Abstract
Several studies have demonstrated that YWHAZ (14-3-3ζ), included in the 14-3-3 family of proteins, is implicated in the initiation and progression of cancers. To detect a novel treatment target for adenocarcinoma of the esophagogastric junction (AEG), we tested whether YWHAZ acted as a cancer-promoting gene through its overexpression in AEG. We analyzed YWHAZ protein expression in 92 consecutive primary AEG tumors, which had been curatively resected in our institution between 2000 and 2010. Overexpression of the YWHAZ protein was frequently detected in primary AEG tumor samples (46% (42/92)). Overexpression of YWHAZ was significantly correlated with Siewert type III tumor, larger tumor size (≥40 mm) and higher rates of lymph node metastasis and recurrence. Patients with YWHAZ-overexpressing tumors had a worse overall rate of survival than those with non-expressing tumors (P = 0.011, log-rank test) in an intensity expression-dependent manner. Patients with YWHAZ-overexpression tumors had worse overall survival rates than those with lower-expression tumors. YWHAZ positivity was independently associated with a worse outcome in the multivariate analysis (P = 0.0015, hazard ratio 4.49 [1.736-13.06]). In conclusion, YWHAZ plays a crucial role in poor outcomes of patients with AEG through its overexpression, which highlights its usefulness as a prognosticator and potential therapeutic target and indicator in AEG.
Keywords: YWHAZ (14-3-3), adenocarcinoma of the esophago-gastric junction, malignant outcome, prognostic factor
Introduction
Over the past few decades, adenocarcinoma of the esophago-gastric junction (AEG) has noticeably increased in Western and Eastern countries [1-5]. In spite of the improvement of diagnosis and treatment technologies such as extended radical resection and chemo and/or chemoradiotherapy, many AEG patients frequently develop metastasis and experience recurrence, and the long-term survival remains poor because of the aggressive and systemic nature of this disease [6].
Various genes have been analyzed to understand the molecular mechanisms of carcinogenesis and improve clinical outcomes for adenocarcinoma of the esophagus and esophago-gastric junction. Various genes with frequent alterations and molecular functions have been identified [7] such as amplification/overexpression of EGFR and ERBB2 [8], hypermethylation or mutation of p16, APC and TP53 [9,10] and overexpression/activation of c-Met and β-catenin [11]. However, in clinical settings, only a few genes have been used as diagnostic biomarkers and/or therapeutic targets [12]. We, therefore, wished to identify novel genes associated with the progression of AEG.
The YWHAZ gene, which encodes the 14-3-3ζ protein, is located on chromosome 8q22.3, and this area is frequently amplified in breast and other cancers [13,14]. YWHAZ has been identified as a clinically relevant prognostic marker for breast cancer, lung cancer, head and neck cancer and hepatocellular carcinoma [15-19] and may allow for the identification of patients with a potentially poor prognosis to receive more aggressive treatment. Recently, we reported that YWHAZ has a crucial role in tumor cell proliferation, migration and invasion through its overexpression associated with the lower expression of miR-375 and highlighted its usefulness as a prognostic factor and potential therapeutic target in gastric cancer [20]. These findings prompted us to determine the clinicopathological and prognostic significance of YWHAZ overexpression/activation in primary AEG. However, to date, there has been no report on the clinical significance of YWHAZ in patients with primary AEG. In this study, we tested whether YWHAZ acted as a cancer-promoting gene through its activation/overexpression in AEG. Our results provided evidence that YWHAZ could be an important molecular marker for determining the malignant properties and a target for molecular therapy in patients with AEG.
Materials and methods
Primary AEG tissue samples
Primary tumor samples of AEG were obtained from 92 consecutive AEG patients, who had undergone curative resection at the Division of Digestive Surgery, Department of Surgery, Kyoto Prefectural University of Medicine (Kyoto, Japan) between 2000 and 2010. Samples were embedded in paraffin after 24 h of formalin fixation. Relevant clinical and survival data were available for all patients. Written consent was always obtained in the formal style and after approval by the local ethics committee. None of these patients underwent endoscopic mucosal resection, palliative resection, preoperative chemotherapy or radiotherapy, and none of them had synchronous or metachronous multiple cancers in other organs. Disease stage was defined in accordance with the International Union Against Cancer tumor-lymph node-metastases (TNM) classification (7th edition) [21]. The mean follow-up period for surviving patients was 46.1 months. In Japanese and Asian patients with AEG defined by the Siewert classification [22], most AEG tumors were classified as a Siewert type II or III [3,4]. Therefore, in this study, we examined these tumors.
Treatments following curative gastrectomy
Of all Stage II or more AEG patients, 37 patients (63% (37/58) received adjuvant chemotherapy while 18 patients (31% (18/58) did not. Eighteen patients received S-1 alone or S-1 based chemotherapy such as S-1 plus cisplatin, S-1 plus taxane or S-1 plus Krestin; five patients received methotrexate plus 5-fluorouracil; four patients received cisplatin plus 5-fluorouracil; four patients received doxifluridine; three patients received uracil-tegafur; two patients received 5-fluorouracil and one patient received taxane as adjuvant chemotherapy. In patients with pStage II or more AEG, there was no significant prognostic difference between the 37 patients with adjuvant chemotherapy and the 18 patients without (P = 0.3044). None of the patients received neoadjuvant chemotherapy, adjuvant radiotherapy or chemoradiotherapy. All patients were examined in the outpatient clinic, in which abdominal ultrasound, computed tomography (CT) and measurements of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels were performed every 3-6 months after surgery.
Immunohistochemistry
Tumor samples were fixed with 10% formaldehyde in PBS, embedded in paraffin, sectioned into 5-µm thick slices and subjected to immunohistochemical staining of the YWHAZ protein with the avidin-biotin-peroxidase method as described by Naoi et al [23,24]. In brief, after deparaffinization, endogenous peroxidases were quenched by incubating the sections for 20 min in 3% H2O2. Antigen retrieval was performed by heating the samples in 10 mmol/L citrate buffer (pH 6.0) at 95°C for 60 min. After treatment with Block Ace (Dainippon Sumitomo Pharmaceutical, Osaka, Japan) for 30 min at room temperature, sections were incubated at 4°C overnight with an anti-YWHAZ (1:500) antibody. The avidin-biotin-peroxidase complex system (Vectastain Elite ABC universal kit; Vector Laboratories Inc., Burlingame, CA) was used for color development with diaminobenzidine tetrahydrochloride. Slides were counterstained with Mayer’s hematoxylin. A formalin-fixed gastric cancer cell line overexpressing YWHAZ (MKN28), in which >50% of cells showed staining of each protein, was used as a positive control, whereas a formalin-fixed gastric cancer cell line with low expression of YWHAZ (HGC27) and MKN28 staining without the YWHAZ antibody was included as a negative control [20].
To evaluate YWHAZ expression, in the percentage of tumor cells showing YWHAZ immunopositivity, primary tumors with at least 10% or more of the total cell population was judged positive, and less than 10% was judged negative. For the intensity of YWHAZ expression, the intensity score (0 = negative, 1 = weak, 2 = moderate, 3 = strong) was examined. Namely, primary tumors with non-detectable YWHAZ expression, which was similar to that of non-tumorous mucosa and stroma, were given an intensity score of 0, whereas those with the greatest YWHAZ expression were given an intensity score of 3. The remaining tumors were categorized with intensity scores of 1 or 2 according to the intensity of immunohistochemical staining for YWHAZ. The expression of YWHAZ was regarded as high expression if both intensity scores were ≥2 and ≥10% of tumor cells showed immunopositivity or low expression if intensity scores ≤1 were and/or <10% using high-powered (×200) microscopy [25,26]. The expression of YWHAZ was evaluated by considering the status of both cytoplasmic and nuclear expression. If YWHAZ protein expression was recognized in both the cytoplasm and nucleus, the highest level of expression in the cytoplasm and nucleus was employed. Also, if YWHAZ protein expression was recognized in either the cytoplasm or nucleus, the level of expression was employed.
Statistical analysis
Clinicopathological variables pertaining to the corresponding patients were analyzed for statistical significance using the chi-squared test or Fisher’s exact test (Tables 1 and 2). Survival curves were estimated using the Kaplan-Meier method, and statistical differences were examined using the log-rank test (Figure 1B and 1C). Univariate and multivariate survival analyses were performed using the likelihood ratio test of the stratified Cox proportional hazards model. The data were stratified for multivariate analysis using both forward and backward stepwise Cox regression procedures (Table 3). Differences were assessed with a two-sided test and were considered statistically significant at P <0.05.
Table 1.
Association between clinicopathologic characteristics and YWHAZ expression
| N | YWHAZ immunoreactivity | * P-value | |||
|---|---|---|---|---|---|
|
| |||||
| High expression | Low expression | ||||
| Total | 92 | 42 | 50 | ||
| Sex | Male | 71 | 31 (74%) | 40 (80%) | |
| Female | 21 | 11 (26%) | 10 (20%) | 0.4810 | |
| Age (y) | Mean | 67 (range:37-83) | |||
| <65 | 38 | 18 (43%) | 20 (40%) | ||
| >65 | 54 | 24 (57%) | 30 (60%) | 0.7816 | |
| Siewert type | Type II | 65 | 25 (60%) | 40 (80%) | |
| Type III | 27 | 17 (40%) | 10 (20%) | 0.0317 | |
| Histology | Differentiated | 50 | 23 (55%) | 27 (54%) | |
| Undifferentiated | 42 | 19 (45%) | 23 (46%) | 0.9417 | |
| Tumor size (mm) | <40 | 35 | 11 (26%) | 24 (48%) | |
| ≥40 | 57 | 31 (74%) | 26 (52%) | 0.0319 | |
| Venous invasion | v0 | 44 | 17 (40%) | 27 (54%) | |
| v1-3 | 48 | 25 (60%) | 23 (46%) | 0.1958 | |
| Lymphatic invasion | ly0 | 39 | 17 (40%) | 22 (44%) | |
| ly1-3 | 53 | 25 (60%) | 28 (56%) | 0.7333 | |
| TNM classification | |||||
| PT categories | pT1 | 28 | 8 (19%) | 20 (20%) | |
| pT2 | 11 | 4 (10%) | 7 (14%) | ||
| pT3 | 27 | 14 (33%) | 13 (26%) | ||
| pT4 | 26 | 16 (38%) | 10 (20%) | 0.0807 | |
| PN categories | pN0 | 51 | 18 (43%) | 33 (66%) | |
| pN1 | 9 | 4 (10%) | 5 (10%) | ||
| pN2 | 11 | 9 (21%) | 2 (4%) | ||
| pN3 | 21 | 11 (26%) | 10 (20%) | 0.0386 | |
| PStage | I | 34 | 10 (24%) | 24 (48%) | |
| II | 23 | 9 (21%) | 14 (28%) | ||
| III | 35 | 23 (55%) | 12 (24%) | 0.0079 | |
| Rucurrence | Absent | 62 | 22 (52%) | 40 (80%) | |
| Present | 30 | 20 (48%) | 10 (20%) | 0.0049 | |
Note: Statistically significant values are in boldface type.
P-values are from χ2 or Fisher’s exact test and were statistically significant at <0.05.
Table 2.
Association between recurrence type and YWHAZ expression
| N | YWHAZ immunoreactivity | * P-value | |||
|---|---|---|---|---|---|
|
| |||||
| Low expression | High expression | ||||
| Total | Absent | 62 | 22 (52%) | 40 (80%) | |
| Present | 30 | 20 (48%) | 10 (20%) | 0.0049 | |
| Hematogenous | |||||
| Absent | 85 | 38 (90%) | 47 (94%) | ||
| Present | 7 | 4 (10%) | 3 (6%) | 0.5254 | |
| Distant orlocal lymph node | |||||
| Absent | 84 | 35 (83%) | 49 (98%) | ||
| Present | 8 | 7 (17%) | 1 (2%) | 0.0129 | |
| Peritoneal | |||||
| Absent | 83 | 35 (83%) | 48 (96%) | ||
| Present | 9 | 7 (17%) | 2 (4%) | 0.0416 | |
| Local | |||||
| Absent | 91 | 41 (98%) | 50 (100%) | ||
| Present | 1 | 1 (2%) | 0 (0%) | 0.2726 | |
| Other | |||||
| Absent | 86 | 41 (98%) | 46 (92%) | ||
| Present | 5 | 1 (2%) | 4 (8%) | 0.2363 | |
Note: Statistically significant values are in boldface type;
P-values are from χ2 or Fisher’s exact test and were statistically significant at <0.05.
Figure 1.
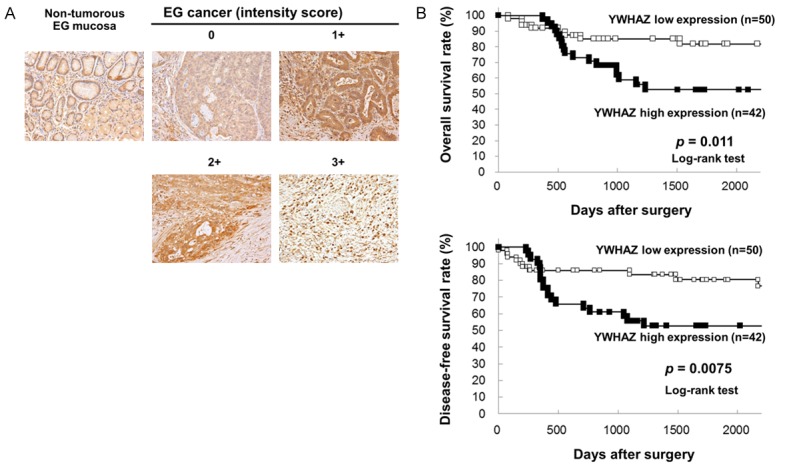
Immunohistochemical-staining analyses and postoperative overall and disease-free survival curves according to the expression of YWHAZ. (A) Specific immunostaining of the YWHAZ protein in primary samples was confirmed. Expression of the DTL protein was observed in both the cytoplasm and nucleus of cancer cells. For scoring YWHAZ expression, the intensity score was defined as 0 = negative, 1 = weak, 2 = moderate, 3 = strong. The YWHAZ high expression group had a significantly poorer prognosis than the low expression group in overall survival (P = 0.011, log-rank test) (B) and disease-free survival (P = 0.0075, log-rank test) (C).
Table 3.
Result of a survival analysis with Cox’s proportional hazard model
| Univariatea | Multivariateb | |||
|---|---|---|---|---|
|
| ||||
| P-value | HRc | 95% CId | P-value | |
| Sex | ||||
| Male vs Female | 0.9427 | - | ||
| Age | ||||
| ≥65 vs <65 | 0.7749 | 2.74 | 1.134-6.967 | 0.0250 |
| Siewert type | ||||
| Type2 vs Type3 | 0.8131 | - | ||
| Histological type | ||||
| Undiffe. vs Diffe. | 0.0486 | - | ||
| Tumor size (mm) | ||||
| ≥40 vs <40 | 0.0185 | - | ||
| Venous invasion | ||||
| Positive vs Negative | <0.0001 | - | ||
| Lymphatic invastion | ||||
| Positive vs Negative | <0.0001 | 5.28 | 1.431-25.51 | 0.0116 |
| PT-stage | ||||
| T4 vs T1-3 | <0.0001 | 4.74 | 2.004-12.21 | 0.0003 |
| PN-stage | ||||
| N3 vs N0-2 | <0.0001 | 3.28 | 1.354-8.607 | 0.0081 |
| YWHAZ expression | ||||
| High vs Low | 0.0108 | 4.49 | 1.736-13.06 | 0.0015 |
Kaplan and Meier method, and the statistical significance was determined by log-rank test;
Multivariate survival analysis was performed using Cox’s proportional hazard model;
HR: hazard ratio;
CI: confidence interval.
Results
Clinicopathological characteristics of AEG patients
The clinical characteristics in 92 consecutive patients with AEG were as follows. Of 92 patients, 65 patients were defined as Siewert type II and 27 patients as Siewert type III based on the tumor location. The study group consisted of 71 male and 21 female patients with a median age of 67 years (range 37-83 years). The median number of retrieved lymph nodes was 29 (range: 2-93). Seventy three patients (79% (73/92)) had more than 15 retrieved lymph nodes. Of 92 patients, 34 patients were staged as pStage I, 23 patients as pStage II and 35 patients as pStage III.
Immunohistochemical analysis of YWHAZ expression in the primary AEG tumors
The clinicopathological significance of YWHAZ expression in primary tumor samples of AEG based on the immunohistochemical staining pattern of this protein was examined. YWHAZ expression was detected in the cytoplasm of AEG cells. We classified 92 AEG tumors into positive and negative groups according to the intensity of YWHAZ staining among tumor cells, as described in materials and methods. In primary cases, YWHAZ protein expression was negative in most of the non-tumorous esophago-gastric mucosal cell population. We divided 92 AEG tumors into a high expression group with both intensity scores ≥2 and ≥10% of tumor cells showing immunopositivity (n = 42 (46%)) and a low expression group with intensity scores ≤1 and/or <10% of tumor cells showing immunopositivity (n = 50 (54%)) according to the intensity of YWHAZ staining among tumor cells (Figure 1A). The high expression group had a significantly poorer prognosis than the low expression group for overall survival (P = 0.011, log-rank test) (Figure 1B) and disease-free survival (P = 0.0075, log-rank test) (Figure 1C).
Association between YWHAZ protein levels and clinicopathological characteristics in primary AEG
The relationship between the expression of the YWHAZ protein and clinicopathological characteristics is summarized in Table 1. The protein expression of YWHAZ was associated with a higher incidence of Siewert type III (P = 0.0317), a larger tumor (≥40 mm) (P = 0.0319), a deeper depth of invasion (P = 0.0807) and higher rates of lymph node metastasis (P = 0.0386) and recurrence (P = 0.0049), whereas the other characteristics including histological grade were not. Recurrences were evident in 30 (33%) of 92 patients. All 30 recurrent patients belonged to pStage II or more. Distant or local lymph node recurrence (YWHAZ high vs. low, 17% (7/42) vs. 2% (1/50), P = 0.0129) and peritoneal recurrence (YWHAZ high vs. low; 17% (7/42) vs. 4% (2/50), P = 0.0416) was found more frequently in YWHAZ high expression cancer. In contrast, there was no significant difference in the rate of hematogenous recurrence between patients with YWHAZ high and low expression (YWHAZ high vs. low; 10% (4/42) vs. 6% (3/50), P = 0.5254) (Table 2).
In the Cox proportional hazard regression model (Table 3), univariate analyses demonstrated that YWHAZ protein expression, venous invasion, lymphatic invasion, pT category and pN category were significantly associated with overall survival. When the data were stratified for multivariate analysis using the Cox-proportional hazards analysis procedures, YWHAZ immunoreactivity in tumor cells remained significant at P = 0.0015 (hazard ratio, 4.49 (1.736-13.06)) for overall survival in all patients, suggesting that YWHAZ immunoreactivity can be an independent predictor of overall survival.
Discussion
YWHAZ is included in the 14-3-3 family of proteins, which are a family of evolutionarily highly conserved acidic proteins expressed in all eukaryotic organisms [27]. Moore and Perez first discovered 14-3-3 in 1967 in fractionated soluble proteins from brain tissue [28]. In mammals, there are seven distinct isoforms, β, γ, ε, ζ, η, σ and τ, which are encoded by seven different genes. 14-3-3 proteins have been found to interact with target proteins involved in the regulation of multiple cellular processes, such as cell cycle control, protein trafficking, anti-apoptosis, metabolism, signal transduction, inflammation and cell adhesion/motility [29,30]. YWHAZ (14-3-3ζ) has been identified as a clinically relevant prognostic marker for breast cancer [16,18], lung cancer [15], head and neck cancer [17], hepatocellular carcinoma [19] and may allow for the identification of patients to receive more aggressive treatments because their tumors are resistant to standard chemotherapies [31,32]. YWHAZ has been implicated in the initiation and progression of cancer and has been shown to be overexpressed in multiple cancer tissues and cell lines such as esophageal cancer [33], pancreatic cancer [34], colon cancer [35], oral cancer [36] and gastric cancer [20] even if gene amplification was not always detected. Mechanisms independent of the increased gene copy number, such as the modulation of gene transcription, protein translation or RNA and protein stability may also contribute to increased protein expression.
In the present study, it was hypothesized that the overexpression/activation of YWHAZ may be related to tumor cell proliferation and/or lower survival rate in patients with AEG. To test this hypothesis, we examined the expression status of YWHAZ in primary tumors of AEG. Consequently, it was demonstrated that YWHAZ was frequently overexpressed in 46% (42/92) of AEG patients, and overexpression of YWHAZ was significantly correlated with larger tumor size (≥40 mm), suggesting cell proliferation. Moreover, overexpression of YWHAZ was a poor prognosticator independent of other prognostic factors. The prognosis of AEG patients was correlated to the intensity of YWHAZ activity in an expression-dependent manner.
The most striking finding in this study was that the AEG patients with a high YWHAZ expression tumor had a significantly higher rate of lymph node metastasis and distant and/or local lymph node recurrence than those without. These results strongly suggested that patients with advanced AEG need a further treatment strategy to achieve better local tumor clearance and to improve prognosis. Recently, the multi-institutional phase II trial of neoadjuvant chemotherapy consisting of 4-weekly S-1plus cisplatin followed by surgery with para-aortic lymph node (PAN) dissection (JCOG0405) achieved safe results and much higher 3- and 5-year survival rates in some patients such as bulky clinical N2 (≥3 cm, or at least two adjacent tumors ≥1.5 cm) or PAN (≥1 cm) metastasis [37]. Mediastinal and/or para-aortic lymph node metastasis of advanced AEG is an important clinical issue [38,39], and a reliable indicator of prognosis and local tumor control for advanced AEG is needed [40]. By evaluating the tumor expression status of YWHAZ in biopsy specimens as an indicator of lymphatic spreading, neoadjuvant therapy potentially leads to downsizing or downstaging of the tumor. This may facilitate its complete resection, which is the cornerstone of cure in oncological surgery.
In conclusion, this is the first report to show that YWHAZ has a crucial oncogenic role and may be a potential therapeutic target and indicator in AEG. We demonstrated the frequent overexpression of the YWHAZ protein and its prognostic value in patients with AEG. Our study has a limitation because its results were retrospectively demonstrated using a single institutional cohort. The long accrual period of the retrospective analysis may reflect possible variations of the treatment. Therefore, studies of larger cohorts are needed to validate these findings before moving to a clinical setting. However, our results provide evidence that YWHAZ is an important molecular marker for determining malignant properties and is a target for molecular therapy in patients with this lethal disease.
Disclosure of conflict of interest
None.
References
- 1.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 2.Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, Shimoda T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662–1665. doi: 10.1111/j.1440-1746.2008.05572.x. [DOI] [PubMed] [Google Scholar]
- 3.Fang WL, Wu CW, Chen JH, Lo SS, Hsieh MC, Shen KH, Hsu WH, Li AF, Lui WY. Esophagogastric junction adenocarcinoma according to Siewert classification in Taiwan. Ann Surg Oncol. 2009;16:3237–3244. doi: 10.1245/s10434-009-0636-9. [DOI] [PubMed] [Google Scholar]
- 4.Hosokawa Y, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Kato Y, Daiko H, Nishimura M, Katsumata K, Sugiyama Y, Kinoshita T. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan. Ann Surg Oncol. 2012;19:677–683. doi: 10.1245/s10434-011-1983-x. [DOI] [PubMed] [Google Scholar]
- 5.Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–1158. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cen P, Banki F, Cheng L, Khalil K, Du XL, Fallon M, Amato RJ, Kaiser LR. Changes in age, stage distribution, and survival of patients with esophageal adenocarcinoma over three decades in the United States. Ann Surg Oncol. 2012;19:1685–1691. doi: 10.1245/s10434-011-2141-1. [DOI] [PubMed] [Google Scholar]
- 7.Clemons NJ, Phillips WA, Lord RV. Signaling pathways in the molecular pathogenesis of adenocarcinomas of the esophagus and gastroesophageal junction. Cancer Biol Ther. 2013;14:782–795. doi: 10.4161/cbt.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.al-Kasspooles M, Moore JH, Orringer MB, Beer DG. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer. 1993;54:213–219. doi: 10.1002/ijc.2910540209. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez MV, Artimez ML, Rodrigo L, Lopez-Larrea C, Menendez MJ, Alvarez V, Perez R, Fresno MF, Perez MJ, Sampedro A, Coto E. Mutation analysis of the p53, APC, and p16 genes in the Barrett’s oesophagus, dysplasia, and adenocarcinoma. J Clin Pathol. 1997;50:212–217. doi: 10.1136/jcp.50.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami K, Brabender J, Lord RV, Groshen S, Greenwald BD, Krasna MJ, Yin J, Fleisher AS, Abraham JM, Beer DG, Sidransky D, Huss HT, Demeester TR, Eads C, Laird PW, Ilson DH, Kelsen DP, Harpole D, Moore MB, Danenberg KD, Danenberg PV, Meltzer SJ. Hypermethylated APC DNA in plasma and prognosis of patients with esophageal adenocarcinoma. J Natl Cancer Inst. 2000;92:1805–1811. doi: 10.1093/jnci/92.22.1805. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MR, Harrison R, Atherfold PA, Campbell MJ, Darnton SJ, Obszynska J, Jankowski JA. Met receptor signaling: a key effector in esophageal adenocarcinoma. Clin Cancer Res. 2006;12:5936–5943. doi: 10.1158/1078-0432.CCR-06-1208. [DOI] [PubMed] [Google Scholar]
- 12.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 13.Garnis C, Coe BP, Ishkanian A, Zhang L, Rosin MP, Lam WL. Novel regions of amplification on 8q distinct from the MYC locus and frequently altered in oral dysplasia and cancer. Genes Chromosomes Cancer. 2004;39:93–98. doi: 10.1002/gcc.10294. [DOI] [PubMed] [Google Scholar]
- 14.Pollack JR, Sorlie T, Perou CM, Rees CA, Jeffrey SS, Lonning PE, Tibshirani R, Botstein D, Borresen-Dale AL, Brown PO. Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci U S A. 2002;99:12963–12968. doi: 10.1073/pnas.162471999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang H, Shen J, Zhao RY, Caraway NP, Katz RL, Stass SA, Jiang F. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007;67:7901–7906. doi: 10.1158/0008-5472.CAN-07-0090. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B, Ling C, Zhou X, Chen T, Chiao PJ, Feng X, Seewaldt VL, Muller WJ, Sahin A, Hung MC, Yu D. 14-3-3zeta Cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell. 2009;16:195–207. doi: 10.1016/j.ccr.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matta A, DeSouza LV, Shukla NK, Gupta SD, Ralhan R, Siu KW. Prognostic significance of head-and-neck cancer biomarkers previously discovered and identified using iTRAQ-labeling and multidimensional liquid chromatography-tandem mass spectrometry. J Proteome Res. 2008;7:2078–2087. doi: 10.1021/pr7007797. [DOI] [PubMed] [Google Scholar]
- 18.Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H, Lan KH, Ensor J, Hittelman W, Hung MC, Yu D. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69:3425–3432. doi: 10.1158/0008-5472.CAN-08-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichl P, Dengler M, van Zijl F, Huber H, Fuhrlinger G, Reichel C, Sieghart W, Peck-Radosavljevic M, Grubinger M, Mikulits W. Axl activates autocrine transforming growth factor-beta signaling in hepatocellular carcinoma. Hepatology. 2015;61:930–941. doi: 10.1002/hep.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura Y, Komatsu S, Ichikawa D, Nagata H, Hirajima S, Takeshita H, Kawaguchi T, Arita T, Konishi H, Kashimoto K, Shiozaki A, Fujiwara H, Okamoto K, Tsuda H, Otsuji E. Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br J Cancer. 2013;108:1324–1331. doi: 10.1038/bjc.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 22.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 23.Naoi Y, Miyoshi Y, Taguchi T, Kim SJ, Arai T, Maruyama N, Tamaki Y, Noguchi S. Connexin26 expression is associated with aggressive phenotype in human papillary and follicular thyroid cancers. Cancer Lett. 2008;262:248–256. doi: 10.1016/j.canlet.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu S, Imoto I, Tsuda H, Kozaki KI, Muramatsu T, Shimada Y, Aiko S, Yoshizumi Y, Ichikawa D, Otsuji E, Inazawa J. Overexpression of SMYD2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis. 2009;30:1139–1146. doi: 10.1093/carcin/bgp116. [DOI] [PubMed] [Google Scholar]
- 25.Tsuda H. Individualization of breast cancer based on histopathological features and molecular alterations. Breast Cancer. 2008;15:121–132. doi: 10.1007/s12282-008-0032-5. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Komatsu S, Ichikawa D, Kawaguchi T, Hirajima S, Miyamae M, Okajima W, Ohashi T, Kosuga T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Tsuda H, Otsuji E. Overexpression of denticleless E3 ubiquitin protein ligase homolog (DTL) is related to poor outcome in gastric carcinoma. Oncotarget. 2015;6:36615–36624. doi: 10.18632/oncotarget.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aitken A. 14-3-3 proteins: a historic overview. Semin Cancer Biol. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Moore BW, Perez VJ, Gehring M. Assay and regional distribution of a soluble protein characteristic of the nervous system. J Neurochem. 1968;15:265–272. doi: 10.1111/j.1471-4159.1968.tb11610.x. [DOI] [PubMed] [Google Scholar]
- 29.Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilker E, Yaffe MB. 14-3-3 Proteins--a focus on cancer and human disease. J Mol Cell Cardiol. 2004;37:633–642. doi: 10.1016/j.yjmcc.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Frasor J, Chang EC, Komm B, Lin CY, Vega VB, Liu ET, Miller LD, Smeds J, Bergh J, Katzenellenbogen BS. Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res. 2006;66:7334–7340. doi: 10.1158/0008-5472.CAN-05-4269. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zou L, Li Q, Haibe-Kains B, Tian R, Li Y, Desmedt C, Sotiriou C, Szallasi Z, Iglehart JD, Richardson AL, Wang ZC. Amplification of LAPTM4B and YWHAZ contributes to chemotherapy resistance and recurrence of breast cancer. Nat Med. 2010;16:214–218. doi: 10.1038/nm.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma R, Samantaray S, Shukla NK, Ralhan R. Transcriptional gene expression profile of human esophageal squamous cell carcinoma. Genomics. 2003;81:481–488. doi: 10.1016/s0888-7543(03)00023-5. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Person MD, Zhu J, Abbruzzese JL, Li D. Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res. 2004;64:9018–9026. doi: 10.1158/0008-5472.CAN-04-3262. [DOI] [PubMed] [Google Scholar]
- 35.Lu B, Xu J, Zhu Y, Zhang H, Lai M. Systemic analysis of the differential gene expression profile in a colonic adenoma-normal SSH library. Clin Chim Acta. 2007;378:42–47. doi: 10.1016/j.cca.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Arora S, Matta A, Shukla NK, Deo SV, Ralhan R. Identification of differentially expressed genes in oral squamous cell carcinoma. Mol Carcinog. 2005;42:97–108. doi: 10.1002/mc.20048. [DOI] [PubMed] [Google Scholar]
- 37.Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–660. doi: 10.1002/bjs.9484. [DOI] [PubMed] [Google Scholar]
- 38.Mine S, Sano T, Hiki N, Yamada K, Nunobe S, Yamaguchi T. Lymphadenectomy around the left renal vein in Siewert type II adenocarcinoma of the oesophagogastric junction. Br J Surg. 2013;100:261–266. doi: 10.1002/bjs.8967. [DOI] [PubMed] [Google Scholar]
- 39.Kurokawa Y, Hiki N, Yoshikawa T, Kishi K, Ito Y, Ohi M, Wada N, Takiguchi S, Mine S, Hasegawa S, Matsuda T, Takeuchi H. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery. 2015;157:551–555. doi: 10.1016/j.surg.2014.08.099. [DOI] [PubMed] [Google Scholar]
- 40.Komatsu S, Ichikawa D, Miyamae M, Kosuga T, Okamoto K, Arita T, Konishi H, Morimura R, Murayama Y, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Fujiwara H, Otsuji E. Positive Lymph Node Ratio as an Indicator of Prognosis and Local Tumor Clearance in N3 Gastric Cancer. J Gastrointest Surg. 2016;20:1565–71. doi: 10.1007/s11605-016-3197-9. [DOI] [PubMed] [Google Scholar]


