Abstract
The Delta-like 1 homolog (DLK1) gene is a paternal imprinting gene located on human chromosome 14q32, a site associated with frequent chromosomal mutations in GIST. The expression level of DLK1 is closely associated with the outcome of tumours. However, no study has reported the DLK1 expression in GIST. Here, we demonstrated that DLK1 showed low expression in GIST patients with low risk according to the modified National Institute of Health (NIH) criteria. With increasing tumour risk level, DLK1 gene and protein expression levels gradually increased. In the test cohort, tissue microarray data showed that DLK1 protein expression was significantly associated with tumour size, mitotic figure count, NIH risk level, and Ki67 expression. In terms of either disease-free survival (DFS) or overall survival (OS), the long-term outcome was significantly better in DLK1-negative patients than in DLK1-positive patients. Univariate and multivariate analyses suggested that DLK1 expression was an independent risk factor influencing tumour DFS. Additionally, for intermediate/high-risk GIST patients received postoperative IM adjuvant therapy, Kaplan-Meier analysis showed that IM adjuvant therapy was associated with a better outcome in DLK1-negative patients than in DLK1-positive patients. All of the above results were verified in the validation cohort. Taken together, DLK1 is a promising prognostic biomarker for GISTs that may help to predict surgical outcomes and guide adjuvant IM therapy.
Keywords: DLK1, imatinib mesylate, GIST
Introduction
Gastrointestinal stromal tumour (GIST) is the most common mesenchymal tumour in the gastrointestinal tract. Among malignancies of the digestive tract, GIST can occur throughout the tract system, most preferentially in the stomach (50-70%), followed by the small intestine (20-30%), with an incidence only second to gastric and colorectal tumours [1]. Thus far, it is clear that functional acquired mutation of the tyrosine kinase receptor encoded by the c-kit gene is an important mediating factor in the development of GIST. However, this mechanism alone cannot fully explain all the problems regarding GIST development and progress. It has been found that activating mutations of the c-kit gene already exist in early GISTs. The proportion of early GISTs with c-kit gene mutations (85%) is similar to that of advanced GISTs (86%). The vast majority of these early tumours with c-kit gene mutations have long-term stability, namely, these patients are in a healthy state of tumour-bearing survival. Only a small proportion of such tumours may undergo progressive growth, forming highly malignant tumours [2]. The above fact suggests that c-kit gene mutation is merely an important early event in the course of GIST rather than the main factor triggering the malignant potential of intermediate/advanced GIST. Additionally, the resistance of GIST to targeted imatinib mesylate (IM) therapy also demonstrates that the disease progression of GIST is not simply determined by the c-kit gene. In addition to the c-kit gene, there must be other factors that participate in and ultimately determine the development and outcome of GIST.
The Delta-like 1 homolog (DLK1) gene is a paternal imprinting gene located human chromosome 14q32, a site associated with frequent chromosomal mutations in GIST [3]. DLK1-encoded proteins are primarily involved in regulating the differentiation of various cells in early embryonic development, whereas in the gastrointestinal tract of healthy adults, there is almost no DLK1 expression [4]. In recent years, researchers have begun to observe the effect of DLK1 as an oncogene or anti-oncogene in tumour development and progression. Abnormal expression of DLK1 has especially been found in tumours with neuroendocrine features, such as human neuroglioma and small cell lung carcinoma [5-7]. Moreover, the expression level of DLK1 is closely associated with the outcome of tumours [8]. However, no study has reported the DLK1 expression in GIST.
In this study, the presence of DLK1 gene and protein expression in fresh GIST tissue was examined by quantitative real-time PCR (qRT-PCR) and Western blotting analysis. The DLK1 expression level in GIST tissue was assayed by a large-scale tissue microarray and immunohistochemical (IHC) staining in a test cohort and a validation cohort. Further, clinicopathological and follow-up data were combined to assess the relationship of DLK1 with the surgical outcome of GIST patients and the efficacy of IM adjuvant therapy.
Materials and methods
Patients and specimens
The present study was designed according to the REMARK guidelines for reporting prognostic biomarkers in oncology [9]. Patient inclusion and exclusion criteria were as follows: (1) postoperative confirmation of GIST by pathological examination (IHC analysis of tumour tissue showed CD117-positive staining); (2) clinical exclusion of a history of other associated tumours; (3) surgery meeting the standard for radical (R0) resection; (4) no preoperative chemotherapy, radiotherapy, and other anti-tumour therapies; and (5) complete clinicopathological and postoperative follow-up data. All tumour tissue specimens and clinical data used in the study were acquired from Renji Hospital, which is affiliated with Shanghai Jiaotong University School of Medicine, Shanghai, China. The hospital ethics committee had approved the use of the data.
In accordance with the revised version of the 2008 NIH criteria [10], fresh-frozen tumour tissue specimens were collected from 36 GIST patients with different risk levels (15 cases of low risk, 11 cases of intermediate risk, and 10 cases of high risk) as the discovery cohort. DLK1 mRNA and protein expression was detected in the tumour tissue by qRT-PCR and Western blotting analysis. The specimens were taken from consecutive GIST patients who were admitted to Renji hospital and treated by radical resection from November 2013 to April 2014, including 27 cases of gastric GIST and 9 cases of non-gastric GIST. Additionally, paraffin-embedded tumour tissue specimens were collected from 412 consecutive GIST patients included in the study, who were admitted to Renji Hospital, and were treated by radical resection from May 2005 to November 2013. These paraffin-embedded tumour tissue specimens were used to prepare tissue microarrays for IHC analysis. Of these, 266 GIST patients treated in the General Hospital were designated as the test cohort, and the remaining 146 GIST patients treated in the West Branch Hospital during the same period were designated as the validation cohort.
The study involved the following clinicopathological parameters: age, gender, tumour location, tumour size, mitotic figure count [number of mitotic figures/50 high-power fields (HPF)], tumour rupture, histological type, mutation analysis, and Ki67 expression in the tumour tissue. For all patients included in the study, the tumour was divided into very low-risk, low-risk, intermediate-risk, and high-risk groups according to the NIH risk classification [10]. Details on the clinicopathological features of patients in the test and validation cohorts are presented in Table 1. Since 2008, we began recommending continuous adjuvant therapy with 400 mg/day IM to intermediate/high-risk GIST patients post R0 resection. The IM adjuvant therapy lasted for at least a year, and patients were required to undergo KIT and PDGFRA mutation detection prior to the adjuvant therapy. Among the patients included in the study, a total of 247 patients received gene mutation detection (test cohort, 149 cases; validation cohort, 98 cases), of which 66 patients received postoperative adjuvant therapy with IM (test cohort, 41 cases; validation cohort, 25 cases). Moreover, all GIST patients received a postoperative follow-up through outpatient visits, with physical examination and abdominal ultrasonography every three months. Postoperative examination by abdominal and pelvic computed tomography or magnetic resonance imaging was required every six months for two years and once a year thereafter. During the follow-up period, suspected tumour recurrences were immediately examined for diagnosis. The last follow-up time was March 2014 in all cases. Disease-free survival (DFS) was defined as the period from the date of tumour resection to the detection of tumour recurrence or last observation. The overall survival (OS) was calculated from the length of time between surgery and death or the last follow-up examination. The test cohort had an overall median follow-up period of 42 months (range, 4-106 months), in which patients of the IM adjuvant therapy group had a median follow-up period of 33 months (range, 8-65 months). The validation cohort had an overall median follow-up period of 45 months (range, 4-106 months), in which patients of the IM adjuvant therapy group had a median follow-up period of 34 months (range, 5-63 months).
Table 1.
Clinical characteristics of GIST patients in test and validation cohort
| Variable | Test cohort (%) N = 266 | Validation cohort (%) N = 146 | P value |
|---|---|---|---|
| Age (year) | |||
| ≤ 60 | 144 (54.1) | 80 (54.8) | 0.918 |
| > 60 | 122 (45.9) | 66 (45.2) | |
| Gender | |||
| Male | 146 (54.9) | 77 (52.7) | 0.681 |
| Female | 120 (45.1) | 69 (47.3) | |
| Tumor site | |||
| Stomach | 156 (58.6) | 82 (56.2) | 0.614 |
| Small bowel | 74 (27.8) | 47 (32.2) | |
| Others | 36 (13.5) | 17 (11.6) | |
| Tumor size (cm) | |||
| ≤ 2.0 | 28 (10.5) | 9 (6.2) | 0.466 |
| 2.1-5.0 | 104 (39.1) | 59 (40.4) | |
| 5.1-10.0 | 84 (31.6) | 52 (35.6) | |
| > 10.0 | 50 (18.8) | 26 (17.8) | |
| Mitoses per 50 HPFs | |||
| ≤ 5 | 199 (74.8) | 109 (74.7) | 0.763 |
| 5-10 | 33 (12.4) | 21 (14.4) | |
| > 10 | 34 (12.8) | 16 (11.0) | |
| Modified NIH criteria | |||
| Very low risk | 25 (9.4) | 7 (4.8) | 0.310 |
| Low risk | 100 (37.6) | 52 (35.6) | |
| Intermediate risk | 37 (13.9) | 25 (17.1) | |
| High risk | 104 (39.1) | 62 (42.5) | |
| Tumor rupture | |||
| No | 237 (89.1) | 128 (87.7) | 0.746 |
| Yes | 29 (10.9) | 18 (12.3) | |
| Histological type | |||
| Fusiform | 184 (69.2) | 95 (65.1) | 0.642 |
| Epitheliod | 47 (17.7) | 31 (21.2) | |
| Mixed | 35 (13.2) | 20 (13.7) | |
| DLK1 | |||
| Negative | 174 (65.4) | 86 (58.9) | 0.201 |
| Positive | 92 (34.6) | 60 (41.1) | |
| Ki67 | |||
| Negative | 231 (86.8) | 126 (86.3) | 0.881 |
| Positive | 35 (13.2) | 20 (13.7) | |
| Tumor mutation type | 0.667 | ||
| KIT exon 11 | 101 (67.8) | 72 (73.5) | |
| KIT exon 9 | 13 (8.7) | 5 (5.1) | |
| PDGFRA | 20 (13.4) | 11 (11.2) | |
| Wide type | 15 (10.1) | 10 (10.2) | |
| Imatinib adjuvant therapy | |||
| No | 225 (84.6) | 121 (82.9) | 0.675 |
| Yes | 41 (15.4) | 25 (17.1) |
qRT-PCR
RNA was extracted from 36 fresh GIST tissues with Trizol (Invitrogen) using standard protocols. Reverse-transcription reactions were performed with random primers and M-MLV Reverse Transcriptase (Invitrogen, CA). Gene expression was quantified using the SYBR green method. The primers were as follows: DLK1, forward CTTTCGGCCACAGCACCTAT, reverse CCTCGCAGAATCCATTTTGGG; and β-actin, forward GCACCCAGCACAATGAAGA, reverse CGATCCACACGGAGTACTTG. qRT-PCR was performed on a StepOnePlus instrument (Applied Biosystems, Foster City, CA, USA). Transcript levels were analysed using comparative cycle threshold numbers and were normalised to β-actin.
Western blotting analysis
Total proteins were extracted from fresh-frozen tumour tissue specimens of six GIST patients (low-risk, three cases; high-risk, three cases) of the discovery cohort. The concentration of protein extracts was measured using a Bio-Rad protein assay kit (BioRad, Hercules, CA, USA). An equal amount of total protein (50 µg) was separated by 10% sulphate polyacrylamide gels and then electroblotted to a nitrocellulose membrane. Beta-actin was used as a loading control. The membrane was blocked with 5% dry milk in Tris-buffered saline for 1 h, followed by incubation with the DLK1 primary antibody (1:1,000, Abcam, Cambridge, UK) overnight at 4°C. Signals on the membranes were detected by an Odyssey infrared imaging system (LI-COR, Lincoln, NE) after incubating with a labelled polymer-HRP anti-rabbit secondary antibody (Dako, Carpinteria, CA, USA) for 1 h at room temperature. Quantification was conducted using ImageJ software.
Tissue microarray and immunohistochemistry
The tissue microarrays were developed by Suzhou Xinxin Biotechnology Co., Ltd (Xinxin Biotechnology Co, Suzhou, China). Tissue paraffin blocks of GIST samples were stained with hematoxylin-eosin to confirm the diagnoses and marked at fixed points with most typical histological characteristics under a microscope. Two 1.6-mm cores per donor block were transferred into a recipient block tissue microarray, and each dot array contained fewer than 160 dots. Three-micron-thick sections were cut from the recipient block and transferred to glass slides with an adhesive tape transfer system for ultraviolet cross linkage. The slides were baked at 56°C for 1 h, de-paraffinised in xylene for 20 min, and rehydrated through a graded series of ethanol concentrations (5 min in 100% ethanol, followed by 5 min in 70% ethanol). Antigen retrieval was performed in a pressure cooker for 5 min with Target Retrieval Solution (Dako, Carpinteria, CA, USA). Endogenous peroxidase activity was blocked using a peroxidase blocking reagent (Dako, Carpinteria, CA, USA) for 5 min. Next, a DLK1 antibody (1:150, Abcam, Cambridge, UK) was applied to cover the specimens for 1 h at room temperature, followed by incubation with anti-rabbit secondary antibody (Dako, Carpinteria, CA, USA) for 30 min at room temperature. Thorough rinsing with Tris-buffered saline and Tween 20 was performed after this incubation. The slides were visualised using diaminobenzidine substrate-chromogen (Dako, Carpinteria, CA, USA) and were washed with deionised water before hematoxylin counterstaining. The slides were then dehydrated through an upstaging series of ethanol concentrations, cleared in xylene, and coverslipped with Digital Picture Exchange mounting medium (Leica Biosystems, Wetzlar, Germany).
The expression of DLK1 was quantified by two independent pathologists, and scoring was conducted according to the ratio and intensity of positive-staining cells: 0, if no staining was observed; 1+, if > 25% of the tumour cells had weak or moderate staining intensity; and 2+, if the tumour cells had strong staining intensity. Given the heterogeneity of protein expression in the tumour cells, the highest scoring from either of the TMA cores was considered the final result. Tumours with 1+ and 2+ expression were interpreted as positive, and tumours with no expression (0 score) were interpreted as negative.
Statistical analysis
Statistical analyses were performed with SPSS 20.0 for Windows (Chicago, IL, USA), and the results were expressed by the mean ± SEM. For comparisons, one-way analyses of variance and chi-squared tests were performed when appropriate. The OS and DFS were calculated according to the Kaplan-Meier method. The log-rank test was used to compare the survival distributions. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. Only variables that were significantly different in univariate analysis were entered into the next multivariate analysis. All statistical tests were two-sided. P-value differences < 0.05 were considered statistically significant.
Results
DLK1 expression increases with increasing tumour risk level of GIST
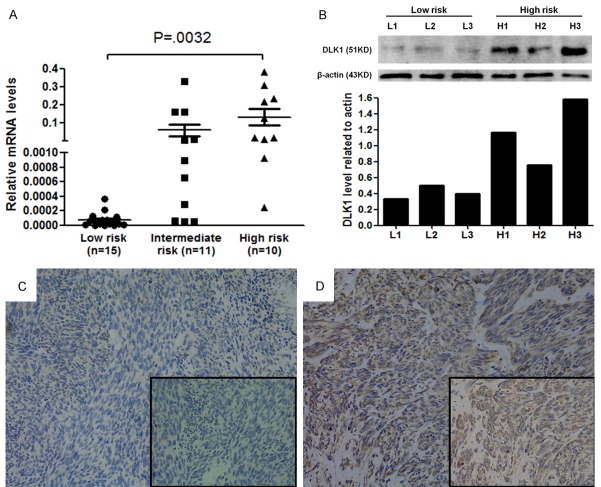
DLK1 expression in tumour tissue was examined by qRT-PCR analysis among 36 GIST patients with different risk levels. With increasing tumour recurrence risk, the DLK1 mRNA relative expression level (2-ΔCt) exhibited a gradual upward trend in the low-risk group (including extremely low-risk and low-risk groups in the NIH classification), intermediate-risk group, and high-risk group (P = 0.008). The DLK1 expression levels were 0.001 ± 0.001 in the low-risk group, 0.061 ± 0.033 in the intermediate-risk group, and 0.131 ± 0.045 in the high-risk group. Pairwise comparisons between the groups showed that the high-risk group had a significantly higher DLK1 mRNA expression level than that of the low-risk group (P = 0.032) (Figure 1A). High-risk and low-risk GIST tissue specimens (three each) were randomly selected from the above 36 specimens for Western blotting analysis of DLK1 protein expression. Similarly, the results showed that DLK1 expression in the tumour tissue was significantly higher in the high-risk GIST group than in the low-risk GIST group (Figure 1B).
Figure 1.
DLK1 expression in GIST tissues. A: DLK1 mRNA levels in GIST tissues of high-risk group were significantly higher than those of low-risk or intermediate-risk group (P = 0.008 and P = 0.039). B: DLK1 protein expression in 3 low-risk and 3 high-risk GIST tissues. C: Representative image of no DLK1 expression in GIST tissues was defined as negative staining. Original magnification: ×200. D: Representative images of strong DLK1 expression in GIST tissues were defined as positive staining. Original magnification: ×200.
DLK1 expression in tumour tissue is an independent risk factor for GIST outcome
Based on the discovery cohort, large-scale tissue microarray analysis was performed to detect DLK1 protein in paraffin-embedded tumour tissue specimens of GIST. In the test cohort, DLK1 showed negative expression (Figure 1C) in 174 patients (65.4%) and positive expression (Figure 1D) in 92 patients (34.6%). In the validation cohort, DLK1 showed negative expression in 86 patients (58.9%) and positive expression in 60 patients (41.1%). The positive expression ratio of DLK1 in the test cohort was similar to that in the validation cohort (P = 0.201). The relationship between the DLK1 expression and clinicopathological features of GIST patients is shown in Table 2. In the test cohort, DLK1 expression level in tumour tissue was closely associated with tumour size (P < 0.001), mitotic figure count/50 HPF (P < 0.001), NIH risk level (P < 0.001), and Ki67 expression (P = 0.004); however, DLK1 expression level was independent of patient age (P = 0.122), gender (P = 0.195), tumour location (P = 0.182), tumour rupture (P = 0.146), histological type (P = 0.589), and gene mutation type (P = 0.388). The above results were verified in the validation cohort.
Table 2.
Relationship between DLK1 expression and clinicopathologic features of GIST patients in test and validation cohort
| Variable | Test cohort (n = 266) | Validation cohort (n = 146) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| DLK1 | P value | DLK1 | P value | |||
|
|
|
|||||
| Negative (%) | Positive (%) | Negative (%) | Positive (%) | |||
| Age (year) | ||||||
| ≤ 60 | 88 (61.1) | 56 (38.9) | 0.122 | 48 (60.0) | 32 (40.0) | 0.866 |
| > 60 | 86 (70.5) | 36 (29.5) | 38 (57.6) | 28 (42.4) | ||
| Gender | ||||||
| Male | 90 (61.6) | 56 (38.4) | 0.195 | 47 (61.0) | 30 (39.0) | 0.616 |
| Female | 84 (70.0) | 36 (30.0) | 39 (56.5) | 30 (43.5) | ||
| Tumor site | ||||||
| Stomach | 109 (69.9) | 37 (30.1) | 0.182 | 55 (67.1) | 27 (32.9) | 0.075 |
| Small bowel | 43 (58.1) | 31 (41.9) | 23 (48.9) | 24 (51.1) | ||
| Others | 22 (61.1) | 14 (38.9) | 8 (47.1) | 9 (52.9) | ||
| Tumor size (cm) | ||||||
| ≤ 2 | 26 (92.9) | 2 (7.1) | < 0.001* | 7 (77.8) | 2 (22.2) | 0.004* |
| > 2 & ≤ 5 | 76 (73.1) | 28 (26.9) | 43 (72.9) | 16 (27.1) | ||
| > 5 & ≤ 10 | 48 (57.1) | 36 (42.9) | 27 (51.9) | 25 (48.1) | ||
| > 10 | 24 (48.0) | 26 (52.0) | 9 (34.6) | 17 (65.4) | ||
| Mitoses per 50 HPFs | ||||||
| ≤ 5 | 147 (73.9) | 52 (26.1) | < 0.001* | 74 (67.9) | 35 (32.1) | 0.001* |
| > 5 & ≤ 10 | 16 (48.5) | 17 (51.5) | 6 (28.6) | 15 (71.4) | ||
| > 10 | 11 (32.4) | 23 (67.6) | 6 (37.5) | 10 (62.5) | ||
| Modified NIH criteria | ||||||
| Very low risk | 23 (92.0) | 2 (8.0) | < 0.001* | 6 (86.7) | 1 (14.3) | 0.002* |
| Low risk | 77 (77.0) | 23 (23.0) | 39 (75.0) | 13 (25.0) | ||
| Intermediate risk | 28 (75.7) | 9 (24.3) | 15 (60.0) | 10 (40.0) | ||
| High risk | 46 (44.2) | 58 (55.8) | 26 (41.9) | 36 (58.1) | ||
| Tumor rupture | ||||||
| No | 159 (67.1) | 78 (32.9) | 0.146 | 73 (57.0) | 55 (43.0) | 0.307 |
| Yes | 15 (51.7) | 14 (48.3) | 13 (72.2) | 5 (27.8) | ||
| Histological type | ||||||
| Fusiform | 124 (67.4) | 60 (32.6) | 0.589 | 59 (62.1) | 36 (37.9) | 0.546 |
| Epitheliod | 29 (61.7) | 18 (38.3) | 16 (51.6) | 15 (48.4) | ||
| Mixed | 21 (60.0) | 14 (40.0) | 11 (55,0) | 9 (45.0) | ||
| Ki67 | ||||||
| Negative | 159 (68.8) | 72 (31.2) | 0.004* | 81 (64.3) | 45 (35.7) | 0.001* |
| Positive | 15 (42.9) | 20 (57.1) | 5 (25.0) | 15 (75.0) | ||
| Tumor mutation type | ||||||
| KIT exon 11 | 55 (54.5) | 46 (45.6) | 0.388 | 46 (63.9) | 26 (36.1) | 0.606 |
| KIT exon 9 | 6 (46.2) | 7 (53.8) | 3 (60.0) | 2 (40.0) | ||
| PDGFRA | 12 (60.0) | 8 (40.0) | 5 (45.5) | 6 (54.5) | ||
| Wide type | 5 (33.3) | 10 (66.6) | 5 (50.0) | 5 (50.0) | ||
P < 0.05.
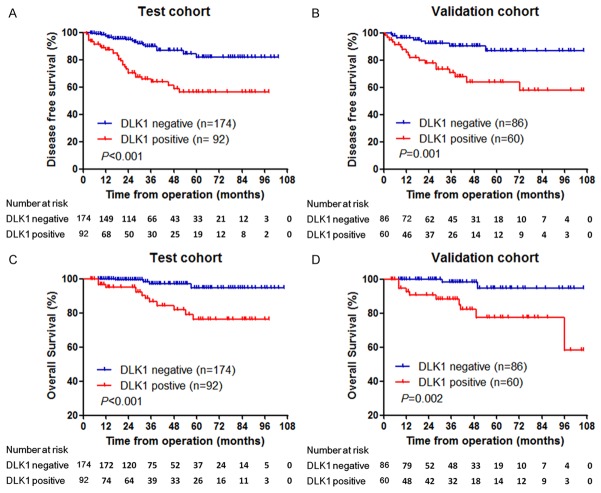
The DFS and OS survival curves of patients were plotted and compared between the DLK1-negative and DLK1-positive groups (Figure 2). In both the test and validation cohorts, DLK1-negative patients had significantly higher DFS and OS rates than DLK1-positive patients. In the test cohort, the five-year DFS and OS rates of DLK1-negative patients were 94.7% and 97.0%, respectively, which was significantly higher than those of DLK1-positive patients, 65.8% and 76.2%, respectively (P < 0.001). Similarly, in the validation cohort, DLK1-negative patients had significantly higher five-year DFS and OS rates than DLK1-positive patients (five-year DFS rate, 86.8% vs. 64.1%, P = 0.001; five-year OS rate, 94.8% vs. 77.5%, P = 0.002).
Figure 2.
Prognostic significance of DLK1 expression in GISTs was assessed by Kaplan-Meier method and log-rank test. A: Comparisons of disease-free survival (DFS) between DLK1 negative and positive groups in the test cohort (P < 0.001). B: Comparisons of DFS between DLK1 negative and positive groups in the validation cohort (P = 0.001). C: Comparisons of overall survival (OS) between DLK1 negative and positive groups in the test cohort (P < 0.001). D: Comparisons of OS between DLK1 negative and positive groups in the validation cohort (P = 0.002).
Moreover, univariate analysis (Table 3) confirmed that in both the test and validation cohorts, DLK1 expression was significantly associated with postoperative recurrence of GIST (test cohort: hazard ratio = 1.357, P < 0.001; validation cohort: hazard ratio = 1.892, P = 0.003), in addition to tumour location, tumour size, mitotic figure count, tumour rupture, and Ki67 expression. Further multivariate analysis (Table 4) showed that DLK1 expression was an independent risk factor influencing tumour DFS (test cohort: hazard ratio = 1.486, P = 0.012; validation cohort: hazard ratio = 1.794, P = 0.024).
Table 3.
Univariate analyses of factors associated with disease free survival (DFS) in test and validation cohort
| Variable | Test cohort (n = 266) | Validation cohort (n = 146) | ||
|---|---|---|---|---|
|
|
|
|||
| DFS hazard ratio (95% Cl) | P value | DFS hazard ratio (95% Cl) | P value | |
| Age (≤ 60, > 60) | 1.381 (0.778-2.451) | 0.270 | 1.135 (0.525-2.456) | 0.747 |
| Gender (male, female) | 0.460 (0.246-0.861) | 0.015* | 0.453 (0.197-1.042) | 0.063 |
| Histological type (fusiform, epitheliod, mixed) | 1.169 (0.795-1.718) | 0.428 | 1.211 (0.741-1.978) | 0.445 |
| Tumor site (stomach, small bowel, colon, others) | 1.615 (1.109-2.354) | 0.013* | 2.248 (1.353-3.734) | 0.002* |
| Tumor size (≤ 2, > 2 & ≤ 5, > 5 & ≤ 10, > 10 cm) | 3.106 (2.129-4.531) | < 0.001* | 4.196 (2.365-7.444) | < 0.001* |
| Mitosis count (≤ 5, > 5 & ≤ 10, > 10/50 HPF) | 3.225 (2.341-4.444) | < 0.001* | 3.678 (2.361-5.730) | < 0.001* |
| Tumor rupture (yes, no) | 3.534 (1.864-6.702) | 0.008* | 5.135 (2.329-11.322) | < 0.001* |
| Ki67 (negative, positive) | 6.773 (3.788-12.110) | < 0.001* | 8.979 (4.096-19.683) | < 0.001* |
| DLK1 (negative, positive) | 1.357 (1.173-1.570) | < 0.001* | 1.892 (1.247-2.871) | 0.003* |
P < 0.05.
Table 4.
Multivariate analyses of factors associated with disease free survival (DFS) in test and validation cohort
| Variable | Test cohort (n = 266) | Validation cohort (n = 146) | ||
|---|---|---|---|---|
|
|
|
|||
| DFS hazard ratio (95% Cl) | P value | DFS hazard ratio (95% Cl) | P value | |
| Tumor site (stomach, small bowel, colon, others) | 1.633 (1.116-2.390) | 0.012* | 2.525 (1.450-4.397) | 0.001* |
| Tumor size (≤ 2, > 2 & ≤ 5, > 5 & ≤ 10, > 10 cm) | 2.354 (1.514-3.661) | < 0.001* | 3.012 (1.589-5.708) | 0.001* |
| Mitosis count (≤ 5, > 5 & ≤ 10, > 10/50 HPF) | 1.701 (1.163-2.488) | 0.006* | 2.885 (1.685-4.938) | < 0.001* |
| Tumor rupture (yes, no) | 1.969 (1.030-3.766) | 0.041* | 10.491 (3.785-29.079) | < 0.001* |
| Ki67 (negative, positive) | 2.972 (1.605-5.505) | 0.001* | - | - |
| DLK1 (negative, positive) | 1.486 (1.092-2.023) | 0.012* | 1.794 (1.078-2.984) | 0.024* |
P < 0.05.
DLK1 expression in tumour tissue can be used as a predictor for the efficacy of postoperative IM adjuvant therapy in GIST
A total of 228 patients were pathologically diagnosed with intermediate/high recurrence risk of GIST after surgery (test cohort, 141 cases; validation cohort, 87 cases). Of these, 66 patients (test cohort, 41 cases; validation cohort, 25 cases) received postoperative IM adjuvant therapy. The 66 patients all underwent KIT and PDGFRA gene testing prior to postoperative IM adjuvant therapy. KIT exon 11 mutation was detected in 56 patients (84.9%), KIT exon 9 mutation was detected in seven patients (10.6%), and PDGFRA mutation was detected in two patients (3.0%), with one wild-type case (1.5%). According to the DLK1 expression results, intermediate/high-risk GIST patients were divided into DLK1-negative and DLK1-positive subgroups. Postoperative DFS curves were compared between patients received simple surgical treatment and IM adjuvant therapy within each subgroup (Figure 3). In the test cohort, 74 (52.5%) patients showed negative expression of DLK1, and 67 (47.5%) patients showed positive expression of DLK1. Within the DLK1-negative subgroup, patients received IM adjuvant therapy had significantly higher DFS than those received simple surgical treatment (five-year DFS rate 81.6% vs. 56.5%, P = 0.022) (Figure 3A). Within the DLK1-positive subgroup, patients received simple surgical treatment and IM adjuvant therapy had five-year DFS rates of 51.9% and 49.2%, respectively, showing no significant difference on the DFS curve (P = 0.612) (Figure 3B). The above results were verified in the validation cohort (Figure 3C, 3D). Kaplan-Meier analysis showed that in the DLK1-negative subgroup, postoperative IM adjuvant therapy benefited intermediate/high-risk GIST patients relative to surgical treatments alone (P = 0.046). However, in the DLK1-positive subgroup, IM adjuvant therapy did not improve DFS in intermediate/high-risk GIST patients (P = 0.704).
Figure 3.
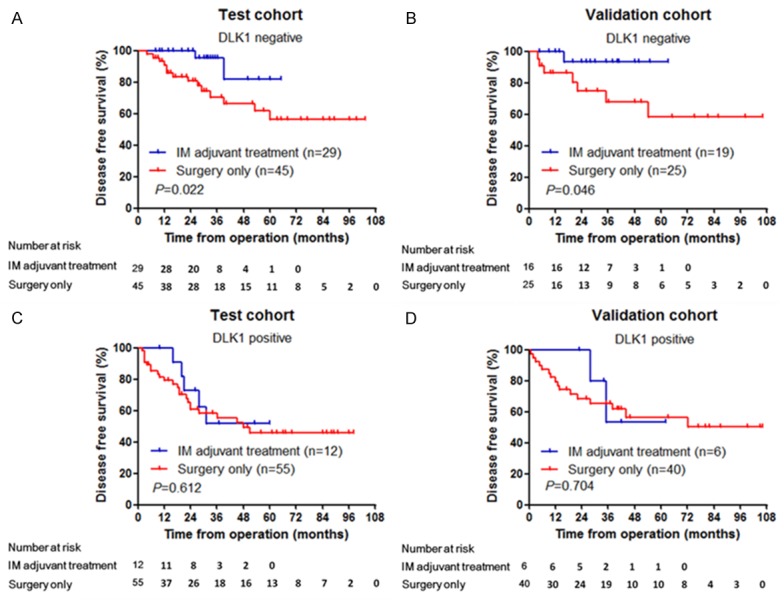
Correlation between DLK1 expression and the efficacy of imatinib adjuvant treatment in intermediate- and high-risk GIST patients. A: In the test cohort, among 74 DLK1-negative intermediate- and high-risk GIST patients, DFS of the patients with imatinib adjuvant treatment was significantly higher than that of the patients without imatinib adjuvant treatment (P = 0.022). B: In the validation cohort, among 41 DLK1-negative intermediate- and high-risk GIST patients, DFS of the patients with imatinib adjuvant treatment was significantly higher than that of the patients without imatinib adjuvant treatment (P = 0.046). C: In the test cohort, among 67 DLK1-positive intermediate- and high-risk GIST patients, there was no significant difference in DFS between the groups with or without imatinib adjuvant treatment (P = 0.612). D: In the validation cohort, among 46 DLK1-positive intermediate- and high-risk GIST patients, there was no significant difference in DFS between the groups with or without imatinib adjuvant treatment (P = 0.704).
Discussion
According to the NCCN guidelines, all GISTs have malignant potential. However, substantial differences exist in the biological behaviours among individual GISTs. The vast majority of low-risk GIST patients have good outcomes, and some very small GISTs do not even require clinical intervention, as they do not progress during life-long follow-up. On the contrary, high-risk GISTs, as determined pathologically, have poor outcomes, and before the advent of IM, more than half of patients still had tumour recurrence within two years even if the tumour was completely resected by surgery [11]. Therefore, accurate assessment and prediction for postoperative recurrence risk of GIST has great implications for the clinical guidance of follow-up treatment. Presently, the tumour location, tumour size, and mitotic figure count are commonly used as the major reference indicators for assessing the GIST risk level in clinics. However, the above indicators are often easily affected by subjective factors, such as the experience of the doctors in pathology [12]. Therefore, Identifying molecular biomarkers associated with the recurrence risk of GIST can provide objective diagnostic indicators for predicting tumour recurrence, further complementing and improving the existing risk evaluation criteria [13,14].
The DLK1 gene encodes a transmembrane protein in the epidermal growth factor (EGF) superfamily. The protein structure and amino acid sequence exhibit high homology with the corresponding structures of Notch ligand (Delta) in Drosophila melanogaster. The transmembrane protein contains an intracellular signal peptide, an intermediate transmembrane region and an extracellular region. The extracellular region is constituted by four EGF-like tandem repeats [15]. A large number of experiments have demonstrated that DLK1 is involved in regulating the differentiation of various cells; DLK1 exhibits high expression in the precursors or stem cells of many tissues. Additionally, DLK1 can be used as a recognition target for isolating and identifying stem cells [16]. Moreover, DLK1 expressed primarily during early embryonic development. As the embryo develops and matures, the tissue expression of DLK1 gradually decreases. In adult tissues, few cells express DLK1, especially pancreatic B cells, adrenal medulla, anterior pituitary, placenta, testis, and ovary cells. There is no DLK1 expression in normal adult gastrointestinal tract tissues [17]. In addition to its normal physiological function, DLK1 has received more attention from researchers for its abnormal expression in a few tumours. In 1993, Laborda first discovered and cloned the DLK1 gene from neuroblastoma cells [18]. In the following studies of other types of tumours, abnormal expression of DLK1 was observed in many tumours, especially those with neuroendocrine properties [5-7]. Yanai et al. [19] found that different levels of DLK1 overexpression existed in various tumour tissues, as which was revealed by IHC analysis. In colon, small cell lung, breast, and pancreatic tumour tissues, the positive rates of DLK1 in the total cell count were 58.6%, 52.5%, 39.0% and 30.8%, respectively. Furthermore, DLK1 expression is associated with the prognostic outcome of a few tumours. Jin et al. [20] reported that in primary hepatic cancer, patients with positive DLK1 expression in tumour tissue was significantly lower OS than those negative for DLK1 expression; multivariate analysis also showed that positive expression of DLK1 was an independent risk factor influencing the survival rate of patients with hepatic cancer. The above studies suggest that DLK1 meets the necessary requirements as a molecular marker for tumour diagnosis. However, to date, no study has reported DLK1 expression in GISTs.
In this study, tumour specimens were collected from GIST patients admitted to and treated in our hospital over approximately the last decade. Combined with the corresponding clinicopathological and follow-up data, the large-scale screening and validation showed that DLK1 is a molecular marker that is associated with the risk level of GIST. First, we detected DLK1 expression in tumour tissue by PCR analysis of the discovery cohort including 36 GIST patients with different risk levels. In GIST tumour tissue of the low-risk group, the DLK1 gene was expressed at significantly low levels with nearly no expression; as the tumour risk level increased, the proportion of patients with a high expression of DLK1 gene gradually increased. The mean level of DLK1 gene expression was significantly higher in the high-risk group than in the low-risk group. Similarly, the Western blotting analysis demonstrated that DLK1 protein expression was significantly higher in tumour tissue of the high-risk GIST group than the low-risk GIST group. We further verified the relationship between the expression of DLK1 and the recurrence risk of GIST by large-scale tissue microarray and IHC analysis in the test and validation cohorts. A comparative analysis of the clinicopathological features showed that the positive expression ratio of DLK1 significantly increased with increases in tumour size, mitotic figure count, NIH rise level, and Ki67 expression. All of the above factors are known to be pathological indicators that are closely associated with the recurrence risk of GIST [21,22]. Thus, our results suggest that DLK1-positive GISTs have a higher degree of invasive and malignancy than DLK1-negative GISTs. The subsequent survival curve analysis further demonstrated that in either the test or validation cohort, DLK1-positive patients had significantly lower DFS and OS than DLK1-negative patients. Moreover, COX multivariate survival analysis showed that DLK1 was an independent risk factor predicting the postoperative recurrence of GIST. Together, the above analysis fully demonstrates that DLK1 exhibits selective high expression in high-risk tumour tissue of GIST, and can be served as a prognostic predictor of GIST patients.
To date, the DLK1 expression level in GISTs has never been studied; therefore, we do not know the exact mechanism of action in the evolution of this disease. We do know from the literature, however, that DLK1 has high homology with the ligand Delta of the Notch signalling pathway. Although DLK1 lacks a DSL domain to bind Notch, it still can bind the Notch receptor through the extracellular EGF-like tandem structure of Notch. This process competitively inhibits the binding of other ligands to Notch1, playing a regulatory role in the Notch pathway [23]. Furthermore, DLK1 is able to regulate the bioactivity of insulin-like growth factor (IGF) through binding IGF binding proteins [24]. Through the above mechanisms, DLK1 is involved in regulating various cellular differentiation processes and is associated with the proliferation activity of tumour cells as well as the regulation of the cell cycle [25,26]. In the present study, we also found that DLK1 expression was associated with cell proliferation cycle-related indicators, such as mitotic figure count and Ki67 expression. This observation suggests that high expression of DLK1 may increase the proliferation activity of GIST tumour cells and enhance the malignant potential of the tumour. However, the above speculations about the functional mechanism of DLK1 in GIST must be verified through further in vitro and in vivo studies.
In addition to the effect on GIST recurrence risk, the present study found that a significant difference existed in the efficacy of postoperative IM adjuvant therapy between DLK1-negative and DLK1-positive patients. It was confirmed in both the test and validation cohorts that DLK1-negative patients receiving postoperative IM adjuvant therapy had a significantly higher DFS than those receiving simple surgical treatment. Among the DLK1-positive patients, however, there was no significant difference in the DFS curve between the IM adjuvant therapy and simple surgical treatment subgroups. According to ACOSOG Z9001, the US Federal Drug Administration and the European Medicines Agency approved IM for postoperative adjuvant therapy of intermediate/high-risk GIST, and IM has become the standard postoperative treatment regimen for GIST [27]. However, an accurate assessment of the drug efficacy prior to IM adjuvant therapy is essential because of the high cost of treatment and side effects of drugs in targeted drug therapy. The present study suggests that in addition to the currently used KIT and PDGFRA gene mutation tests, DLK1 could be used as a potential biomarker for precise guidance of IM adjuvant therapy. For intermediate/high-risk GIST patients with negative expression of DLK1, postoperative IM adjuvant therapy significantly reduced the long-term recurrence rate; however, for patients with positive expression of DLK1, the efficacy of IM adjuvant therapy was not significant. It is worth noting that the vast majority of patients in the present study only received one year of IM adjuvant therapy and that DLK1-positive patients suffering tumour recurrence all had the recurrent event after withdrawal of IM. Thus, future studies should examine whether the period of postoperative IM adjuvant therapy should be extended or if other targeted therapies should be selected for intermediate/high-risk GIST patients.
In conclusion, the present study examined the differential expression of DLK1 in R0 resection specimens from GIST tumour tissues of different risk levels. The results showed that the DLK1 expression was significantly increased in high-risk GISTs and was closely associated with the prognostic outcome of DLK1 patients as well as the efficacy of IM adjuvant therapy. In addition to the NIH risk classification, DLK1 expression can be used as an effective molecular biomarker to assess the recurrence risk of GIST in patients after surgery. Moreover, DLK1 has guidance value for individualised postoperative IM adjuvant therapy of GIST.
Acknowledgements
This study was supported by Project of Shanghai Health and Family Planning Commission (No. 201440610).
Disclosure of conflict of interest
None.
References
- 1.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–83. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 2.Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567–1572. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunawan B, von Heydebreck A, Sander B, Schulten HJ, Haller F, Langer C, Armbrust T, Bollmann M, Gasparov S, Kovac D, Füzesi L. An oncogenetic tree model in gastrointestinal stromal tumours (GISTs) identifies different pathways of cytogenetic evolution with prognostic implications. J Pathol. 2007;211:463–470. doi: 10.1002/path.2128. [DOI] [PubMed] [Google Scholar]
- 4.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 5.Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, Said JW, Black KL, Koeffler HP. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006;25:1852–1861. doi: 10.1038/sj.onc.1209219. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao CC, Huang CC, Sheen JM, Tai MH, Chen CM, Huang LL, Chuang JH. Differential expression of delta-like gene and protein in neuroblastoma, ganglioneuroblastoma and ganglioneuroma. Mod Pathol. 2005;18:656–662. doi: 10.1038/modpathol.3800335. [DOI] [PubMed] [Google Scholar]
- 7.Khoury H, Suarez-Saiz F, Wu S, Minden MD. An upstream insulator regulates DLK1 imprinting in AML. Blood. 2010;115:2260–2263. doi: 10.1182/blood-2009-03-212746. [DOI] [PubMed] [Google Scholar]
- 8.Falix FA, Aronson DC, Lamers WH, Hiralall JK, Seppen J. DLK1, a serum marker for hepatoblastoma in young infants. Pediatr Blood Cancer. 2012;59:743–745. doi: 10.1002/pbc.24024. [DOI] [PubMed] [Google Scholar]
- 9.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. Reporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–91. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 11.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–74. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 13.Braconi C, Bracci R, Bearzi I, Bianchi F, Sabato S, Mandolesi A, Belvederesi L, Cascinu S, Valeri N, Cellerino R. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293–1298. doi: 10.1093/annonc/mdn040. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi U, Nakayama R, Honda K, Ichikawa H, Hasegawa T, Shitashige M, Ono M, Shoji A, Sakuma T, Kuwabara H, Shimada Y, Sasako M, Shimoda T, Kawai A, Hirohashi S, Yamada T. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J. Clin. Oncol. 2008;26:4100–8. doi: 10.1200/JCO.2007.14.2331. [DOI] [PubMed] [Google Scholar]
- 15.Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23:1717–25. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishina H. hDlk-1: a cell surface marker common to normal hepatic stem/progenitor cells and carcinomas. J Biochem. 2012;152:121–123. doi: 10.1093/jb/mvs069. [DOI] [PubMed] [Google Scholar]
- 17.da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24:306–16. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Laborda J, Sausville EA, Hoffman T, Notario V. DLK, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem. 1993;268:3817–3820. [PubMed] [Google Scholar]
- 19.Yanai H, Nakamura K, Hijioka S, Kamei A, Ikari T, Ishikawa Y, Shinozaki E, Mizunuma N, Hatake K, Miyajima A. Dlk-1, a cell surface antigen on foetal hepatic stem/progenitor cells, is expressed in hepatocellular, colon, pancreas and breast carcinomas at a high frequency. J Biochem. 2010;148:85–92. doi: 10.1093/jb/mvq034. [DOI] [PubMed] [Google Scholar]
- 20.Jin ZH, Yang RJ, Dong B, Xing BC. Progenitor gene DLK1 might be an independent prognostic factor of liver cancer. Expert Opin Biol Ther. 2008;8:371–7. doi: 10.1517/14712598.8.4.371. [DOI] [PubMed] [Google Scholar]
- 21.Rossi S, Miceli R, Messerini L, Bearzi I, Mazzoleni G, Capella C, Arrigoni G, Sonzogni A, Sidoni A, Toffolatti L, Laurino L, Mariani L, Vinaccia V, Gnocchi C, Gronchi A, Casali PG, Dei Tos AP. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35:1646–56. doi: 10.1097/PAS.0b013e31822d63a7. [DOI] [PubMed] [Google Scholar]
- 22.Zhao WY, Xu J, Wang M, Zhang ZZ, Tu L, Wang CJ, Lin TL, Shen YY, Liu Q, Cao H. Prognostic value of Ki67 index in gastrointestinal stromal tumors. Int J Clin Exp Pathol. 2014;7:2298–304. [PMC free article] [PubMed] [Google Scholar]
- 23.Falix FA, Aronson DC, Lamers WH, Gaemers IC. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim Biophys Acta. 2012;1822:988–995. doi: 10.1016/j.bbadis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Nueda ML, García-Ramírez JJ, Laborda J, Baladrón V. DLK1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 cells. J Mol Biol. 2008;379:428–442. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Zhang X, Zhang M, Zhu JD, Zhang YL, Lin Y, Wang KS, Qi XF, Zhang Q, Liu GZ, Yu J, Cui Y, Yang PY, Wang ZQ, Han ZG. Up-regulation of DLK1 as an imprinted gene could contribute to human hepatocellular carcinoma. Carcinogenesis. 2007;28:1094–1103. doi: 10.1093/carcin/bgl215. [DOI] [PubMed] [Google Scholar]
- 26.Nueda ML, Baladrón V, Sánchez-Solana B, Ballesteros MA, Laborda J. The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J Mol Biol. 2007;367:1281–1293. doi: 10.1016/j.jmb.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 27.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]