Abstract
The G-protein coupled chemoattractant receptor formylpeptide receptor-2 (FPR2 in human, Fpr2 in mice) is expressed by mouse colon epithelial cells and plays a critical role in mediating mucosal homeostasis and inflammatory responses. However, the biological role of FPR2 in human colon is unclear. Our investigation revealed that a considerable number of human colon cancer cell lines expressed FPR2 and its ligands promoted cell migration and proliferation. Human colon cancer cell lines expressing high levels of FPR2 also formed more rapidly growing tumors in immunocompromised mice as compared with cell lines expressing lower levels of FPR2. Knocking down of FPR2 from colon cancer cell lines highly expressing FPR2 reduced their tumorigenicity. Clinically, FPR2 is more highly expressed in progressive colon cancer, associated with poorer patient prognosis. These results suggest that FPR2 can be high-jacked by colon cancer cells for their growth advantage, thus becoming a potential target for therapeutic development.
Keywords: Colon, cancer, FPR2, tumorigenesis, prognosis
Introduction
Formylpeptide receptors (FPRs) belong to the G-protein coupled receptor GPCR family, which are increasingly recognized as important mediators of inflammatory and immune responses [1,2]. Under physiologic conditions, FPRs expressed by various normal cell types are essential for host defense against microbial infection, as well as for the control of inflammation, immune responses, and epithelial homeostasis [3,4]. However, some malignant tumors cells also express FPRs and respond to bacterial or endogenous agonists by chemotaxis and proliferation to promote their growth and invasion. For instance, human gastric cancer cells aberrantly express FPRs, which mediate epithelial-mesenchymal transition, proliferation, migration, and cell resistance to apoptosis [5]. The prototype PFRs, FPR1, selectively expressed by cells of the more highly malignant glioblastoma multiforme (GBM), responds to an endogenous chemotactic ligand anexin 1 (Anx A1) released by necrotic GBM cells [6]. Activated FPR1 cooperated with the epidermal growth factor receptor (EGFR) to enhance the survival, invasiveness, and production of angiogenic factors by GBM cells [7-12]. Human breast cancer cells also express FPR1 and its variant 2, which interact with a shared ligand Anx A1 to enhance tumor cell proliferation [13]. In addition, FPR1 expressed by human liver cancer cells enhances cell chemotaxis, invasion, proliferation, and production of angiogenic factors. To illustrate the important contribution of FPR1 to cancer progression, silencing FPR1 markedly reduced the tumorigenic capacity of human GBM and liver cancer cells in immunocompromised mice [7-13].
FPRs are also expressed by crypt epithelial cells in human colon. Activation of FPR1 by bacterial chemotactic peptide fMLF promotes epithelial growth and restoration of the mucosal integrity [14]. Recently, Fpr2, the mouse ortholog of human FPR2, shows a prominent role in maintaining the normal growth and renewal of colonic epithelial cells. Epithelial cells in colon mucosa of Fpr2-deficient mice displayed defects in commensal bacterium-dependent homeostasis, absence of responses to stimulation by the bacterial chemotactic peptide fMLF, shortened colon crypts, reduced acute inflammatory responses to dextran sulfate sodium challenge, delayed mucosal restoration after chronic injury, and increased azoxymethane-induced tumorigenesis [12,15]. These observations indicate the potential for Fpr2 to mediate anti-cancer responses in mouse colon epithelia. However, the role of FPR2 in the development of human colon cancer is unclear. Herein, we report that FPR2 is more highly expressed in colon cancer cell lines for their growth advantage and in colon cancer tissues associated with poorer patient survival.
Materials and methods
Reagents
FPR2 agonist peptides MMK-1 and W-peptide (WKYMVm, W-pep) were synthesized at the Department of Biochemistry, Colorado State University (Fort Collins, CO) [16]. The human cathelicidin LL37 peptide was purchased from InvivoGen (San Diego, CA). Human-EGF and fMLF was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against p-p38 (Thr180/Tyr182) and p38 MAPK, p-Erk1/2 and Erk1/2, p-IκB-α (Ser32) and IκB-α, p-AKT (Ser473/Thr308) and AKT and EGFR, GAPDH and horseradish peroxidase (HRP)-linked anti-rabbit IgG antibody for Western blotting were purchased from Cell Signaling Technology (Beverly, MA). FPR2 antibody was obtained from Novus Biologicals (Littleton, CO). Human FPR2 siRNA Lentiviral particle support reagents (control shRNA lentiviral particles, polybrene, puromycin dihydrochloride) were obtained from Santa Cruz Biotechnology (Dallas, TX).
Animals
Female nude mice (AthymicNcr-nu/nu) were purchased from Charles River Laboratories Inc. (Frederick, Maryland, USA). All mice were housed in the animal facility at Frederick National Laboratory for Cancer Research (Frederick, Maryland, USA) and were used at the age of 6 weeks. Animal care was provided in accordance with procedures outlined in the Guide for Care and Use of Laboratory Animals (National Research Council, 1996, National Academy Press, Washington D.C.).
Cell lines and culture
The human colon cancer cell lines used in this study were COLO 205, HCT116, HT-29, HCT-15, HCC-2998, KM12, KM20L2, LoVo, SW620, WiDr and T84, all of which were obtained from American Type Culture Collection and maintained in National Cancer Institute DCTD Tumor Repository. Human embryonic kidney (HEK) 293 cell line stably expressing human FPR2 (FPR2/293 cells) was a gift of Drs. P. M. Murphy and J. L. Gao (National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD).
WiDr cell line was cultured in Eagle’s Minimum Essential Medium (EMEM) containing 10% FCS. T84 cell line was cultured in 1:1 mixture of Ham’s F12 medium and DMEM containing 10% FCS. All other human cancer cell lines were cultured in RPMI-1640 medium containing 10% FCS. FPR2/293 cells were maintained in the presence of 1 mg/ml geneticin (Invitrogen Life Technologies) in complete DMEM containing 10% FCS, 2 mM glutamine, 25 mM HEPES, 100 U/ml penicillin, and 100 ug/ml streptomycin.
Human colon cancer tissue collection
A total of 127 formalin-fixed paraffin-embedded specimens of primary human colorectal cancer (CRC) were obtained from archival blocks of colectomy samples at institute of Pathology, Southwestern Hospital, Third Military Medical University, Chongqing, and Nanfang Hospital, Guangzhou, China. All patients gave informed consent to use excess pathological specimens for research purposes. Tissues were diagnosed as colon adenocarcinoma and staged by pathologists. The protocols were approved by the Protection of Human Subjects and Ethics Committee in these two hospitals. The use of human tissues was approved by the institutional review board of the Third Military Medical University in accordance with international guidelines.
RT-PCR
Total RNA was extracted from cells with an RNeasy mini kit and depleted of contaminating DNA with RNase-free DNase (Qiagen, Valencia, CA). The first strand cDNA was synthesized with the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, IN). The primers for human FPR2 were designed to yield a 600 bp product. RT-PCR of FPR2 transcript was performed and PCR products were resolved by 1.5% agarose gel electrophoresis and visualized with ethidium bromide (EB) staining.
Immunoblotting
Subconfluent colon cancer cells grown in 60-mm dishes to were cultured overnight in FCS-free medium. After treatment with FPR2 agonist peptides at different concentrations or at different time points, the cells were lysed with 1 × SDS sample buffer (62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM dithiothreitol), sonicated for 12 s, and then heated at 100°C for 5 min. The cell lysate was centrifuged at 12,000 rpm (4°C) for 5 min and protein concentration in the supernatant was measured by Micro BCA Protein Assay System (Pierce, Rockford, IL). Proteins were electrophoresed on 10% SDS-PAGE precast gels (Invitrogen) and transferred onto ImmunoBlot Poly-vinylidene membranes (Bio-Rad), which were then blocked with 5% nonfat milk and incubated with primary antibodies overnight at 4°C. After incubation with a HRP-conjugated secondary antibody, the protein bands were detected with a Super Signal Chemiluminescent Substrate (Pierce, Rockford, IL) and BIOMAX-MR film (Eastman Kodak Co. Rochester, NY).
Histology, immunohistochemistry and immunofluorescence
Paraffin-embedded human colon cancer specimens were sectioned at 5 μm thickness, dewaxed, rehydrated, and stained with H&E for pathological diagnosis. For immunohistochemistry, the tissue sections were incubated in 0.3% (v/v) hydrogen peroxide for 20 min to quench endogenous peroxidase activity before adding 10% (v/v) normal goat serum for 1 h. Sections were then incubated at 4°C overnight with the anti-human FPR2 antibody followed by biotinylated goat anti-rabbit antibody for 30 min at room temperature (RT) and streptavidin-HRP for 20 min. Reaction on sections were visualized with diaminobenzidine and counterstained with hematoxylin. The expression of FPR2 was evaluated as the percentage of positive cells in a specimen and by staining intensity [17]. At least three fields in each coded tumor section were examined by two pathologists without knowledge of sample identities. The percentage of positive cells was quantitated and scored as 0 for positive staining of ≤ 1% of total counted cells, 1 for positive staining of 2-25% cells, 2 for positive staining of 26-50% cells, 3 for positive staining of 51-75% cells, and 4 for positive staining of > 75% of total cells examined. The intensity was graded as follows: 0, no signal; 1, weak; 2, moderate; and 3, strong staining. A total staining scores of 0-12 was calculated and graded as negative (-, scores 0-1), weak (+, scores 2-4), moderate (++, scores 5-8), or strong (+++, scores 9-12). Based on these criteria, low expression was defined for samples with negative and weak FPR2 staining, and high expression included staining samples with moderate and strong staining.
For staining of FPR2 on cell lines, colon cancer cells were seeded at 2.0 × 104 cells/well on 8-well chamber slides (NalgeNunc International Co., Naperville, IL) for 24 h. The cells were fixed in 2% paraformaldehyde for 20 min at RT, washed with PBS 3 times for 5 min each, and incubated with 5% normal goat serum (Sigma-Aldrich) in PBS plus 0.05% Tween 20 for 1 h to block nonspecific antibody binding. The samples were then incubated with anti-human FPR2 antibody at 1:50 dilution for 2 h at RT followed with PE-conjugated goat anti-rabbit IgG (BD Pharmingen) in PBS containing 1% BSA for 60 min. After staining with DAPI to visualize nuclei, the samples were analyzed under a laser-scanning confocal fluorescence microscope (ZeissLSM510 NLO Meta). The intensity of green fluorescence detected for FPR2 was analyzed with Image J (NIH software).
Chemotaxis
Chemotaxis assays were performed in 48-well chemotaxis chambers (NeuroProbe, Gaithersburg, MD). The upper and lower compartments of the chambers were separated by a 10 μm pore-sized polycarbonate filter (GE Osmonics Labstore, Minnetonka, MN) coated with 50 μg/ml collagen type I (BD Biosciences, San Jose, CA). A 28-30 μl aliquot of chemoattractants was placed in the wells of the lower compartment, and 50 μl of human colon cancer cells (each at 2 × 106 cells per ml of RPMI 1640 medium containing 1% bovine serum albumin and 25 mM HEPES) were placed in the wells of the upper compartment. After 4 h incubation at 37°C, the filters were collected, removed of non-migrating cells, rinsed with PBS, fixed and stained with Three-Step solution (Richard-Allan Scientific, Kalamazoo, MI). Migrated cells were counted in 5 random fields at 400 magnifications under light microscopy. The results were expressed as the mean ± SEM of the chemotaxis index (CI), representing the fold increase in the number of migrated cells in response to chemoattractants over spontaneous cell migration (to control medium).
Cell monolayer scratching (wound-healing) assays
The wound-healing assays were performed according to the published procedures [10]. Briefly, equal number of human colon cancer cells (1.2 × 106/ml) was planted in six-well tissue culture plates. When the cells reached confluence, a 250 μl pipette tip (Thermo Fisher Scientific, Waltham, MA) was used to scratch thus creating a gap in the cell monolayer. Detached tumor cells were immediately removed by replacement with fresh culture medium containing 0.5% FCS with or without FPR2 agonists at different concentrations. Same fields of scratch were subsequently observed under light microscopy at different time points. The scratch-healing status was monitored for up to 72 h or at indicated time points. Each scratch was photographed and the size was measured for the width at three positions by using Image-Pro Plus software (Media Cybernetics, Rockville, MD). Results were quantitated by calculating the mean width (± SE) of the wound.
Human colon cancer cell proliferation
Colon cancer cell proliferation was quantitated at different time points in the presence of FPR2 agonist MMK-1 using a colorimetric Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies INC., Rockville, MD) [18]. The cells were cultured in 96-well plates at 3000 cells per well in RPMI 1640 with 10% FCS. After 24 h, the medium was replaced with fresh RPMI 1640 containing 0.5% FCS with or without the presence of FPR2 agonist at different concentrations. At the indicated time points, the plates were measured for absorbance at 450 nm with a microplate reader. The results were expressed as the mean ± SE.
Lentiviral particle transduction of FPR2 shRNA
Colon cancer cells were grown to 50% confluence prior to viral infection. The culture medium was replaced with 1 ml mixture of RPMI-1640 with Polybrene® (sc-134220) at a final concentration of 5 μg/ml per well. The cells were then added with 10 μl FPR2 shRNA (h) lentiviral particles (sc-40123-V) or control shRNA (h) lentiviral particles (sc-108080). The plate was gently swirled and incubated at 37°C overnight. At day 3, culture medium was replaced with 1 ml RPMI-1640 without Polybrene overnight. Stable clones expressing the FPR2 shRNA or Control shRNA via Puromycin dihydrochloride (sc-108071) were selected for further analysis.
Tumor implantation
Human colon cancer cells or FPCK-1-1 cells (2 × 106) in 100 μl PBS were subcutaneously injected into the right flank of nude mice. The weight of mice and the tumor size were monitored every other day and tumor volume was calculated as follows: Volume (cm3) = length × width × width × 0.52. At the indicated days, mice were euthanized and tumors were harvested for frozen or paraffin sections. In survival experiments, mice were euthanized when tumors exceeded 2 cm in diameter or contained visible necrosis.
Statistical analysis
All experiments were performed at least three times. Representative and reproducible results were shown. Statistical analysis was performed with Prism Version 6.0 software (GraphPad Software, La Jolla, CA). All statistical tests were two-sided. The significance of the differences was assessed by Student’s t test. P < 0.05 was considered as statistically significant. Mouse survival curves were plotted as Kaplan-Meier plots (Prism Version 6.0).
Results
The expression of functional FPR2 by selected human colon cancer cell lines
The expression of FPR2 by different colon cancer cell lines was investigated. High levels of FPR2 mRNA (Figure 1A, 1B) and protein (Supplementary Figure 1) were expressed by a considerable proportion of human colon cancer cell lines. Immunofluorescence staining detected FPR2 mainly on the membrane of human colon cancer cells (Figure 2A). The human cell lines expressing FPR2 migrated in response to FPR2 ligands MMK-1 (Figure 2B-E) and W-peptide (Supplementary Figure 2A-D). Also, FPR2 agonist MMK-1 enhanced the proliferation of FPR2 expressing colon cancer cells (Figure 3A) and in a model of human cancer cell monolayer scratching assay, MMK-1 stimulated a more rapid closure of the wound by FPR2 expressing human colon cancer cells (Figure 3B, 3C). Since activation of the MEK/ERK pathway is required for cell proliferation and migration [19], we examined the capacity of FPR2 agonists to activate ERK1/2 in human colon cancer cell lines. Figure 3D, 3E and Supplementary Figure 3A, 3B show that the phosphorylation of ERK1/2 was induced in FPR2 expressing human colon cancer cell lines by FPR2 agonist peptides. Thus, FPR2 highly expressed by selected human colon cancer cell lines enhances cell motility and growth, which may be contributing factors for increased progressiveness of clinical colon cancer.
Figure 1.
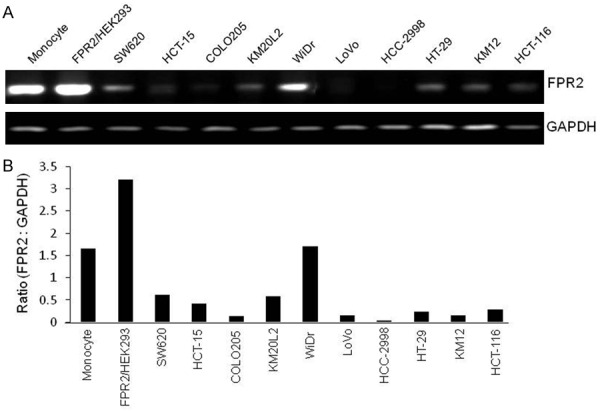
The expression of functional FPR2 by selected human colon cancer cell lines. A. FPR2 mRNA expression in human colon cancer cell lines. Monocytes and FPR2/HEK293 cells are used as positive control; SW620, HCT-15, COLO205, KM20L2, WiDr, LoVo, HCC-2998, HT-29, KM12 and HCT-116 are human colon cancer cell lines. B. Ratio of density for PCR products of FPR2 mRNA versus GAPDH mRNA.
Figure 2.
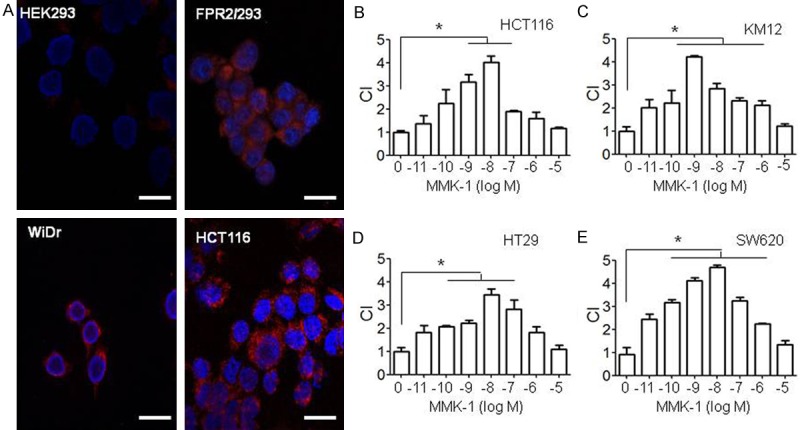
FPR2 expressed on cell membrane and FPR2-mediated colon cancer cell migration in response to the FPR2 ligand MMK-1. A. Immunofluorescence staining showing FPR2 expressed on cell membrane of human colon cancer cell lines and human embryonic kidney epithelial (HEK293) transfected with FPR2 (FPR2/293) and parent HEK293 cells are used as control. WiDr and HCT-116 are human colon carcinoma cells. Red: FPR2, Blue: DAPI. Scale Bar: 50 µm. B-E. Human colon cancer cell lines HCT116, KM12, HT29 and SW620 migrated in response to the FPR2 ligands MMK-1. The results are expressed as the mean ± SE of the chemotaxis index (CI), representing the fold increase in the number of migrated cells in response to chemoattractants over spontaneous cell migration (to control medium). *P < 0.05.
Figure 3.
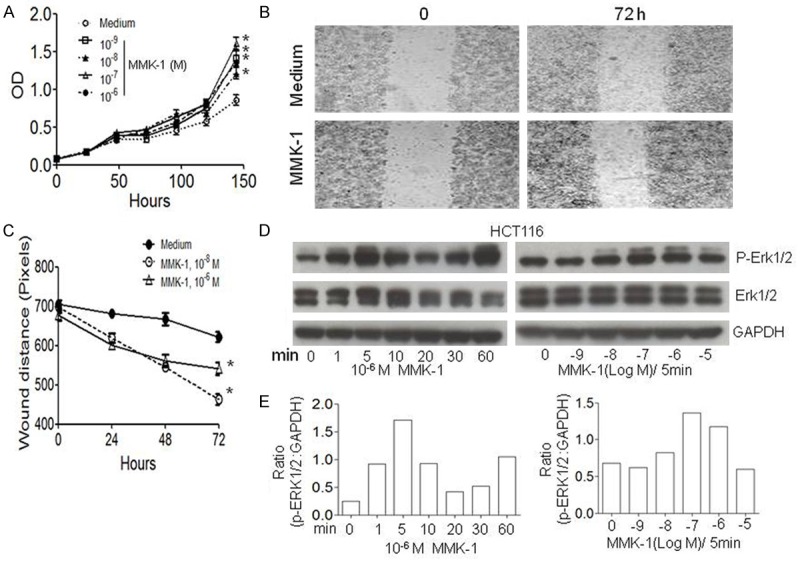
FPR2-mediated proliferation of human colon cancer cell lines. A. Proliferation of human colon cancer cell line HCT116 in response to MMK-1. *P < 0.05. B, C. In creased rate of closure of scratching wound on the monolayer of human colon cancer cell line SW620 in response to MMK-1. *P < 0.05. D: ERK1/2 phosphorylation in HCT116 cells induced by MMK-1. GAPDH protein was used as a loading control. E. Ratio of density for p-ERK1/2 versus GAPDH in HCT116 cells after stimulation with MMK-1. Activation of ERK1/2 induced by MMK-1 at different time points (Left) with different ligand concentrations (Right).
Reduced migration and proliferation shown by human cancer cells with FPR2 silencing
To verify the function of FPR2 in mediating human colon cell migration and proliferation, FPR2 expressed in a human colon cancer cell line was silenced by using siRNA. The colon cell line was stably transfected with lentiviral particles containing FPR2 shRNA (FPR2-KD) or control shRNA (Mock). The expression of FPR2 mRNA was down-regulated in FPR2-KD cells but not in Mock cells as confirmed with RT-PCR (Figure 4A). FPR2-KD colon cancer cells showed reduced migration (Figure 4B) and proliferation (Figure 4C), and decreased ERK1/2 phosphorylation (Figure 4D) in response to stimulation by the FPR2 agonist MMK-1. These results enabled further experiments to examine the contribution of FPR2 to the progresses of xenograft tumors in nude mice.
Figure 4.
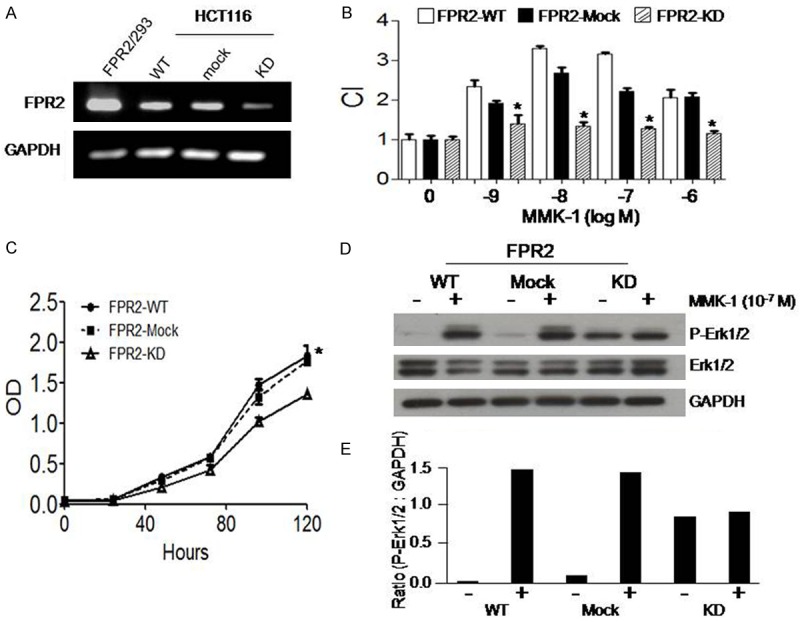
Reduced migration and proliferation of human colon cancer cells with FPR2 silencing. A. The expression of FPR2 mRNA in WT, Mock and FPR2 knockdown (KD) HCT116 colon cancer cells. RT-PCR product of GAPDH was used as a loading control. RT-PCR products of FPR2/293 cells were used as a positive control. B. Reduced migration of FPR2-KD HCT116 cells in response to MMK-1. The results are expressed as the mean ± SE of the chemotaxis index (CI). *P < 0.05, significantly reduced migration of FPR2-KD HCT116 cells as compared to WT and Mock cells. C. Reduced proliferation of FPR2-KD HCT116 cells in response to MMK-1. *P < 0.05. D. Reduced phosphorylation of ERK1/2 in FPR2-KD HCT116 cells stimulated with MMK-1. E. Ratio of density for p-ERK1/2 versus GAPDH in WT, Mock and FPR2 knockdown (KD) HCT116 cells after stimulation with MMK-1.
Reduced tumorigenicity of human colon cancer cells with silenced FPR2
To evaluate the role of FPR2 in human cancer cell tumorigenesis and invasiveness in vivo, the capacity of colon cancer cell lines expressing high levels of FPR2 to form tumors in nude mice was compared to those expressing lower levels of FPR2. Figure 5A shows that human colon cancer cells highly expressing FPR2 formed more rapidly growing xenograft tumors in nude mice as compared with cells expressing lower levels of FPR2. We then injected FPR2-WT, FPR2-Mock and FPR2-KD human colon cancer cells into nude mice and found that tumors formed by FPR2-KD cells, whose FPR2 expression was silenced, grew more slowly as compared to tumors formed by WT and Mock colon cancer cells (Figure 5B, 5C). The survival of mice bearing tumors formed by FPR2-KD cells was significantly longer as compared to mice bearing tumors formed by FPR2-Mock cells (Figure 5D). Pathological analysis shows smaller size of tumors formed by FPR2-KD cells as compared to tumors formed by FPR2-WT or FPR2-Mock cells (Figure 5E). Tumors formed by FPR2 expressing colon cancer cells invaded and caused the disruption of surrounding connective tissues. There was no formation of solid capsules surrounding the tumors, which also contained visible necrotic foci. In contrast, tumors formed by FPR2-KD colon cancer cells were incapsuled with no disruption of surrounding connective tissues (Figure 5F). These results indicate that human colon cancer cells expressing higher levels of FPR2 are more tumorigenic with increased invasiveness.
Figure 5.
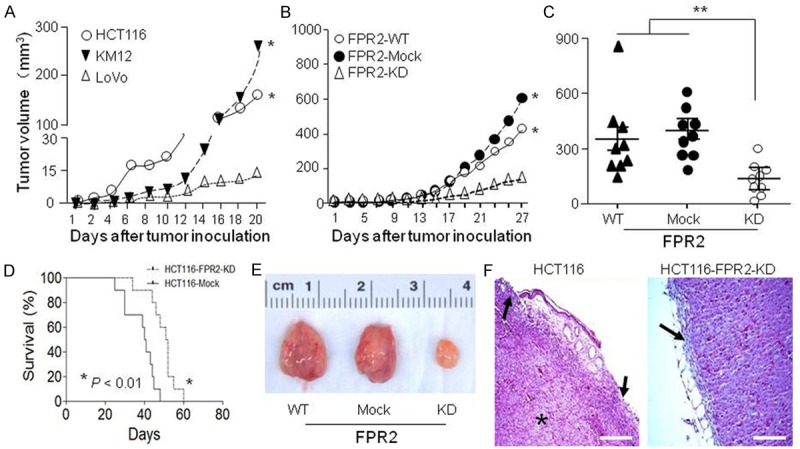
Reduced tumorigenicity of human colon cancer cells with silenced FPR2. A. Tumors formed in vivo by human colon cancer cells. HCT116, KM12 cells (FPR2 high) and LoVo cells (FPR2 low) were injected subcutaneously into the right flanks of nude mice (8-10 mice per group). Mice were examined for tumor formation at indicated time points. B. The size of tumors formed by WT, Mock and FPR2-KD HCT116 cells at different days. C. Tumor size at day 25. D. Survival curve of mice bearing HCT116-Mock or HCT116-FPR2-KD cells. The results were analyzed with Log-rank (Mantel-Cox) Test and Gehan-Breslow-Wilcoxon Test. *P < 0.01. E. Tumors at day 25 after inoculation. F. Histology of tumors formed by WT, Mock and FPR2-KD HCT116 cells at day 25. Black arrow: capsule; Star: necrosis; Scale bar = 100 µm.
High level FPR2 in more progressive human colon cancer
The observations that FPR2 is more highly expressed by aggressive human colon cancer cell lines with higher malignancy promoted us to investigate the relevance of FPR2 to colon cancer progression in patients. In surgically resected samples collected from 127 patients, immunohistochemical scoring revealed that although normal colon epithelial cells expressed low to moderate levels of FPR2 (Scores < 2), high levels of FPR2 (Scores > 2) was detected in colon cancer tissues of 50 patients (39%) (Figure 6A). FPR2 expression was correlated with the malignant behavior of colon cancer as shown by increased invasiveness. Table 1 showed that in 54 cases of lesions within the mucosa of the colon, there were 47 low FPR2 expressing cases (87%), 7 high FPR2 expressing cases (13%); in 37 cases of lesions invading muscularis, there were 22 low FPR2 expressing cases (59.5%), 15 high FPR2 expressing cases (40.5%); in 36 cases of lesions invading serosal layer, there were only 8 low FPR2 expressing cases (22%), 28 high FPR2 expressing cases (78%). Survival analysis of patients after surgery showed a significant correlation between the expression of FPR2 in colon cancer and shortened survival of patients (Figure 6B). These results indicate that FPR2 is preferentially expressed in more highly invasive colon cancer associated with rapid progression and poor patient prognosis.
Figure 6.
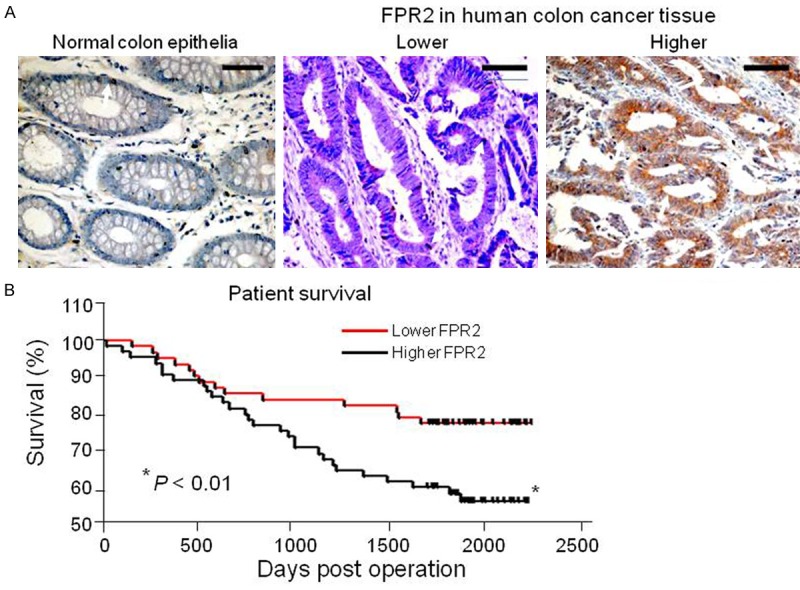
FPR2 expressing highly in more progressive colon cancer of patients. A. The expression of FPR2 in human colon cancer. Human colon cancer specimens were incubated in 0.3% (v/v) hydrogen peroxide to quench endogenous peroxidase activity before adding 10% (v/v) normal goat serum for 1 h. Sections were then incubated at 4°C overnight with the anti-human FPR2 antibody followed by biotinylated goat anti-rabbit antibody for 30 min at room temperature (RT) and streptavidin-HRP for 20 min. Reaction on sections were visualized with diaminobenzidine and counterstained with hematoxylin. The expression of FPR2 was evaluated as the percentage of positive cells in a specimen and by staining intensity. Brown color: FPR2. Scale bar: 30 µm. B. The correlation between FPR2 expression and patient survival. *P < 0.05, significantly shortened survival of patients with high FPR2 expressing colon cancer as compared to the patients bearing low FPR2 expressing colon cancer.
Table 1.
Increased invasiveness of FPR2 high human colon cancer
| Invasion depth (case number and %) | ||||
|---|---|---|---|---|
|
|
||||
| Mucosa | Muscularis | Serosa | ||
| FPR2 levels | Lower | 47 (87%) | 22 (59.5%) | 8 (22%) |
| Higher | 7 (13%) | 15 (40.5%)* | 28 (78%)* | |
| Total Cases | 54 | 37 | 36 | |
P < 0.0001, compared with tumors limited in the mucosa.
Discussion
We have shown in this study that the G-protein coupled chemoattractant receptor FPR2 is selectively expressed at higher levels by more aggressive human colon cancer cells in association with poor patient prognosis. We also revealed that a number of human colon cancer cell lines express high levels of FPR2 and formed more rapidly growing tumors in immunocompromised mice. Our results thus suggest that FPR2 in human colon cancer contributes to cancer progression.
FPR family consists of three members FPR1, FPR2 and FPR3 in human [1,2]. FPR1 is a high affinity receptor for the bacterial peptide formyl-methionyl-leucyl-phenylalanine (fMLF) and mediates fMLF-induced phagocyte chemotaxis and activation. FPR1 also mediates the leukocyte chemotactic activity of an endogenous ligand, neutrophil granule protein cathepsin G [20]. In vivo, FPR1 protects host in defense against infection by Listeria monocytogenes [21]. FPR2 and its mouse counterpart Fpr2 are low affinity receptors for fMLF, but they interact with a greater number of endogenous chemotactic agonist peptides produced during inflammation and immune responses [2]. FPR3 in human recognizes a chemotactic peptide fragment derived from Heme-binding protein that chemoattracts dendritic cells DCs [22]. In mice, Fpr2 is likely a receptor that functions as both human FPR2 and FPR3 [21,23]. Although FPRs were first identified on phagocytic leukocytes, these receptors are also expressed by many nonhematopoietic cells including intestinal epithelial cells and cancer cells originated from different organs such as human breast cancer [13], liver cancer [12], glioblastoma multiforme (GBM) and gastric cancer [5].
Accumulating evidence shows that FPRs acts as a double-edged sword in colon epithelial cells. FPR1 was expressed in crypt epithelial cells in the human colon. Activation of FPR1 by bacterial fMLF promotes epithelial growth and restoration of the mucosal integrity [14]. In mice, some agonists such as Anx A1, fMLF, and viable Lactobacillus rhamnosus GG, stimulate Fpr1 on intestinal epithelial cells, leading to the generation of reactive oxygen species via enterocyte NADPH oxidase 1 and rapid phosphorylation of focal adhesion kinase and ERK/MAPK [24]. Activated FPR1 also mediates the migration and proliferation of enterocytes adjacent to colonic wounds [25]. Intestinal crypts of the Fpr1-deficient mice contain increased number of proliferating epithelial cells and show slower migration along the crypt-villus axis, despite their normal intestinal tissue architecture, suggesting that Fpr1 may be important in maintaining the homeostasis of the intestinal epithelia with yet unclear mechanistic basis and consequences [14].
Interestingly, opposing roles of FPR1 in cancer has also been reported. For example, FPR1 silencing in GC cells (shFPR1) significantly enhanced xenograft tumor growth with higher HIF-1α and VEGF mRNA levels. Moreover, the in vitro production of proangiogenic factors or IL-1α was higher in shFPR1 GC cells [5]. This is in contrast to observations with human GBM cells in which FPR1 was activated by an endogenous ligand Anx A1 released by necrotic tumor cells and cooperate with transactivated EGFR to promote GBM progression [26].
In mouse colon, commensal bacterial lysates and fMLF activate ERK/MAPK in an FPR-dependent manner [27], without clear identity of the receptor subtypes. It has been reported that activation of FPRs (FPR1, 2, 3) induced epithelial-to-mesenchymal transition, proliferation, resistance to apoptosis and migration of human GC epithelial cells in culture, which were reversed by blocking each FPR [5]. Our previous study showed that Fpr2 was essential for the homeostatic integrity of colon epithelia [15]. This was supported by the observation that the colon of Fpr1 and Fpr2 double deficient mice showed shortened crypts comparable to that observed in Fpr2 single-deficient mice suggesting a more important role for Fpr2 [12]. In this study, we found that an immortalized human colon epithelial cell line without the capacity to form tumors in mice also expressed functional FPR2 (Data not shown). However, in a considerable number of human colon cancer cell lines, FPR2 was highly expressed and promoted tumor growth. Thus, FPR2 is high-jacked by cancer cells for their benefit.
The mechanistic basis for FPR2 to be high-jacked by colon cancer cells for progression remains to be elucidated. In human GBM, the increased global methylation in tumor cells enabled NFκB to more efficiently bind to FPR1 promoter and displace the binding of tumor suppressor p53, resulting in markedly enhanced transcription and translation of FPR1 [9-11]. Preliminary studies revealed similar mechanistic basis for increased expression of FPR2 in human colon cancer cell lines (data not shown). Recently, an FPR2 ligand cathelicidin LL-37 was shown to be increased in colon cancer tissues. Macrophages in colon cancer are a major source of LL-37 and upon contact with tumor cells, macrophages release LL-37 to stimulate the proliferation of tumor cells. LL-37 activates the Wnt/β-catenin signaling pathway in tumor cells by inducing the phosphorylation of PTEN, resulting in stabilization and nuclear translocation of β-catenin [28], important for epithelial mesenchymal transition. On the other hand, it has also been reported that treatment of human colon cancer cell line HCT116 with LL-37 induced apoptotic cell death [29]. Thus, structurally unrelated FPR2 ligands including highly hydrophobic LL-37 may trigger diverse intracellular signals [19]. This calls for better understanding of the regulation and signaling of FPR2 in cancer versus normal cells for future development of precision therapeutics.
Acknowledgements
The authors thank Dr. A. Tominaga of the Department of Molecule and Cellular Biology, Kochi University, Kochi, Japan for providing FPCK-1-1, a normal human colon epithelial cell line; Dr. J. J. Oppenheim for critically reviewing the manuscript; Ms. C. Lamb and Ms. S. Livingstone for secretarial assistance. This project was funded in part by federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E and was supported in part by the Intramural Research Program of the NCI, NIH.
Disclosure of conflict of interest
None.
Authors’ contribution
Y.X. and X.Y., performed the experiments, and analyzed the data; X.W., J.Z., W.G., T.Y., S.H., R.W., T.W. performed the experiments; G.S. and X.B. edited the manuscript, K.C. analyzed the data and wrote the manuscript; J.M.W. conceived, designed, and supervised this research, analyzed, interpreted data, and revised the manuscript.
Supporting Information
References
- 1.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 3.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 4.Le Y, Yang Y, Cui Y, Yazawa H, Gong W, Qiu C, Wang JM. Receptors for chemotactic formyl peptides as pharmacological targets. Int Immunopharmacol. 2002;2:1–13. doi: 10.1016/s1567-5769(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 5.Prevete N, Liotti F, Visciano C, Marone G, Melillo RM, de Paulis A. The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene. 2015;34:3826–3838. doi: 10.1038/onc.2014.309. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Liu Y, Yao X, Ping Y, Jiang T, Liu Q, Xu S, Huang J, Mou H, Gong W, Chen K, Bian X, Wang JM. Annexin 1 released by necrotic human glioblastoma cells stimulates tumor cell growth through the formyl peptide receptor 1. Am J Pathol. 2011;179:1504–1512. doi: 10.1016/j.ajpath.2011.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for Treatment of Glioblastoma: Molecular Basis to Overcome Resistance. Curr Cancer Drug Targets. 2012;12:197–209. doi: 10.2174/156800912799277557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao XH, Ping YF, Chen JH, Chen DL, Xu CP, Zheng J, Wang JM, Bian XW. Production of angiogenic factors by human glioblastoma cells following activation of the G-protein coupled formylpeptide receptor FPR. J Neurooncol. 2008;86:47–53. doi: 10.1007/s11060-007-9443-y. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Hu J, Bian X, Chen K, Gong W, Dunlop NM, Howard OM, Wang JM. Transactivation of the epidermal growth factor receptor by formylpeptide receptor exacerbates the malignant behavior of human glioblastoma cells. Cancer Res. 2007;67:5906–5913. doi: 10.1158/0008-5472.CAN-07-0691. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Chen K, Chen J, Gong W, Dunlop NM, Howard OM, Gao Y, Bian XW, Wang JM. The G-protein-coupled formylpeptide receptor FPR confers a more invasive phenotype on human glioblastoma cells. Br J Cancer. 2010;102:1052–1060. doi: 10.1038/sj.bjc.6605591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Chen K, Huang J, Gong W, Dunlop NM, Howard OM, Bian X, Gao Y, Wang JM. Regulation of the leucocyte chemoattractant receptor FPR in glioblastoma cells by cell differentiation. Carcinogenesis. 2009;30:348–355. doi: 10.1093/carcin/bgn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Chen K, Xiang Y, Yoshimura T, Su S, Zhu J, Bian XW, Wang JM. New development in studies of formyl-peptide receptors: critical roles in host defense. J Leukoc Biol. 2016;99:425–435. doi: 10.1189/jlb.2RI0815-354RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khau T, Langenbach SY, Schuliga M, Harris T, Johnstone CN, Anderson RL, Stewart AG. Annexin-1 signals mitogen-stimulated breast tumor cell proliferation by activation of the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 2011;25:483–496. doi: 10.1096/fj.09-154096. [DOI] [PubMed] [Google Scholar]
- 14.Babbin BA, Jesaitis AJ, Ivanov AI, Kelly D, Laukoetter M, Nava P, Parkos CA, Nusrat A. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J Immunol. 2007;179:8112–8121. doi: 10.4049/jimmunol.179.12.8112. [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Liu M, Liu Y, Yoshimura T, Shen W, Le Y, Durum S, Gong W, Wang C, Gao JL, Murphy PM, Wang JM. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J Clin Invest. 2013;123:1694–1704. doi: 10.1172/JCI65569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu JY, Le Y, Gong W, Dunlop NM, Gao JL, Murphy PM, Wang JM. Synthetic peptide MMK-1 is a highly specific chemotactic agonist for leukocyte FPRL1. J Leukoc Biol. 2001;70:155–161. [PubMed] [Google Scholar]
- 17.Shi H, Chen S, Jin H, Xu C, Dong G, Zhao Q, Wang W, Zhang H, Lin W, Zhang J, Davidovic L, Yao L, Fan D. Downregulation of MSP58 inhibits growth of human colorectal cancer cells via regulation of the cyclin D1-cyclin-dependent kinase 4-p21 pathway. Cancer Sci. 2009;100:1585–1590. doi: 10.1111/j.1349-7006.2009.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, Iwabuchi Y, Shibata H. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–2571. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 19.Cattaneo F, Parisi M, Ammendola R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci. 2013;14:7193–7230. doi: 10.3390/ijms14047193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun R, Iribarren P, Zhang N, Zhou Y, Gong W, Cho EH, Lockett S, Chertov O, Bednar F, Rogers TJ, Oppenheim JJ, Wang JM. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J Immunol. 2004;173:428–436. doi: 10.4049/jimmunol.173.1.428. [DOI] [PubMed] [Google Scholar]
- 21.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migeotte I, Riboldi E, Franssen JD, Gregoire F, Loison C, Wittamer V, Detheux M, Robberecht P, Costagliola S, Vassart G, Sozzani S, Parmentier M, Communi D. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devosse T, Guillabert A, D’Haene N, Berton A, De Nadai P, Noel S, Brait M, Franssen JD, Sozzani S, Salmon I, Parmentier M. Formyl peptide receptor-like 2 is expressed and functional in plasmacytoid dendritic cells, tissue-specific macrophage subpopulations, and eosinophils. J Immunol. 2009;182:4974–4984. doi: 10.4049/jimmunol.0803128. [DOI] [PubMed] [Google Scholar]
- 24.Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Neish AS, Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam A, Leoni G, Wentworth CC, Kwal JM, Wu H, Ardita CS, Swanson PA, Lambeth JD, Jones RM, Nusrat A, Neish AS. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–655. doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Liu Y, Yao X, Ping Y, Jiang T, Liu Q, Xu S, Huang J, Mou H, Gong W, Chen K, Bian X, Wang JM. Annexin 1 released by necrotic human glioblastoma cells stimulates tumor cell growth through the formyl peptide receptor 1. Am J Pathol. 2011;179:1504–1512. doi: 10.1016/j.ajpath.2011.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wentworth CC, Jones RM, Kwon YM, Nusrat A, Neish AS. Commensal-epithelial signaling mediated via formyl peptide receptors. Am J Pathol. 2010;177:2782–2790. doi: 10.2353/ajpath.2010.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li D, Liu W, Wang X, Wu J, Quan W, Yao Y, Bals R, Ji S, Wu K, Guo J, Wan H. Cathelicidin, an antimicrobial peptide produced by macrophages, promotes colon cancer by activating the Wnt/beta-catenin pathway. Oncotarget. 2015;6:2939–2950. doi: 10.18632/oncotarget.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuroda K, Fukuda T, Isogai H, Okumura K, Krstic-Demonacos M, Isogai E. Antimicrobial peptide FF/CAP18 induces apoptotic cell death in HCT116 colon cancer cells via changes in the metabolic profile. Int J Oncol. 2015;46:1516–1526. doi: 10.3892/ijo.2015.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


