Abstract
Cervical cancer is one of the most lethal malignancies amongst women, partially because it is unresponsive to many chemotherapeutic drugs. The mechanism underlying cisplatin (DDP) resistance in cervical cancer remains largely elusive. In this study, by detecting the 12 most reported down-regulated miRNAs in chemotherapy-sensitive and -resistant cervical cancer cells, we found that miR-497 was significantly reduced in chemotherapy-resistant HeLa/DDP cells and contributed to DDP chemosensitivity. Transketolase (TKT), a thiamine-dependent enzyme that plays a role in the channeling of excess glucose phosphates to glycolysis in the pentose phosphate pathway, was identified as a direct target of miR-497. TKT expression in clinical specimens was characterized by immunohistochemistry and the result showed that TKT was highly expressed in 81.1% (60/74) of samples examined. Data from Oncomine databases revealed that TKT was significantly up-regulated in cervical cancer tissues compared to normal controls. Gain-of-function and loss-of-function studies showed that the miR-497/TKT axis was a critical modulator in DDP chemosensitivity as demonstrated by cell viability and apoptosis assays. Mechanistically, DDP chemosensitivity induced by the miR-497/TKT axis was associated with glutathione (GSH) depletion and reactive oxygen species (ROS) generation, and GSH treatment effectively abrogated miR-497/TKT-mediated chemosensitivity. In conclusion, these findings suggest that a deregulated miR-497/TKT axis has important implications in the cervical cancer cellular response to DDP, and thus targeting this axis may be a promising way to improve chemosensitivity in cervical cancer.
Keywords: MicroRNA-497, pentose phosphate pathway, transketolase, chemosensitivity, reactive oxygen species, cervical cancer
Introduction
Cervical cancer is the third most common malignancy and the fourth leading cause of cancer-related death amongst women worldwide [1]. Long and persistent human high-risk papillomavirus (HPV) infection is a critical factor for the initiation and development of cervical cancer [2]. The expression levels of the oncoproteins HPV E6 and E7 are closely associated with the severity of the cancer [3]. The 5-year survival rates in patients with locally advanced or metastatic cervical cancer is only about 30-50%. When patients are diagnosed at the advanced or metastatic stages, the prognosis is extremely poor and chemoradiotherapy is recommended as the standard therapy [4-6]. However, the clinical outcome of this therapy is far from satisfactory. One of the key potential reasons is chemoresistance induced by inner oncogenic signaling of cancer cells. Therefore, it is of great importance to uncover the underlying mechanisms involved in cervical cancer chemoresistance.
MicroRNAs (miRNAs), as important modulators of gene expression via binding to the 3’-untranslated region (3’-UTR) of target messenger RNAs and suppression of their expression, are involved in many important intracellular processes in cervical cancer, such as cell proliferation [7], invasive capacity [8], differentiation [9], mitophagy [10], as well as chemoresistance [11]. Numerous studies have demonstrated the upstream regulators of altered miRNA expression in cervical cancer, especially the E6-p53-miRNA pathway, however, little is known about the downstream modifiers of these perturbed miRNAs [12-14]. Previously, we reported that HPV E6/p53 mediated suppression of miR-34a results in elevated expression of lactate dehydrogenase A, which promotes cell proliferation and invasion through enhancing the Warburg effect [15]. However, the putative miRNAs and their targets underlying chemoresistance in cervical cancer remain largely unexplored.
De-regulated reactive oxygen species (ROS) are proposed to correlate with drug resistance and oncogenesis [16]. Indeed, ROS is a doubled-edged sword. On the one hand, ROS can activate cellular oncogenic pathways to facilitate malignant phenotypes. On the other hand, excessive ROS results in increased cytochrome c, which is released into the cytoplasm and triggers apoptosis or “programmed cell death” [17]. In cancer cells, reprogrammed cellular metabolism drives substrate utilization toward a dependence on glucose, a phenomenon known as the Warburg effect, which provides cancer cells with sufficient building blocks though the pentose phosphate pathway (PPP) [18]. Besides, the PPP also produces NADPH throughglucose-6-phosphate dehydrogenase. NADPH supports the antioxidant glutathione pathway by converting oxidized glutathione (GSSG) to reduced glutathione (GSH), which is ultimately utilized by glutathione peroxidase to reduce H2O2 to H2O [19]. Therefore, the PPP may be involved in chemoresistance through regulation of ROS levels.
In this study, using a functional screen, we identified miR-497 as a regulator in cisplatin (DDP) chemoresistance in cervical cancer. Then we confirmed transketolase (TKT), an enzyme involved in the PPP, as a downstream target of miR-497. Furthermore, we demonstrated that the miR-497/TKT axis modulates the GSH and ROS levels, which subsequently promote DDP chemoresistance in cervical cancer.
Materials and methods
Cell culture and reagents
The human cervical cancer cell lines, HeLa, HeLa/DDP, and SiHa, were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum and 1% antibiotics (penicillin and streptomycin, Invitrogen, USA) and 5% CO2 in a humidified incubator at 37°C. Cisplatin and GSH were purchased from Sigma (Shanghai, China).
Transfection
Hsa-miR-NC and hsa-miR-497 precursors or anti-miR-497 and control inhibitors were synthesized by Ambion (Austin, TX, USA). A total of 100 nM of miR-497 precursors or inhibitors and corresponding negative controls were diluted separately in 200 μL of Opti-MEM medium. After incubation for 15 min at room temperature, the oligonucleotides were added and the cells were incubated for 48 hr. The expression levels of miRNAs were measured by stem-loop qRT-PCR analysis. Small interfering RNAs (siRNAs) targeting TKT were synthesized by GenePharma (Shanghai, China). Cells were transfected with Lipofectamine® RNAiMAX reagent (Invitrogen, CA, USA) following the manufacturer’s instructions.
Immunohistochemistry
The commercial cervical cancer tissue microarrays (CXC1021) used in this study was purchased from Shanghai superbiotek Inc (Shanghai, China). After deparaffinizing, rehydrating, antigen retrieval, and blocking endogenous peroxidases, the section was washed in 1 × phosphate buffer solution (PBS) three times, blocked in 10% bovine serum albumin, followed by incubation of primary anti-TKT antibody (Abcam, USA, ab112997) at 4°C overnight. After incubation in horseradish peroxidase-conjugated secondary antibodies for 1 hr at room temperature, the slide was visualized by 3,3’-diaminobenzidine tetrahydrochloride (DAB) and counterstained with hematoxylin. A combination of a proportion score and an intensity score was performed to evaluate TKT staining. Negative and weak staining was regarded as low expression, and moderate and strong staining was regarded as high expression. Scoring was conducted independently by two pathologists.
Cell viability and apoptosis assays
Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, a total of 4 × 103 cells per well were seeded in the 96-well plate and allowed to attach overnight. DDP or siRNAs were freshly prepared before each experiment. Then MTT solution at 5 mg/ml was added to each well and incubated for additional 4 hr at 37°C and dimethyl sulfoxide (DMSO) was added to each well to resolve the crystallized product. The absorbance at 590 nm was read on a spectrophotometer. Each experiment was performed in triplicate. Cell apoptosis was detected by the Apo-ONE caspase-3/7 assay according to the manufacturer’s (Promega, G7790) instructions. CellTiter-Blue (Promega, G8081) was used to monitor cell number and relative Caspase-3/7 activity was estimated as the ratio of Apo-ONE/CellTiter-Blue signals.
Measurement of lactate production and glucose consumption
Lactate levels were determined using the Lactate Assay kit (BioVision, USA) and glucose levels were measured using a glucose assay kit (Sigma-Aldrich, USA) according to the manufacturer’s instructions. All values were normalized to total protein levels (BCA Protein Assay Kit, Thermo Scientific, USA).
Western blotting
Total cells lysate was prepared in the presence of protease and phosphatase inhibitors. The protein concentrations were determined by BCA Protein Assay Kit (Thermo Scientific, USA). Proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred into a nitrocellulose membrane (Bio-Rad, USA). Then, the membrane was blocked with 5% non-fat milk and incubated with primary antibodies against TKT (Abcam, USA, ab112997) or β-actin (Abcam, USA, ab8226). After rinsing with TBS, the membrane was incubated with secondary antibodies at room temperature for 30 min. Finally, the immunoreactivity was visualized with an enhanced chemiluminescence substrate (Thermo Scientific, USA).
Total RNA extraction and real-time PCR
Total RNA was isolated from SiHa or HeLa cells using TRIzol® reagent (TaKaRa, Biotech Co., Ltd, Dalian, China). Complementary DNA (cDNA) was synthesized using the PrimeScript™ Reverse Transcription Reagents Kit (TaKaRa, Tokyo, Japan). Quantitative real-time PCR was performed using SYBR Premix Ex Taq II (TaKaRa, Biotech Co., Ltd, Dalian, China) according to the manufacturer’s instructions. Relative mRNA expression was determined using the 2-ΔΔCT method and normalized to β-actin. The primers for TKT are: forward, 5’-TCCACACCATGCGCTACAAG-3’; reverse, 5’-CAAGTCGGAGCTGATCTTCCT-3’. The miRNAs were detected with specific primers and probes using TaqMan® MicroRNA Assays (Applied Biosystems, USA). The relative miRNA expression was calculated and normalized to U6B snRNA. All experiments were performed in triplicate.
Luciferase reporter assay
TKT 3’-UTR regions containing predicted miR-497 binding sites and corresponding mutant sites were amplified by PCR from genomic DNA, and the PCR fragments were inserted into the untranslated region (UTR) downstream of the luciferase gene in the pMIR-reporter luciferase vector (Ambion). After the indicated treatment, SiHa or HeLa cells were co-transfected with the testing firefly luciferase reporter plasmid together with a Renilla luciferase plasmid. Then cells were harvested and dualluciferase activities were measuredusing Dual-Glo® Luciferase Assay System (Promega, Cat. E2920). Renilla signal was used to calculate relative luciferase activity.
Measurement of glutathione and ROS
Glutathione was measured according to the manufacturer’s protocol (Cayman’s Glutathione Assay Kit) from HeLa and SiHa cells treated with miR-497 or TKT siRNA. DCFDA assay was performed 24 h after treatment with either siRNAs or miR-497. HeLa and SiHa cells were incubated with 5 mM 2’,7’-dichlorodihydrofluorescein diacetate (DCFDA, Invitrogen) for 30 min. Excess DCFDA was removed by washing with 1 × PBS three times, and labeled cells were then trypsinized and re-suspended in 1 × PBS. Oxidation of DCFDA to the highly fluorescent 2’,7’-dichloro-fluorescein (DCF) is proportionate to ROS generation and was analyzed by flow cytometry.
Statistical analysis
Each experiment was repeated at least three times independently. All values were reported as mean ± SDs. Statistical significance between experimental and control groups was determined by two-tailed unpaired Student’s t-test or one-way ANOVA. P < 0.05 was considered statistically significant.
Results
miR-497 is associated with cisplatin chemosensitivity in cervical cancer
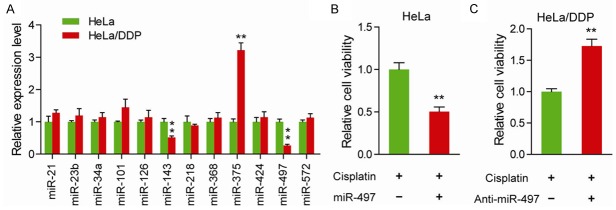
To determine the potential roles of aberrant miRNAs on the chemoresistance of cervical cancer cells, the level of the 12 most reported down-regulated miRNAs in cervical cancer were measured. As shown in Figure 1A, compared to the chemotherapy-sensitive HeLa cells, three miRNAs (miR-143, miR-375 and miR-497) were significantly altered in chemotherapy-resistant HeLa/DDP cells. As the oncogenic roles ofmiR-143 have been reported in cervical cancer [20] and up-regulated miR-375 has been reported in chemotherapy-resistant HeLa/DDP cells, we therefore focused on the role of miR-497 in this study. To further confirm the implications of miR-497 on chemosensitivity, we detected the toxic response of cervical cancer cells in the presence of cisplatin (Figure 1B and 1C). In HeLa cells, treatment of miR-497 markedly improved the cisplatin chemosensitivity (Figure 1B). And just as anticipated, suppression of miR-497 significantly decreased the cisplatin chemosensitivity in HeLa/DDP cells (Figure 1C). Taken together, these data indicate that deregulated miR-497 is critically involved in cisplatin chemosensitivity in cervical cancer.
Figure 1.
miR-497 is associated with cisplatin chemosensitivity in cervical cancer. A. Relative expression of indicated miRNAs in HeLa and HeLa/DDP cells. B. Cell viability was measured in HeLa cells with the introduction of miR-497 mimics or control mimics in the presence of cisplatin. C. Cell viability was measured in HeLa/DDP cells with introduction of miR-497 inhibitors or control inhibitors in the presence of cisplatin. **P < 0.01.
TKT is a direct target of miR-497
Next, we determined the downstream targets of miR-497usingthe prediction program miRBase (http://www.mirbase.org). One of the potential targets, TKT, is of great interest as its role via the PPP reduces chemoresistance through generation of NADPH and GSH, as well as avoids ROS-mediated DNA damage (Figure 2A). Expectedly, treatment withmiR-497 in HeLa and SiHa cells significantly down-regulated TKT expression at both mRNA level (Figure 2B) and protein level (Figure 2C). Then wild type and mutant type of 3’-UTRs of TKT were introduced into luciferase reporter plasmids. Indeed, miR-497 remarkably decreased the luciferase activity of the plasmid with wildtype but not mutant types of TKT 3’-UTR in HeLa and SiHa cells (Figure 2D), suggesting that TKT is a direct target for miR-497 in cervical cancer. Furthermore, by immunohistochemical analysis of 74 cases of cervical squamous carcinoma, we observed that TKT was mainly distributed in the cytoplasm and highly expressed in 81.1% (60/74) of cases examined (Figure 2E). Consistently, data derived from three independent Oncomine databases showed that TKT was commonly up-regulated in cervical cancer tissues compared with their normal controls (Figure 2F and 2G).
Figure 2.
TKT is a direct target of miR-497. (A) In silico algorithms predicted TKT contains one 7-mer putative miR-497-binding site on its 3’-UTRs. The mRNA (B) and protein (C) levels of TKTin miR-497 mimics-treated HeLa and SiHa cells were measured. β-actin was loaded as a control. (D) The direct physical interaction between miR-497 and TKT 3’-UTRs in HeLa and SiHa cells was demonstrated by luciferase reporter assay. (E) Immunohistochemical analysis of TKT expression in 74 cases of cervical squamous carcinoma. Oncomine data (F and G) demonstrated a significant increase in TKT expression in cervical cancer tissues compared to the normal control tissues. *P < 0.05, **P < 0.01. ***P < 0.001.
miR-497/TKT axis contributes to cisplatin chemoresistance in cervical cancer
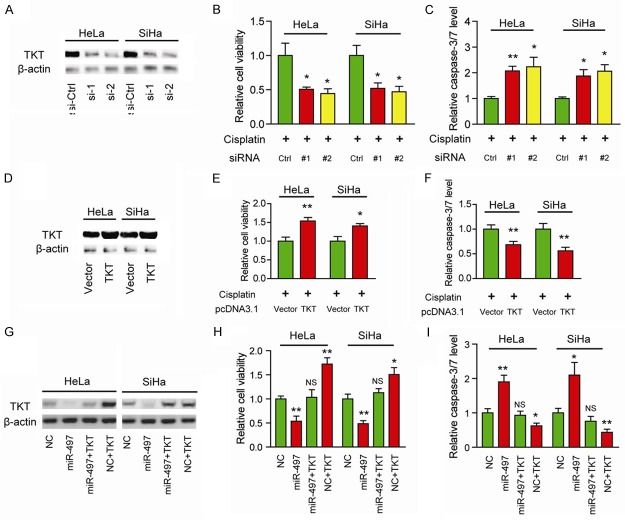
To examine whether TKT is associated with cisplatin chemosensitivity in cervical cancer, we performed loss-of-function and gain-of-function studies. As shown in Figure 3A, two specific siRNAs targeting TKT resulted in a marked decrease in TKT protein levels. Consistently, silencing of TKT significantly improved cisplatin chemosensitivity as demonstrated by reduced cell viability (Figure 3B) and increased caspase-3/7 activity (Figure 3C). To further confirm this observation, we over-expressed TKT in HeLa and SiHa cells (Figure 3D). Conversely, overexpression of TKT increased cell viability (Figure 3E) and decreased cell apoptosis (Figure 3F) in the presence of cisplatin. And the decreased protein level of TKT induced by miR-497 was fully restored by overexpression of TKT (Figure 3G). Indeed, overexpression of TKT remarkably blocked the activities of miR-497 in cisplatin chemosensitivity (Figure 3H and 3I). Taken together, these findings suggest that TKT functionally mediates the role of miR-497 in the regulation of cisplatin chemosensitivity.
Figure 3.
TKT contributes to cisplatin chemoresistance in cervical cancer. (A) Protein level of TKT in specific TKT siRNA-treated HeLa and SiHa cells was measured by Western blotting. The cell viability (B) and caspase-3/7 activity (C) in TKT siRNA-treated HeLa and SiHa cells were measured in the presence of cisplatin. (D) Over-expression efficiency of TKT in HeLa and SiHa cells were measured by Western blotting. The cell viability (E) and caspase-3/7 activity (F) in TKT over-expressed HeLa and SiHa cells were measured in the presence of cisplatin. (G) Protein levels of TKT in the TKT-overexpressed or control cells upon miR-497 treatment was measured by Western blotting. The cell viability (H) and caspase-3/7 activity (I) in HeLa and SiHa cells with indicated treatment were measured in the presence of cisplatin; p value was calculated by comparing to NC. *P < 0.05, **P < 0.01; NS, not significant.
miR-497/TKT axis promotes cisplatin chemoresistance by regulating generation of ROS
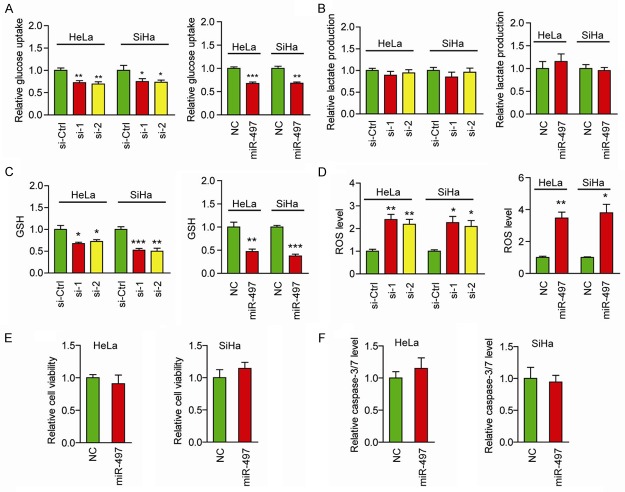
Given TKT is a key factor in the PPP, which provides NADPH to produce GSH, and ROS play crucial roles in the cytotoxic effects of anticancer agents, we supposed that miR-497/TKT axis might facilitate cisplatin chemoresistance by affecting the generation of ROS. As PPP is a branch of glycolysis, and this shift is also known as the Warburg effect, we first tested the effects of TKT knockdown on glucose consumption and lactate production. Surprisingly, knockdown of TKT whether by siRNAs or miR-497 resulted in a distinct decrease in glucose uptake (Figure 4A). However, consistent with our previous findings [15], knockdown of TKT showed no discernable effect on lactate production (Figure 4B). We found that GSH level was reduced by TKT siRNAs or miR-497 (Figure 4C). Conversely, ROS levels were significantly up-regulated by TKT siRNAs and miR-497 (Figure 4D). Furthermore, to determine whether addition of GSH could protect cervical cancer cells from chemotherapy, HeLa and SiHa cells were incubated with 1 mM GSH and 50 μg/ml DDP, and cell viability as well as cell apoptosis were determined. MiR-497 failed to influence cisplatin chemosensitivity as revealed by unaltered cell viability (Figure 4E) and caspase-3/7 activity (Figure 4F). Collectively, these results suggest that the miR-497/TKT axis may facilitate the generation of GSH to reduce ROS level, and ultimately promote cisplatin chemoresistance of cervical cancer cells.
Figure 4.
miR-497/TKT axis promotes cisplatin chemoresistance through regulating generation of ROS. Effects of siRNAs or miR-497-mediated suppression of TKT on glucose uptake (A), lactate production (B), GSH levels (C), and ROS levels (D). The cell viability (E) and caspase-3/7 activity (F) in miR-497-treated HeLa and SiHa cells were measured in the presence of 1 mM GSH and 50 μg/ml cisplatin. *P < 0.05, **P < 0.01. ***P < 0.001.
Discussion
Resistance to current chemotherapy drugs remains the major impediment to successful cancer treatment. Cisplatin, one of the most effective chemotherapeutic agents, is widely used in the treatment of cervical cancer. However, the molecular mechanism underlying acquired resistance to this drug in cervical cancer cells remains unclear. In the current study, we identified a new network involved in cisplatin chemoresistance in cervical cancer.
First, by detecting the differentially expressed miRNAs between HeLa and HeLa/DDP cells, we found that miR-497 was down-regulated in cisplatin-resistant cancer cells and associated with chemosensitivity. Deregulated miR-497 has been reported in many human malignancies, including liver cancer [21], colorectal cancer [22], neuroblastoma [23], ovarian cancer [24], breast cancer [25], lung cancer [26], and cervical cancer [27-30]. Notably, miR-497 can modulate chemosensitivity through multiple targets. In colorectal cancer, miR-497 increases chemosensitivity to 5-fluorouracil treatment through regulating the expression of kinase suppressor of ras 1 (KSR1) [31]. In ovarian cancer, miR-497 decreases cisplatin resistance by targeting mTOR/P70S6K signaling [24]. In pancreatic cancer, miR-497 re-sensitizes cancer cells to gemcitabine and erlotinib by targeting fibroblast growth factor 2 and fibroblast growth factor receptor 1 [32]. In the current study, we demonstrated that TKT was a downstream target of miR-497. Treatment with miR-497 markedly reduced TKT expression and improved cisplatin chemosensitivity. Similar effects were also observed by genetic silencing of TKT. Whether other targets of miR-497 contribute to cisplatin chemoresistance remains unclear, however, our findings at least to some extent demonstrate that elevated TKT protects cervical cancer cells from cisplatin treatment. Therefore, the results from other groups and our study, as a proof of principle, suggest that miR-497 is a critical modulator in the regulation of chemoresistance.
TKT, an enzymein the PPP, catalyzes the conversion of sedoheptulose 7-phosphate and D-glyceraldehyde 3-phosphate to D-ribose 5-phosphate and D-xylulose 5-phosphate. Accumulated studies have concentrated on the paralogs of TKT, TKTL1 [33-37], however, little is known about the oncogenic function of TKT in tumors. Recently, it has been reported that TKT is up-regulated in metastatic peritoneal implants and promotes ovarian cancer cell proliferation but has no effect on motility or invasion [38]. As its activity in the production of NAPDH to counteract oxidative stress, TKT is needed for tumor growth. Xu et al. demonstrated that TKT expression is tightly regulated by oxidative stress sensor pathways in cancers. Specially, genetic knockdown or pharmacologic inhibition of TKT in liver and other cancer cells disturbs the redox homeostasis and sensitizes cancer cells to existing targeted therapy [39]. Consistent with this notion, we found that silencing of TKT resulted in decreased GSH and increased ROS, which ultimately expose cancer cells to oxidative stress and thereby improves cisplatin chemosensitivity. And neutralization of ROS with GSH fully blocked the chemosensitivity induced by miR-497.
Collectively, our study revealed that: i) down-regulated miR-497 was associated with cisplatin-resistance in cervical cancer; ii) TKT is a regulator downstream from miR-497; iii) deregulated miR-497/TKT axis contributes to the cisplatin-resistance phonotype through regulation of GSH and ROS generation. Besides, our study also provides evidence that the PPP is beneficial to chemoresistance and highlights that targeting TKT may have therapeutic benefits in the treatment of cervical cancer.
Acknowledgements
Project Supported by the Natural Science Foundation of China (No. 81472431), Project Supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (No. ykk14154), Key project of social development plan of Jiangsu Province (No. BE2015606).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Lukka H, Johnston M. Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer: a meta-analysis. Clin Oncol (R Coll Radiol) 2004;16:160–161. doi: 10.1016/j.clon.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Martinez A, Molano F, Lloret M, Falcon-Vizcaino O, Garcia-Hernandez JA. Concurrent chemotherapy and radiotherapy for cervical cancer. Eur J Gynaecol Oncol. 2003;24:160–162. [PubMed] [Google Scholar]
- 6.Thomas GM. Improved treatment for cervical cancer--concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340:1198–1200. doi: 10.1056/NEJM199904153401509. [DOI] [PubMed] [Google Scholar]
- 7.Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang L, Han B, Meng J, Yan Z, Yan X, Jiao S. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6:25266–25280. doi: 10.18632/oncotarget.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang HW, Wang F, Wei Q, Zhao YF, Liu M, Li X, Tang H. miR-20a promotes migration and invasion by regulating TNKS2 in human cervical cancer cells. FEBS Lett. 2012;586:897–904. doi: 10.1016/j.febslet.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Leung CO, Deng W, Ye TM, Ngan HY, Tsao SW, Cheung AN, Pang RT, Yeung WS. miR-135a leads to cervical cancer cell transformation through regulation of beta-catenin via a SIAH1-dependent ubiquitin proteosomal pathway. Carcinogenesis. 2014;35:1931–1940. doi: 10.1093/carcin/bgu032. [DOI] [PubMed] [Google Scholar]
- 10.Li QQ, Zhang L, Wan HY, Liu M, Li X, Tang H. CREB1-driven expression of miR-320a promotes mitophagy by down-regulating VDAC1 expression during serum starvation in cervical cancer cells. Oncotarget. 2015;6:34924–34940. doi: 10.18632/oncotarget.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi M, Du L, Liu D, Qian L, Hu M, Yu M, Yang Z, Zhao M, Chen C, Guo L, Wang L, Song L, Ma Y, Guo N. Glucocorticoid regulation of a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. J Pathol. 2012;228:148–157. doi: 10.1002/path.3997. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zhao KN. HPV-p53-miR-34a axis in HPV-associated cancers. Ann Transl Med. 2015;3:331. doi: 10.3978/j.issn.2305-5839.2015.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Li B, Xie X. The roles and clinical significance of microRNAs in cervical cancer. Histol Histopathol. 2016;31:131–139. [PubMed] [Google Scholar]
- 14.Liu W, Gao G, Hu X, Wang Y, Schwarz JK, Chen JJ, Grigsby PW, Wang X. Activation of miR-9 by human papillomavirus in cervical cancer. Oncotarget. 2014;5:11620–11630. doi: 10.18632/oncotarget.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Su J, Xue SL, Yang H, Ju LL, Ji Y, Wu KH, Zhang YW, Zhang YX, Hu JF, Yu MM. HPV E6/p53 mediated down-regulation of miR-34a inhibits Warburg effect through targeting LDHA in cervical cancer. Am J Cancer Res. 2016;6:312–320. [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Li J, Zong L, Chen X, Chen K, Jiang Z, Nan L, Li X, Li W, Shan T, Ma Q, Ma Z. Reactive Oxygen Species and Targeted Therapy for Pancreatic Cancer. Oxid Med Cell Longev. 2016;2016:1616781. doi: 10.1155/2016/1616781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Preter G, Neveu MA, Danhier P, Brisson L, Payen VL, Porporato PE, Jordan BF, Sonveaux P, Gallez B. Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation. Oncotarget. 2016;7:2910–2920. doi: 10.18632/oncotarget.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecker PA, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H491–500. doi: 10.1152/ajpheart.00721.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Yu X, Guo X, Tian Z, Su M, Long Y, Huang C, Zhou F, Liu M, Wu X, Wang X. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol Med Rep. 2012;5:753–760. doi: 10.3892/mmr.2011.696. [DOI] [PubMed] [Google Scholar]
- 21.Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y, Li PY, Wang M, Lin JS, He XX. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget. 2015;6:29527–29542. doi: 10.18632/oncotarget.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu Y, Yu H, Shi X, Xu K, Tang Q, Liang B, Hu S, Bao Y, Xu J, Cai J, Peng W, Cao Q, Yin P. microRNA-497 inhibits invasion and metastasis of colorectal cancer cells by targeting vascular endothelial growth factor-A. Cell Prolif. 2016;49:69–78. doi: 10.1111/cpr.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano A, Paris-Coderch L, Jubierre L, Martinez A, Zhou X, Piskareva O, Bray I, Vidal I, Almazan-Moga A, Molist C, Roma J, Bayascas JR, Casanovas O, Stallings RL, Sanchez de Toledo J, Gallego S, Segura MF. MicroRNA-497 impairs the growth of chemoresistant neuroblastoma cells by targeting cell cycle, survival and vascular permeability genes. Oncotarget. 2016;7:9271–87. doi: 10.18632/oncotarget.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S, Fu GB, Tao Z, OuYang J, Kong F, Jiang BH, Wan X, Chen K. MiR-497 decreases cisplatin resistance in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget. 2015;6:26457–26471. doi: 10.18632/oncotarget.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YY, Kuo WH, Hung JH, Lee CY, Lee YH, Chang YC, Lin WC, Shen CY, Huang CS, Hsieh FJ, Lai LC, Tsai MH, Chang KJ, Chuang EY. Deregulated microRNAs in triple-negative breast cancer revealed by deep sequencing. Mol Cancer. 2015;14:36. doi: 10.1186/s12943-015-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu A, Lu J, Wang W, Shi C, Han B, Yao M. Role of miR-497 in VEGF-A-mediated cancer cell growth and invasion in non-small cell lung cancer. Int J Biochem Cell Biol. 2016;70:118–125. doi: 10.1016/j.biocel.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang D, Wang F, Xu D, Guo Y, Cui W. Serum miRNAs panel (miR-16-2*, miR-195, miR-2861, miR-497) as novel non-invasive biomarkers for detection of cervical cancer. Sci Rep. 2015;5:17942. doi: 10.1038/srep17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Huo M, Mu M, Liu J, Zhang J. [miR-497 suppresses proliferation of human cervical carcinoma HeLa cells by targeting cyclin E1] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30:597–600. [PubMed] [Google Scholar]
- 29.Luo M, Shen D, Zhou X, Chen X, Wang W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery. 2013;153:836–847. doi: 10.1016/j.surg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Lajer CB, Garnaes E, Friis-Hansen L, Norrild B, Therkildsen MH, Glud M, Rossing M, Lajer H, Svane D, Skotte L, Specht L, Buchwald C, Nielsen FC. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer. 2012;106:1526–1534. doi: 10.1038/bjc.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Jiang CF, Li DM, Ge X, Shi ZM, Li CY, Liu X, Yin Y, Zhen L, Liu LZ, Jiang BH. MicroRNA-497 inhibits tumor growth and increases chemosensitivity to 5-fluorouracil treatment by targeting KSR1. Oncotarget. 2016;7:2660–2671. doi: 10.18632/oncotarget.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Wang T, Cao Z, Huang H, Li J, Liu W, Liu S, You L, Zhou L, Zhang T, Zhao Y. MiR-497 downregulation contributes to the malignancy of pancreatic cancer and associates with a poor prognosis. Oncotarget. 2014;5:6983–6993. doi: 10.18632/oncotarget.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwaab J, Horisberger K, Strobel P, Bohn B, Gencer D, Kahler G, Kienle P, Post S, Wenz F, Hofmann WK, Hofheinz RD, Erben P. Expression of Transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. BMC Cancer. 2011;11:363. doi: 10.1186/1471-2407-11-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langbein S, Zerilli M, Zur Hausen A, Staiger W, Rensch-Boschert K, Lukan N, Popa J, Ternullo MP, Steidler A, Weiss C, Grobholz R, Willeke F, Alken P, Stassi G, Schubert P, Coy JF. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94:578–585. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Moralli S, Tarrado-Castellarnau M, Alenda C, Castells A, Cascante M. Transketolase-like 1 expression is modulated during colorectal cancer progression and metastasis formation. PLoS One. 2011;6:e25323. doi: 10.1371/journal.pone.0025323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krockenberger M, Engel JB, Schmidt M, Kohrenhagen N, Hausler SF, Dombrowski Y, Kapp M, Dietl J, Honig A. Expression of transketolase-like 1 protein (TKTL1) in human endometrial cancer. Anticancer Res. 2010;30:1653–1659. [PubMed] [Google Scholar]
- 37.Chen H, Yue JX, Yang SH, Ding H, Zhao RW, Zhang S. Overexpression of transketolase-like gene 1 is associated with cell proliferation in uterine cervix cancer. J Exp Clin Cancer Res. 2009;28:43. doi: 10.1186/1756-9966-28-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricciardelli C, Lokman NA, Cheruvu S, Tan IA, Ween MP, Pyragius CE, Ruszkiewicz A, Hoffmann P, Oehler MK. Transketolase is upregulated in metastatic peritoneal implants and promotes ovarian cancer cell proliferation. Clin Exp Metastasis. 2015;32:441–455. doi: 10.1007/s10585-015-9718-1. [DOI] [PubMed] [Google Scholar]
- 39.Xu IM, Lai RK, Lin SH, Tse AP, Chiu DK, Koh HY, Law CT, Wong CM, Cai Z, Wong CC, Ng IO. Transketolase counteracts oxidative stress to drive cancer development. Proc Natl Acad Sci U S A. 2016;113:E725–734. doi: 10.1073/pnas.1508779113. [DOI] [PMC free article] [PubMed] [Google Scholar]