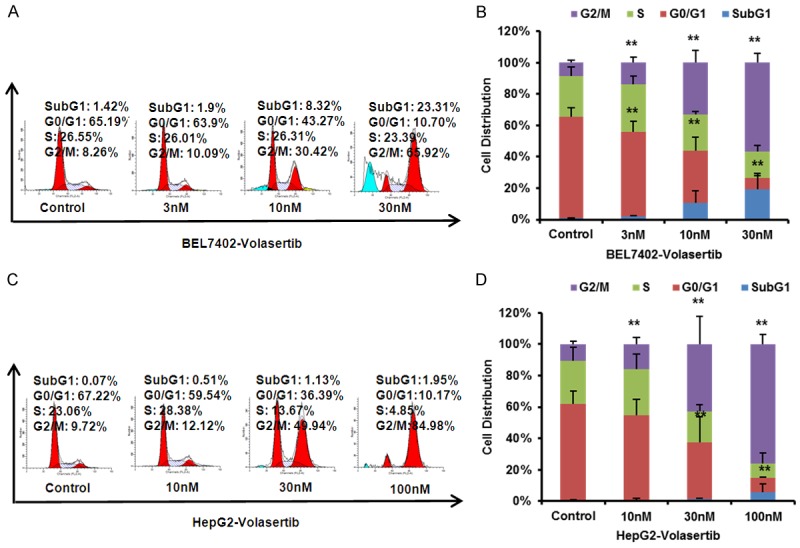
Volasertib induces cell cycle arrest at G2/M Phase in HCC cells. BEL7402 (A) and HepG2 (B) cells were treated with volasertib at the indicated concentrations. The distribution of cell cycle was detected by FCM with PI staining. The percentages of sub G1, G1/G0, S, G2/M phase were calculated using ModFit LT 3.0 software. The representative charts, quantified results (C, D) of three independent experiments were shown. *P<0.05 and **P<0.01 vs. corresponding control.
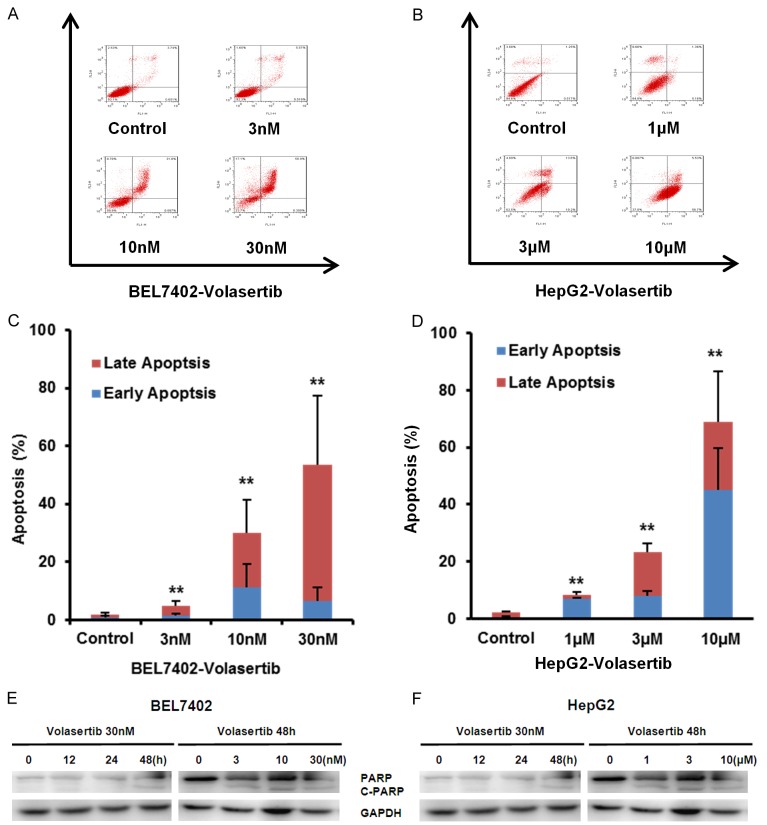
Volasertib induces apoptosis in HCC cells
To further determine whether volasertib is able to induce apoptosis in HCC cells, both BEL7402 and HepG2 were treated with volasertib at the indicated concentrations for 48 hr, stained with Annexin V/PI and examined by FCM. As shown in Figure 3A-D, volasertib induced apoptosis in both BEL7402 and HepG2 cells in a dose-dependent manner. To investigate the molecular mechanism of cell apoptosis by volasertib, the apoptosis related proteins are detected by Western blot. The marker of apoptosis, the cleaved PARP, was increased in a dose- and time-dependent manner after volasertib treatment (Figure 3E, 3F).
Figure 3.
Volasertib induces apoptosis in HCC cells. BEL7402 (A) and HepG2 (B) cells were treated with volasertib at the indicated time and concentrations. The apoptosis was detected by FCM with Annexin V/PI staining. The proportions of Annexin V+/PI- and Annexin V+/PI+ cells indicated the early and late stage of apoptosis. The protein expression was examined by Western blot, and GAPDH was used as loading control. The representative charts, quantified results (C, D) and Western blot results (E, F) of three independent experiments were shown. *P<0.05 and **P<0.01 vs. corresponding control.
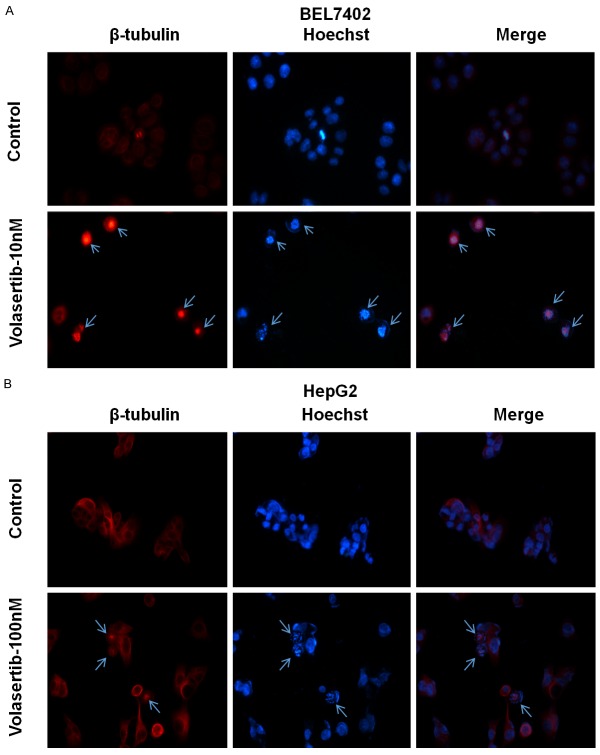
Volasertib promotes spindle abnormalities in HCC cells
PLK1 plays a critical role in the centrosome maturation and maintenance of the bipolar spindle [4]. To test whether volasertib promotes spindle abnormalities, both BEL7402 and HepG2 cells were treated with volasertib at the indicated concentrations for 24 hr and stained with Hoechst 33342 (to visualize chromosomal DNA, blue) and β-tubulin antibody (to visualize tubulin spindles, red). As shown in Figure 4, both BEL7402 and HepG2 cells after volasertib treatment exhibited profound abnormalities in spindle formation, which lead to misalignment of chromosomes.
Figure 4.
Volasertib promotes spindle abnormalities in HCC cells. BEL7402 (A) and HepG2 (B) cells were treated with 10 nM and 100 nM of volasertib for 24 h respectively. Cells were fixed and stained with Hoechst 33342 (to stain DNA, blue) as well as with β-tubulin antibody (to visualize tubulin spindles, red). Cells were analyzed by immunofluorescence microscopy. Arrows indicate the spindle abnormalities of cells.
Volasertib enhances ROS accumulation in HCC cells
Reactive oxidative species (ROS) is important to involve in the sensitivity of cancer cells to anticancer agents [34]. To examine the role of ROS in the anticancer effect of volasertib in HCC cells, the ROS fluorescent probe dihydroethidium (DHE) was used to stain both BEL7402 and HepG2 cells after volasertib treatment. As shown in Figure 5, volasertib dose- and time-dependently enhanced the fluorescent intensity of DHE in both cells, suggesting that volasertib can enhance ROS accumulation in HCC cells.
Figure 5.
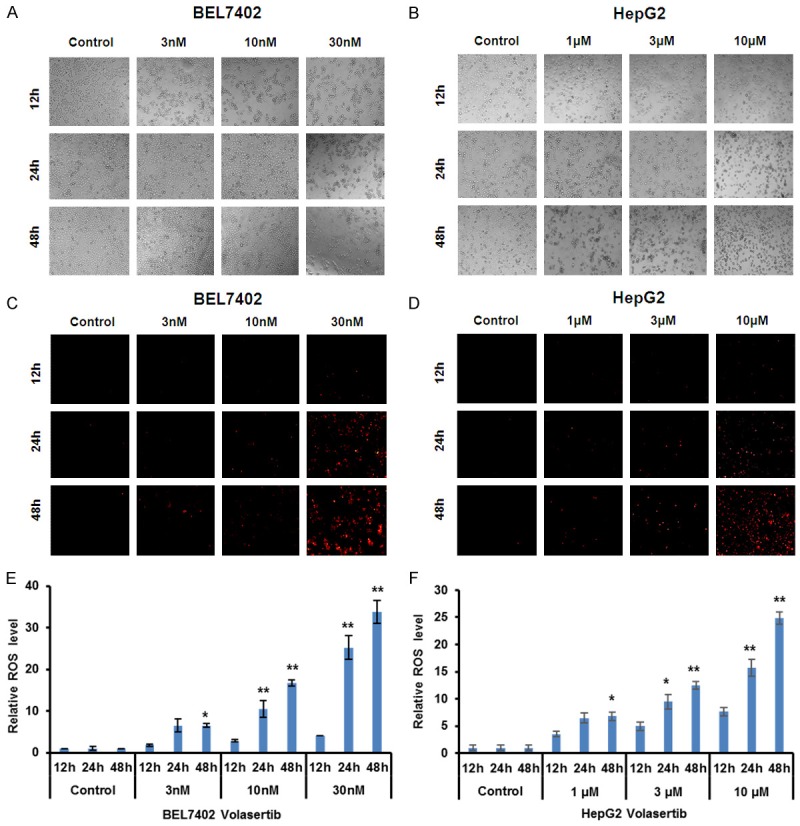
Volasertib enhances ROS accumulation in HCC cells. BEL7402 and HepG2 cells were treated with volasertib at the indicated times and concentrations, stained with DHE and photographed under florescent microscope. The representative micrographs (bright field - A, B and fluorescence - C, D) and quantified results (E, F) of three independent experiments were shown. *P<0.05; **P<0.01 vs. corresponding control.
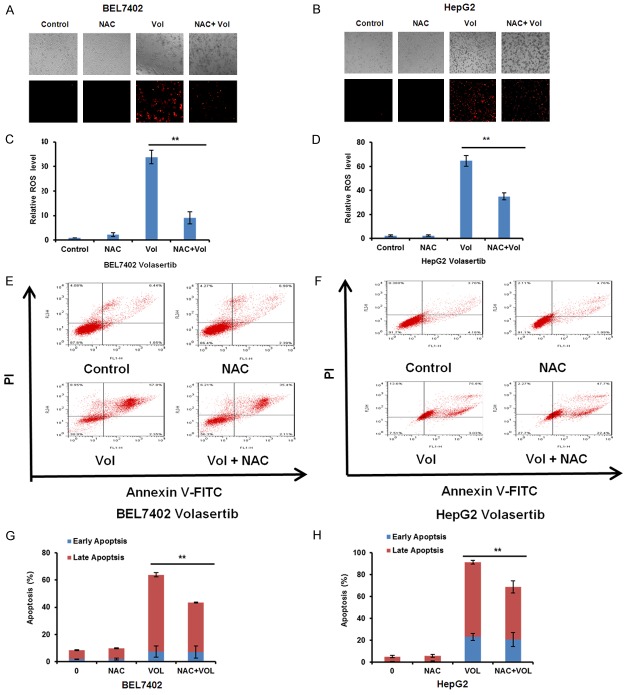
Inhibition of ROS partially rescues volasertib-induced apoptosis in HCC cells
To further study the role of ROS in volasertib-induced apoptosis in HCC cells, both BEL7402 and HepG2 cells were treated with volasertib for 48 h with or without the ROS scavenger NAC at 5 mM pretreated for 1 h and stained with DHE. As shown in Figure 6A-D, the volasertib-induced DHE fluorescent signal was dramatically reversed by NAC in both cells. Additionally, the volasertib-induced apoptosis was partially inverted by NAC (Figure 6E-H), suggesting volasertib can induce both ROS dependent and independent apoptosis in HCC cells.
Figure 6.
Inhibition of ROS partially rescues volasertib-induced apoptosis in HCC cells. BEL7402 (A) and HepG2 (B) cells were treated with volasertib at 30 nM and 10 μM respectively for 48 h in the presence or absence of 5 mM NAC pretreatment for 1 h, stained with DHE and photographed under florescent microscope. The representative micrographs and quantified results (C, D) of three independent experiments were shown. The apoptosis was detected by FCM with Annexin V/PI staining. The representative charts (E, F) and quantified results (G, H) of three independent experiments were shown. Vol: Volasertib. *P<0.05 and **P<0.01 versus corresponding control.
Volasertib suppresses the subcutaneous xenograft growth of HCC in nude mice
To further estimate the antitumor effects of volasertib in vivo, we generated the subcutaneous xenograft tumor models by transplanting both BEL7402 and HepG2 cells into nude mice. As shown in Figure 7, volasertib at 15 mg/kg significantly inhibited the tumor growth by reducing the volume and weight of both BEL7402 and HepG2 tumors. The inhibition rates of tumor growth in BEL7402 and HepG2 was 75.4% and 52.9%, respectively. Additionally, the weight of nude mice did not exhibit the statistical difference during experiments, which suggests volasertib at the indicated dose did not cause toxicities in mice (Figure 7G).
Figure 7.
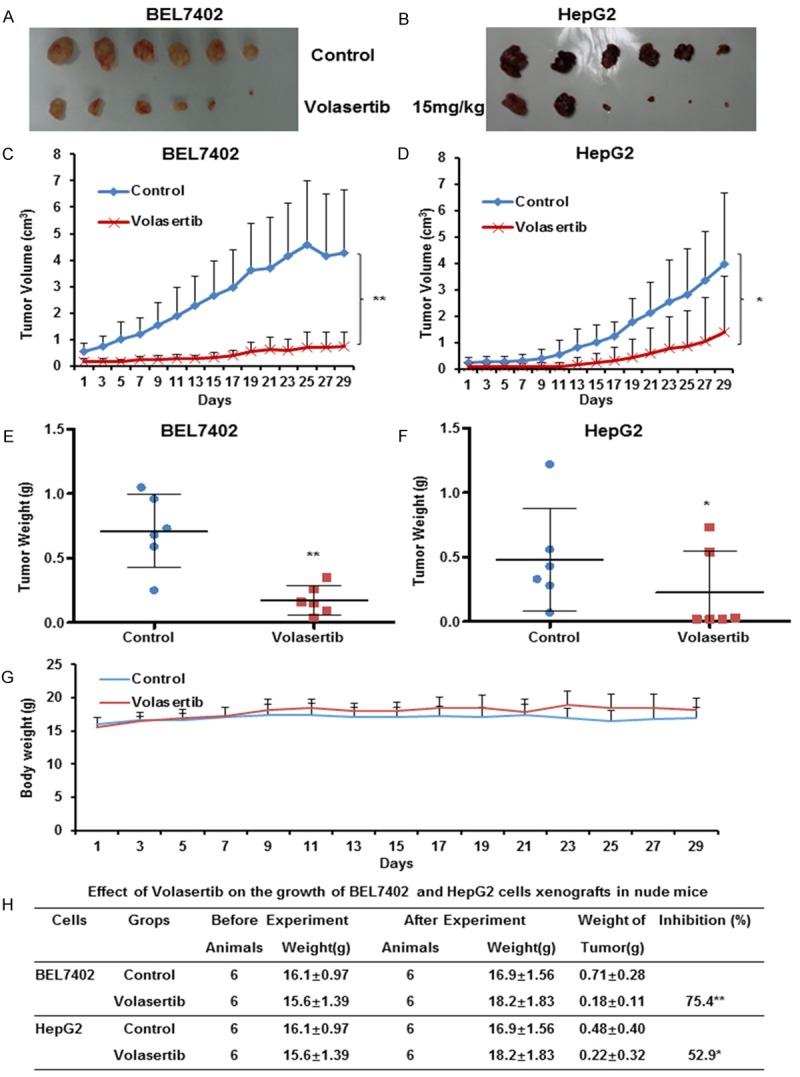
Volasertib suppresses the subcutaneous xenograft growth of HCC in nude mice. Each mouse was injected subcutaneously with BEL7402 and HepG2 cells (2×106 in 100 μl of medium) under the shoulder. When the tumors developed into 0.3×0.3 cm2 (two perpendicular diameters) in size, mice were randomized into two groups, and were intraperitoneally injection with vehicle (0.5% carboxymethyl cellulose) and volasertib (15 mg/kg) every two days, respectively. The body weights of mice and the two perpendicular diameters of the tumor were recorded. The mice were anaesthetized after experiment, and tumor tissue was excised from the mice and weighed. The original tumors (A, B), tumor volume (C, D), tumor weight (E, F), body weight (G) and summary data (H) were shown. The values presented are the means ± SD for each group. *P<0.05 and **P<0.01 vs. corresponding control.
Discussion
In this study, we show that volasertib can potently induce cell growth inhibition, cell cycle arrest at G2/M phase and apoptosis with abnormal spindle formation in human HCC cells in vitro and in vivo, which are consistent with previous reports that have demonstrated that volasertib is able to induce mitotic arrest and apoptosis by targeting PLK1 in several other cancer models [13,14]. Furthermore, volasertib also increases the intracellular ROS levels, and pretreated with ROS scavenger NAC partially reverses volasertib-induced apoptosis. It has been reported that another PLK1 inhibitor BI 2536 alone or in combination with histone deacetylase (HDAC) inhibitor vorinostat could also enhance the intracellular ROS production to trigger cell death either in imatinib mesylate-sensitive or -resistant BCR/ABL+ leukemia cells, and the ROS scavenger Mn (III) tetrakis (4-benzoic acid)porphyrin chloride (MnTBAP) blocks ROS generation as well as lethality in BI 2536/vorinostat-treated cells [35]. Similarly, BI 2536 alone or combined with microtubule-destabilizing drug vincristine dramatically promotes the production of ROS to induce cell apoptosis in rhabdomyosarcoma cells, and the antioxidants MnTBAP, NAC as well as α-tocopherol significantly rescue from BI 2536/vincristine-induced apoptosis [36]. Therefore, ROS plays an important role in cancer treatment with PLK1 inhibitors including volasertib, which usually triggers cancer cell death by increasing the intracellular ROS.
Currently, the clinical data indicate that volasertib has the promising clinical efficacy in a wide range of malignancies. The phase I clinical trials of volasertib show a favorable pharmacokinetic profile and encouraging preliminary antitumour activity with manageable toxicities such as anaemia, neutropenia and thrombocytopenia [37-40]. But volasertib has insufficient antitumor activity as a monotherapy in the phase II trial of locally advanced or metastatic urothelial cancer [41]. Accordingly, multiple groups have investigated the combined anticancer effects of volasertib and other chemotherapeutic drugs in the preclinical and clinical studies. Combination of volasertib with microtubule-interfering drugs including vincristine, vinblastine, vinorelbine or eribulin shows the synergistic induction of apoptosis in Ewing sarcoma and other pediatric malignant cells [42,43]. Volasertib alone or in combination with azacitidine, cytarabine, decitabine and quizartinib is highly potent in multiple preclinical models of acute myeloid leukemia (AML) [44]. Moreover, volasertib powerfully inhibits the growth of bladder and cervical cancer cells, and potentiates the activity of cisplatin [17,45]. Inspiringly, a recent phase I study has demonstrated that volasertib plus cisplatin or carboplatin has manageable safety and antitumor activity without pharmacokinetics interference [46]. Another phase I study shows that volasertib combined with nintedanib also has manageable safety profile and antitumor effect without clearly pharmacokinetic disturbance [47]. But volasertib in combination with afatinib exhibits limited antitumor activity in a phase I study of 57 patients with advanced solid tumors [48]. In a phase II trial including 87 patients with AML, co-treatment with volasertib and cytarabine significantly prolongs median overall survival and median event-free survival but increases frequency of side effects compared with cytarabine alone [49]. But the results of another phase II trial in patients with advanced non-small-cell lung cancer exhibits that volasertib in combination with pemetrexed does not improve efficacy without increasing adverse events and pharmacokinetics interference compared with pemetrexed alone [50]. However, the combination of volasertib with other anticancer agents for the treatment of HCC still needs to be tested in the future.
In summary, our study demonstrated that volasertib could potently induce cell growth inhibition, cell cycle arrest and apoptosis in HCC cells in vitro and in vivo. The beneficial therapy of volasertib appears to be potential treatment strategy in patients with HCC.
Acknowledgements
This work was supported by funds from the Chinese National Natural Science Foundation No. 31271444 and No. 81201726 (Z. S.), and No. 31171304 (X. W.), the Guangdong Natural Science Funds for Distinguished Young Scholar No. 2014A030306001 (Z. S), the Guangdong Special Support Program for Young Talent No. 2015TQ01R350 (Z. S.) and the Science and Technology Program of Guangdong No. 2016A050502027 (Z. S.) and No. 2013B090500109 (X. W.), the Science and Technology Program of Guangzhou No. 201510010123 and No. 201300000041 (X. W.), and the Foundation for Research Cultivation and Innovation of Jinan University No. 21616119 (Z. S.).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.Smith AD, Dunk AA, Tuttle-Newhall JE, Trotter JF. Hepatocellular carcinoma. Lancet. 2004;363:898–899. doi: 10.1016/S0140-6736(04)15750-4. [DOI] [PubMed] [Google Scholar]
- 4.Zitouni S, Nabais C, Jana SC, Guerrero A, Bettencourt-Dias M. Polo-like kinases: structural variations lead to multiple functions. Nat Rev Mol Cell Biol. 2014;15:433–452. doi: 10.1038/nrm3819. [DOI] [PubMed] [Google Scholar]
- 5.Gjertsen BT, Schoffski P. Discovery and development of the Polo-like kinase inhibitor volasertib in cancer therapy. Leukemia. 2015;29:11–19. doi: 10.1038/leu.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degenhardt Y, Lampkin T. Targeting Polo-like kinase in cancer therapy. Clin Cancer Res. 2010;16:384–389. doi: 10.1158/1078-0432.CCR-09-1380. [DOI] [PubMed] [Google Scholar]
- 7.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 8.He ZL, Zheng H, Lin H, Miao XY, Zhong DW. Overexpression of polo-like kinase1 predicts a poor prognosis in hepatocellular carcinoma patients. World J Gastroenterol. 2009;15:4177–4182. doi: 10.3748/wjg.15.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun W, Su Q, Cao X, Shang B, Chen A, Yin H, Liu B. High expression of polo-like kinase 1 is associated with early development of hepatocellular carcinoma. Int J Genomics. 2014;2014:312130. doi: 10.1155/2014/312130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mok WC, Wasser S, Tan T, Lim SG. Polo-like kinase 1, a new therapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2012;18:3527–3536. doi: 10.3748/wjg.v18.i27.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Z, Wu J, Dang H, Lin H, Zheng H, Zhong D. Polo-like kinase 1 contributes to the tumorigenicity of BEL-7402 hepatoma cells via regulation of Survivin expression. Cancer Lett. 2011;303:92–98. doi: 10.1016/j.canlet.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Studach LL, Rakotomalala L, Wang WH, Hullinger RL, Cairo S, Buendia MA, Andrisani OM. Polo-like kinase 1 inhibition suppresses hepatitis B virus X protein-induced transformation in an in vitro model of liver cancer progression. Hepatology. 2009;50:414–423. doi: 10.1002/hep.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, Haslinger C, Garin-Chesa P, Adolf GR. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094–3102. doi: 10.1158/1078-0432.CCR-08-2445. [DOI] [PubMed] [Google Scholar]
- 14.Gorlick R, Kolb EA, Keir ST, Maris JM, Reynolds CP, Kang MH, Carol H, Lock R, Billups CA, Kurmasheva RT, Houghton PJ, Smith MA. Initial testing (stage 1) of the Polo-like kinase inhibitor volasertib (BI 6727), by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2014;61:158–164. doi: 10.1002/pbc.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X. Targeting Polo-Like Kinases: A Promising Therapeutic Approach for Cancer Treatment. Transl Oncol. 2015;8:185–195. doi: 10.1016/j.tranon.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandwein JM. Targeting polo-like kinase 1 in acute myeloid leukemia. Ther Adv Hematol. 2015;6:80–87. doi: 10.1177/2040620715571077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie FF, Pan SS, Ou RY, Zheng ZZ, Huang XX, Jian MT, Qiu JG, Zhang WJ, Jiang QW, Yang Y, Li WF, Shi Z, Yan XJ. Volasertib suppresses tumor growth and potentiates the activity of cisplatin in cervical cancer. Am J Cancer Res. 2015;5:3548–3559. [PMC free article] [PubMed] [Google Scholar]
- 18.W CS. Tables for convenient calculation of median-effective dose (LD50 or ED50) and instructions in their use. Biometrics. 1952;8:249–263. [Google Scholar]
- 19.Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, Talele TT, Fu LW, Chen ZS. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Jiang QW, Cheng KJ, Mei XL, Qiu JG, Zhang WJ, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Zhang X, Lv M, Chen MW, Wei X, Shi Z. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget. 2015;6:32790–32804. doi: 10.18632/oncotarget.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv M, Qiu JG, Zhang WJ, Jiang QW, Qin WM, Yang Y, Zheng DW, Chen Y, Huang JR, Wang K, Wei MN, Cheng KJ, Shi Z. Wallichinine reverses ABCB1-mediated cancer multidrug resistance. Am J Transl Res. 2016;8:2969–80. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Gong L, Ou R, Zheng Z, Chen J, Xie F, Huang X, Qiu J, Zhang W, Jiang Q, Yang Y, Zhu H, Shi Z, Yan X. Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecol Oncol. 2016;140:537–544. doi: 10.1016/j.ygyno.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z, Li Z, Li ZJ, Cheng K, Du Y, Fu H, Khuri FR. Cables1 controls p21/Cip1 protein stability by antagonizing proteasome subunit alpha type 3. Oncogene. 2015;34:2538–2545. doi: 10.1038/onc.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Z, Liang YJ, Chen ZS, Wang XW, Wang XH, Ding Y, Chen LM, Yang XP, Fu LW. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol Ther. 2006;5:39–47. doi: 10.4161/cbt.5.1.2236. [DOI] [PubMed] [Google Scholar]
- 26.Gong LH, Chen XX, Wang H, Jiang QW, Pan SS, Qiu JG, Mei XL, Xue YQ, Qin WM, Zheng FY, Shi Z, Yan XJ. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid Med Cell Longev. 2014;2014:906804. doi: 10.1155/2014/906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen XX, Xie FF, Zhu XJ, Lin F, Pan SS, Gong LH, Qiu JG, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Shi Z, Yan XJ. Cyclin-dependent kinase inhibitor dinaciclib potently synergizes with cisplatin in preclinical models of ovarian cancer. Oncotarget. 2015;6:14926–14939. doi: 10.18632/oncotarget.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y, Jiang QW, Wu JY, Qiu JG, Zhang WJ, Mei XL, Shi Z, Di JM. Regulation of migration and invasion by Toll-like receptor-9 signaling network in prostate cancer. Oncotarget. 2015;6:22564–22574. doi: 10.18632/oncotarget.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Lan SJ, Liu QR, Liu JM, Chen XQ. Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol Pharmacol. 2013;83:1109–1119. doi: 10.1124/mol.112.083634. [DOI] [PubMed] [Google Scholar]
- 30.Shi Z, Park HR, Du Y, Li Z, Cheng K, Sun SY, Fu H, Khuri FR. Cables1 complex couples survival signaling to the cell death machinery. Cancer Res. 2015;75:147–158. doi: 10.1158/0008-5472.CAN-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, Fu LW, Ambudkar SV, Chen ZS. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem Pharmacol. 2009;77:781–793. doi: 10.1016/j.bcp.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei XL, Yang Y, Zhang YJ, Li Y, Zhao JM, Qiu JG, Zhang WJ, Jiang QW, Xue YQ, Zheng DW, Chen Y, Qin WM, Wei MN, Shi Z. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. 2015;5:3311–3324. [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu JG, Zhang YJ, Li Y, Zhao JM, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Shi Z. Trametinib modulates cancer multidrug resistance by targeting ABCB1 transporter. Oncotarget. 2015;6:15494–15509. doi: 10.18632/oncotarget.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue YQ, Di JM, Luo Y, Cheng KJ, Wei X, Shi Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid Med Cell Longev. 2014;2014:765832. doi: 10.1155/2014/765832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasmahapatra G, Patel H, Nguyen T, Attkisson E, Grant S. PLK1 inhibitors synergistically potentiate HDAC inhibitor lethality in imatinib mesylate-sensitive or -resistant BCR/ABL+ leukemia cells in vitro and in vivo. Clin Cancer Res. 2013;19:404–414. doi: 10.1158/1078-0432.CCR-12-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hugle M, Belz K, Fulda S. Identification of synthetic lethality of PLK1 inhibition and microtubule-destabilizing drugs. Cell Death Differ. 2015;22:1946–1956. doi: 10.1038/cdd.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, Taton M, Fritsch H, Glomb P, Munzert G. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2012;48:179–186. doi: 10.1016/j.ejca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Lin CC, Su WC, Yen CJ, Hsu CH, Su WP, Yeh KH, Lu YS, Cheng AL, Huang DC, Fritsch H, Voss F, Taube T, Yang JC. A phase I study of two dosing schedules of volasertib (BI 6727), an intravenous polo-like kinase inhibitor, in patients with advanced solid malignancies. Br J Cancer. 2014;110:2434–2440. doi: 10.1038/bjc.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nokihara H, Yamada Y, Fujiwara Y, Yamamoto N, Wakui H, Nakamichi S, Kitazono S, Inoue K, Harada A, Taube T, Takeuchi Y, Tamura T. Phase I trial of volasertib, a Polo-like kinase inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2016;34:66–74. doi: 10.1007/s10637-015-0300-0. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi Y, Yamauchi T, Kiyoi H, Sakura T, Hata T, Ando K, Watabe A, Harada A, Taube T, Miyazaki Y, Naoe T. Phase I trial of volasertib, a Polo-like kinase inhibitor, in Japanese patients with acute myeloid leukemia. Cancer Sci. 2015;106:1590–1595. doi: 10.1111/cas.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stadler WM, Vaughn DJ, Sonpavde G, Vogelzang NJ, Tagawa ST, Petrylak DP, Rosen P, Lin CC, Mahoney J, Modi S, Lee P, Ernstoff MS, Su WC, Spira A, Pilz K, Vinisko R, Schloss C, Fritsch H, Zhao C, Carducci MA. An open-label, single-arm, phase 2 trial of the Polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer. 2014;120:976–982. doi: 10.1002/cncr.28519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss LM, Hugle M, Romero S, Fulda S. Synergistic induction of apoptosis by a polo-like kinase 1 inhibitor and microtubule-interfering drugs in Ewing sarcoma cells. Int J Cancer. 2016;138:497–506. doi: 10.1002/ijc.29725. [DOI] [PubMed] [Google Scholar]
- 43.Abbou S, Lanvers-Kaminsky C, Daudigeos-Dubus E, LE Dret L, Laplace-Builhe C, Molenaar J, Vassal G, Geoerger B within the ITCC Biology and Preclinical Evaluation Committee. Polo-like Kinase Inhibitor Volasertib Exhibits Antitumor Activity and Synergy with Vincristine in Pediatric Malignancies. Anticancer Res. 2016;36:599–609. [PubMed] [Google Scholar]
- 44.Rudolph D, Impagnatiello MA, Blaukopf C, Sommer C, Gerlich DW, Roth M, Tontsch-Grunt U, Wernitznig A, Savarese F, Hofmann MH, Albrecht C, Geiselmann L, Reschke M, Garin-Chesa P, Zuber J, Moll J, Adolf GR, Kraut N. Efficacy and mechanism of action of volasertib, a potent and selective inhibitor of Polo-like kinases, in preclinical models of acute myeloid leukemia. J Pharmacol Exp Ther. 2015;352:579–589. doi: 10.1124/jpet.114.221150. [DOI] [PubMed] [Google Scholar]
- 45.Brassesco MS, Pezuk JA, Morales AG, de Oliveira JC, Roberto GM, da Silva GN, Francisco de Oliveira H, Scrideli CA, Tone LG. In vitro targeting of Polo-like kinase 1 in bladder carcinoma: comparative effects of four potent inhibitors. Cancer Biol Ther. 2013;14:648–657. doi: 10.4161/cbt.25087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Awada A, Dumez H, Aftimos PG, Costermans J, Bartholomeus S, Forceville K, Berghmans T, Meeus MA, Cescutti J, Munzert G, Pilz K, Liu D, Schoffski P. Phase I trial of volasertib, a Polo-like kinase inhibitor, plus platinum agents in solid tumors: safety, pharmacokinetics and activity. Invest New Drugs. 2015;33:611–620. doi: 10.1007/s10637-015-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Braud F, Cascinu S, Spitaleri G, Pilz K, Clementi L, Liu D, Sikken P, De Pas T. A phase I, dose-escalation study of volasertib combined with nintedanib in advanced solid tumors. Ann Oncol. 2015;26:2341–6. doi: 10.1093/annonc/mdv354. [DOI] [PubMed] [Google Scholar]
- 48.Machiels JP, Peeters M, Herremans C, Surmont V, Specenier P, De Smet M, Pilz K, Strelkowa N, Liu D, Rottey S. A phase I study of volasertib combined with afatinib, in advanced solid tumors. Cancer Chemother Pharmacol. 2015;76:843–851. doi: 10.1007/s00280-015-2860-2. [DOI] [PubMed] [Google Scholar]
- 49.Dohner H, Lubbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM, Lepretre S, Reman O, Turlure P, Ottmann OG, Muller-Tidow C, Kramer A, Raffoux E, Dohner K, Schlenk RF, Voss F, Taube T, Fritsch H, Maertens J. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–1433. doi: 10.1182/blood-2014-03-560557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis PM, Leighl NB, Hirsh V, Reaume MN, Blais N, Wierzbicki R, Sadrolhefazi B, Gu Y, Liu D, Pilz K, Chu Q. A Randomized, Open-Label Phase II Trial of Volasertib as Monotherapy and in Combination With Standard-Dose Pemetrexed Compared With Pemetrexed Monotherapy in Second-Line Treatment for Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2015;16:457–465. doi: 10.1016/j.cllc.2015.05.010. [DOI] [PubMed] [Google Scholar]