Abstract
The clinical potential of transplantation is often reduced by T cell-mediated alloresponses that cause graft rejection or graft-versus-host disease. Integrin-mediated adhesion between alloreactive T cells and antigen-presenting cells is essential for allorejection. The identity of the signaling events needed for the activation of integrins such as LFA-1 is poorly understood. Here, we identified a novel role of the protein tyrosine phosphatase SHP-1 in the regulation of murine LFA-1-mediated adhesion in an allograft setting. Upon alloactivation, SHP-1 activity is reduced, resulting in an increase in LFA-1 adhesion compared to that for syngeneically activated T cells. The importance of these differential activation properties was further indicated by small interfering RNA (siRNA) knockdown of SHP-1 in syngeneically and allogeneically stimulated T cells. Mechanistically, SHP-1 modulated the binding of SLP-76 to ADAP by dephosphorylation of the YDGI tyrosine motif of ADAP, a known docking site for the Src family kinase Fyn. This novel key role of SHP-1 in the regulation of LFA-1-mediated adhesion may provide a new insight into T cell-mediated alloresponses and may pave the way to the development of new immunosuppressive pharmaceutical agents.
INTRODUCTION
Recipient-derived antigen-presenting cells (APCs) are pivotal for the induction of alloresponses (1). In patients, alloresponses cause significant treatment-related morbidity and mortality, such as graft-versus-host disease (GVHD) or graft rejection. Alloreactive T cells are largely responsible for these detrimental effects (2). In this context, T cell function depends on integrin-mediated adhesion and migration.
The β2 integrins are preferentially expressed among the 12 integrins on lymphocytes. Of these, αLβ2 (leukocyte function-associated antigen 1 [LFA-1], also termed CD11a [αL chain of LFA-1]-CD18 [β2 chain of LFA-1]) binds to the ligands ICAM-1, -2, and -3 (intracellular adhesion molecules 1, 2, and 3). The ligands ICAM-1 and -2 are expressed on endothelial cells that line blood vessels on the surface of APCs (3). Following the initial adhesive interaction between potentially alloreactive T cells and allogeneic APCs such as dendritic cells (DCs), LFA-1 facilitates the stable formation of the “immunological synapse,” which enhances T cell activation and subsequent effector functions (4, 5). Hence, LFA-1 has emerged as an attractive therapeutic target for the treatment of various inflammatory diseases (6). Immunosuppressive effects induced by LFA-1 antagonists are of substantial interest, since ligation of a T cell receptor (TCR) generates intracellular signals leading to activation of LFA-1-mediated cell adhesion, a process termed “inside-out” signaling. So far, the molecular processes underlying the signaling events between TCR activation and LFA-1 clustering are not fully understood. Adaptor proteins such as ADAP have been identified as key molecules in the TCR “inside-out” pathway (7), and their potential influence in T cell alloreactivity has been discussed (8).
Here, we identified the protein tyrosine phosphatase (PTP) SHP-1 as a key regulator of LFA-1-mediated adhesion in primary murine T cells, with particular involvement in alloactivation. We demonstrate for the first time in vitro and in vivo that SHP-1 activity is significantly reduced upon alloactivation, resulting in an increase in the allogeneic activation of T cells and their adhesion to major histocompatibility complex (MHC)-mismatched APCs. Furthermore, we found that SHP-1 expression impairs the adhesion-associated signaling cascade via SLP-76→ADAP→LFA-1 by modulating a specific event, namely, the binding of SLP-76 to ADAP by dephosphorylation of the ADAP YDGI tyrosine motif. Our findings suggest the possible use of a novel pharmaceutic approach that specifically targets SHP-1 in the modulation of alloresponses in transplantation.
MATERIALS AND METHODS
Mice.
C57BL/6 (Charles River, Sulzfeld, Germany) and B10.A (Taconic Laboratories, Ry, Denmark) mice were used in agreement with approved protocols of the state government of Lower Saxony, Germany.
Bone marrow-derived DC generation.
Bone marrow was harvested from the long bones of the femur, tibia, and fibula of C57BL/6 or B10.A mice. Red cells were lysed in red blood cell (RBC) lysis buffer, and the single-cell suspension was washed with phosphate-buffered saline (PBS), incubated at 2 × 106 cells/ml in Iscove modified Dulbecco medium (IMDM) supplemented with 10% fetal calf serum (FCS), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 mg/ml streptomycin, 100 U/ml penicillin, 50 mg/ml gentamicin, 0.5 mg/ml amphotericin B (Fungizone), 0.05 mM 2-mercaptoethanol, 150 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF), and 6% supernatant from the interleukin-4 (IL-4)-expressing cell line EL4-IL-4 in 6-well plates for 8 days, and cultured for an additional 2 days with oligodeoxynucleotides with the sequence TCGTCGTTTTTCGGTCGTTTT as a maturing agent at 2 μg/ml. On day 10, DCs were harvested, washed 3 times with PBS, and used for priming of T cells.
Induction and expansion of T cells.
DCs derived from bone marrow of C57BL/6 (allogeneic stimulator) or B10.A (syngeneic stimulator) mice were added to splenocytes from B10.A mice (responder) at a ratio of 1:10 and cultured in serum-free AIM-V medium supplemented with 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 mg/ml streptomycin, 100 U/ml penicillin, 50 mg/ml gentamicin, 0.5 mg/ml amphotericin B, 0.05 mM 2-mercaptoethanol, 20 U/ml IL-2, and 4 ng/ml IL-7 in 24-well plates. After 5 days, magnetic beads coated with anti-CD3 and anti-CD28 antibodies prepared as described previously (9) were added to the culture (4 × 106 beads/ml) and left for 2 days. After removing the beads, the cells were expanded for 5 days, restimulated with the appropriate DCs for 1 day, and harvested.
Generation of fibroblasts.
Skin samples of B10.A mice were cut into squares of 0.5 to 1.0 cm. The skin slices were transferred into 6-well plates, weighted down with glass cover slides, and cultivated in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 mg/ml streptomycin, and 100 U/ml penicillin. The medium was changed every 2 to 3 days, and after 4 to 7 days, the skin slices were removed from the plates and the outgrowing cells were trypsinized, harvested, and cultured in DMEM.
The fibroblasts were genetically modified by insertion of an enhanced green fluorescent protein (eGFP)-expressing sequence via lentiviral transduction. Lentiviral particles were produced by transduction into HEK293T cells. To generate eGFP-expressing cells, the fibroblasts were transduced at passage 2 with the lentiviral particles (multiplicity of infection [MOI] of >20). Selection was achieved by treatment with 200 μg/ml Zeocin, and eGFP expression was confirmed by fluorescence microscopy. The eGFP-expressing fibroblasts were expanded until passage 5 to 8, and their purity was confirmed by fluorescence-activated cell sorter (FACS) analysis with an antibody specific for mouse embryonic fibroblasts (see Fig. 3A).
FIG 3.
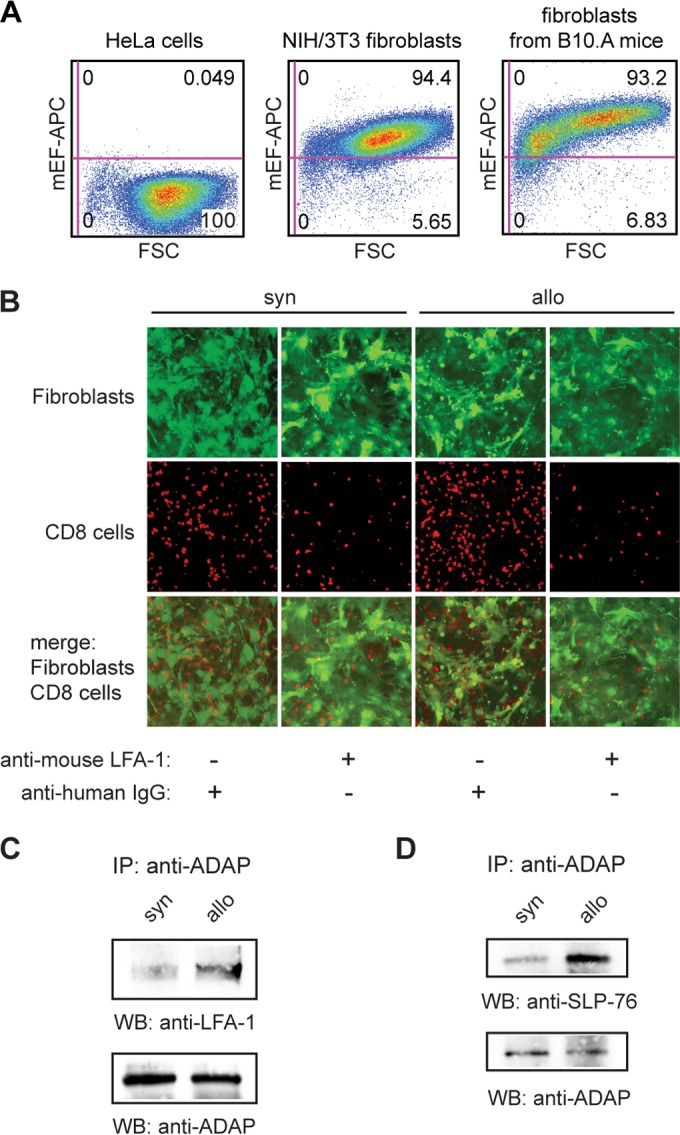
Alloactivation enhances LFA-1-mediated adhesion to MHC-mismatched fibroblasts and interaction of ADAP with LFA-1 and SLP-76. (A) Characterization of in vitro-generated and expanded fibroblasts by flow cytometry with an antibody specific for mouse embryonic fibroblasts (mEF) in comparison to HeLa cells and NIH 3T3 fibroblasts. (B) Syngeneically (syn) or allogeneically (allo) stimulated T cells were seeded on plates cultured with syngeneic eGFP-expressing fibroblasts. The mixed cultures were incubated with anti-human IgG (negative control) or anti-mouse LFA-1 antibody (each at 20 μg/ml) for 2 h at 37°C. After washing and fixing, the adherent T cells were probed with antibody against CD8 and, together with fibroblasts, were visualized by fluorescence microscopy. (C and D) Lysates of syngeneically (syn) or allogeneically (allo) stimulated T cells were immunoprecipitated (IP) and probed (WB) with antibodies as indicated.
Mixed-leukocyte reaction (MLR).
Splenocytes from C57BL/6 (allogeneic stimulator) or B10.A (syngeneic stimulator) mice were irradiated with 30 Gy and cocultured with splenocytes from B10.A mice (responder) at a ratio of 1:5 (4 × 105 stimulator cells were added to 2 × 106 responder cells in a total of 200 μl/well [96-well plates]) in RPMI medium supplemented with 10% FCS, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 mg/ml streptomycin, 100 U/ml penicillin, 50 mg/ml gentamicin, and 0.5 mg/ml amphotericin B for 48 h.
HCT.
Fully MHC-mismatched allogeneic or syngeneic hematopoietic stem cell transplantation (HCT) was performed using C57BL/6 (H-2b) mice as recipients and B10.A (H-2a) or C57BL/6 mice as donors. The recipients were cotransplanted with 15 × 106 (allogeneic) or 3 × 106 (syngeneic) T cell-depleted bone marrow cells and 20 × 106 allogeneic or syngeneic splenocytes by tail vein injection at 4 h after total body irradiation (10.5 Gy). To accelerate GVHD, allogeneically transplanted mice were injected again with 40 × 106 splenocytes after 8 days. Analysis of CD8+ T cells was performed between day 15 and 17 according to a clinical GVHD scoring system based on weight loss, skin integrity, posture, fur texture, and activity that was described previously (10).
Adhesion assays with recombinant ICAM-1.
Nunc MaxiSorp flat-bottom 96-well plates were coated with anti-human Fc antibody (Jackson ImmunoResearch, Hamburg, Germany) at 4 μg/ml (50 μl/well; diluted in TSM buffer [10 mM Tris-HCl {pH 8.0}, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2]) by incubation for 1 h at 37°C, washed once with 100 μl of TSM buffer, and blocked with 100 μl of 1% bovine serum albumin (BSA)–TSM buffer for 30 min at 37°C. Plates were washed once with 100 μl of TSM buffer and loaded overnight at 4°C with recombinant mouse ICAM-1/Fc (R&D Systems, Bad Nauheim, Germany; 4 μg/ml [50 μl/well] in TSM buffer). For controls, ICAM-1/Fc was replaced with 50 μl of TSM buffer per well. After being washed once with TSM buffer (100 μl/well), T cells (5 × 104 cells/well in 50 μl of PBS) were seeded into the plates and incubated for 1 h at 37°C, and nonadherent cells were removed by gently washing the plates 3 times with warmed 0.5% BSA–TSM (100 μl/well). Adherent cells were harvested from the plates using 50 mM EDTA–PBS (100 μl/well) and counted, and the adhesion was calculated.
Adhesion to fibroblasts and immunofluorescence microscopy.
Fibroblasts from B10.A mice (2 × 105) were seeded into 24-well plates with 1 ml DMEM. After 24 h, the fibroblasts were washed once with PBS (37°C), T cells (0.7 × 106 in 1 ml AIM-V medium [37°C]) were added, and the mixed culture was incubated for 2 h at 37°C. Thereafter, the cells were washed 3 times with AIM-V medium (37°C), fixed in 4% paraformaldehyde at room temperature for 1 min, and washed 3 times with PBS. The cells were then blocked in antibody dilution buffer (3% BSA, 0.1% Tween 20–PBS) for 15 min and incubated for 1 h with anti-CD8 antibody at room temperature. Thereafter, cells were washed 3 times with PBS, incubated with Rhodamine Red-X-conjugated secondary antibody in antibody dilution buffer for 30 min at room temperature, and finally washed three times with PBS. Images were obtained on an IX71 microscope with a 4× objective using CellP 2.8 software (Olympus, Hamburg, Germany).
PTP activity and dephosphorylation assays.
For PTP activity assays, T cells were lysed in cell lysis buffer (50 mM HEPES [pH 7.0], 150 mM NaCl, 1 mM MgCl2, 10% glycerol, 1% Triton X-100), and SHP-1, SHP-2, or ADAP was immunoprecipitated (IP) in IP buffer (20 mM HEPES [pH 7.2], 150 mM NaCl, 10% glycerol, 0.1% Triton X-100, 1 mM dithiothreitol [DTT]). The pellets were washed twice with IP buffer and twice with assay buffer (25 mM HEPES [pH 7.2], 50 mM NaCl, 2.5 mM EDTA, 5 mM DTT) and finally resuspended in 300 μl assay buffer. The assays were initiated by the addition of 20 mM para-nitrophenyl-phosphate (pNPP), and PTP activity was detected by measuring the conversion of pNPP at 405 nm after 30 min at 37°C. Dephosphorylation assays were performed with glutathione S-transferase (GST)–SHP-1 or GST–SHP-2 fusion protein as described previously (11).
siRNA-based knockdown of SHP-1.
For functional silencing experiments, T cells were transfected with SHP-1 small interfering RNA (siRNA) (pool of 3 target-specific 20- to 25-nucleotide [nt] siRNAs; Santa Cruz, Heidelberg, Germany) or control siRNA (Santa Cruz) by nucleofection (Lonza, Cologne, Germany) using an optimized protocol for murine T cells. In brief, 1 × 106 cells were washed once with PBS, resuspended in 100 μl Nucleofector solution supplemented with 200 pmol siRNA, transferred into an appropriate cuvette, and transfected with the Nucleofector program X-001. Transfected cells were transferred into 2 ml AIM-V medium additionally supplemented with component B (10 μg/ml; Lonza). After 48 h, the cells were used for assays as described below.
Statistical analysis.
Analyses were performed with an unpaired t test that compares the means of two groups.
Expression constructs and transient expression of protein.
The sequences of 5′ hemagglutinin (HA)-tagged human ADAP (accession number BC015933.1) and corresponding Y → F point mutants (synthesized by GenScript, Piscataway, NJ) were subcloned into BamHI- and XhoI-restricted vector pCS2+. Additional expression constructs were used: pJ3omega-SHP-1 WT and pJ3omega-SHP-1 C/S (gifts from Ben Neel, Toronto, ON, Canada), pRc/CMV-SHP-2 and pRc/CMV-SHP-2 C/S (gifts from Zhizhuang Joe Zhao, Oklahoma City, OK), and pRK5 c-Fyn (gift from Filippo Giancotti via Addgene, plasmid 16032).
Transient expression of protein in NIH 3T3 fibroblasts was performed with PolyFect transfection reagent (Qiagen, Hilden, Germany). Allogeneically stimulated T cells were transfected with HA-tagged wild-type ADAP or ADAP point mutants (Y595F, Y625F, and Y697F) by nucleofection (Lonza, Cologne, Germany) using an optimized protocol for murine T cells. In brief, 1 × 106 cells were washed once with PBS, resuspended in 100 μl Nucleofector solution supplemented with 2.5 μg plasmid, transferred into an appropriate cuvette, and transfected with the Nucleofector program X-001. Transfected cells were transferred into 2 ml AIM-V medium additionally supplemented with component B (10 μg/ml; Lonza). After 24 h, the cells were used for assays as described below.
Preparation of cell lysates, IPs, and Western blotting.
Cell extracts were prepared by solubilization in radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) containing protease and phosphatase inhibitors (10 μg/ml aprotinin, 5 μg/ml phenylmethylsulfonyl fluoride, 4 μg/ml leupeptin, 10 μg/ml antipain, 4 μg/ml pepstatin, 50 mM NaF, and 2 mM Na3VO4). Immunoprecipitations (IPs) from 0.5 to 3.0 mg total cell lysate were performed by incubation overnight at 4°C in IP buffer (20 mM Tris HCl [pH 7.5], 1 mM EDTA, 150 mM NaCl, 5% glycerol, 0.5% Tween 20) containing protease and phosphatase inhibitors as described above, 2 μg of rabbit polyclonal or mouse monoclonal antibody, and protein A-Sepharose or protein G-Sepharose, respectively. The pellets were washed 3 times with RIPA buffer, resuspended in sample buffer, and separated by SDS-PAGE. Antibodies used for precipitation and blotting are listed in detail below. For redetection with another antibody, the prior antibody was removed completely from the blot by incubation in blot-stripping buffer (60 mM Tris [pH 6.8], 2% SDS, 0.1% β-mercaptoethanol) for 20 min at 50°C while shaking gently.
Antibodies.
For precipitation, blotting, and immunostaining, the following antibodies were used: polyclonal antibodies to ADAP (07-546; Merck Millipore, Schwalbach, Germany), HA (sc-805; Santa Cruz, Heidelberg, Germany), LFA-1 (sc-15327; Santa Cruz), SHP-1 (sc-287; Santa Cruz), SHP-2 (sc-280; Santa Cruz), and c-Fyn (4023; CST, Frankfurt, Germany) and monoclonal antibodies to CD8 (554854; BD Bioscience, Heidelberg, Germany), GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (G8795; Sigma-Aldrich, Taufkirchen, Germany), phosphotyrosine pY99 (sc-7020; Santa Cruz), SHP-1 (sc-7289; Santa Cruz), SHP-2 (sc-7384; Santa Cruz), and SLP-76 (05-1426; Merck Millipore).
Flow cytometry.
Flow cytometry was performed using a FACSCanto instrument (BD Bioscience, Heidelberg, Germany). The following antibodies were used: rat anti-CD4–phycoerythrin (PE), rat anti-CD8–allophycocyanin (APC), rat anti-CD25–PE, rat anti-CD44–PE, rat anti-CD62L–PE, and hamster anti-CD69–PE (all from BD Bioscience, Heidelberg, Germany), mouse anti-LFA-1 (Bio X Cell, West Lebanon, NH), and rat anti-Feeder–APC (clone mEF-SK4; Miltenyi Biotec, Bergisch Gladbach, Germany).
For analysis of the enzymatic activity of SHP-1 in CD8+ thymocytes, anti-SHP-1 Y536 antibody (PAB25887; Abnova via Biozol, Eching, Germany) was used. In brief, for blocking of antibody Fc receptors, thymocytes from one mouse were preincubated with anti-CD16/32 antibody (BD Bioscience; 0.25 μg in 50 μl PBS) for 20 min at 4°C. The thymocytes were then stained with anti-CD8–PE antibody (BD Bioscience; 2 μg in 100 μl flow cytometry buffer [PBS, 0.5% BSA]) for 20 min at 4°C, washed once with PBS, and fixed in 2% paraformaldehyde (100 μl) for 10 s at room temperature (fixation was stopped by addition of 1 ml PBS). Thereafter, the cells were permeabilized in 0.2% Triton X-100–PBS (500 μl) for 5 min at room temperature, washed once with flow cytometry buffer, incubated with anti-SHP-1 Y536 antibody (1:200 dilution; 50 μl flow cytometry buffer) for 20 min at 4°C, washed once with flow cytometry buffer, incubated with Cy5 secondary antibody in flow cytometry buffer for 20 min at 4°C, and finally washed twice with flow cytometry buffer.
Purification of CD8+ T cells.
CD8+ T cells from splenocytes or MLR were purified by positive selection with the MiniMACS separation system (Miltenyi Biotech, Bergisch Gladbach, Germany).
RESULTS
Characterization of in vitro-generated and expanded T cells.
To study the role of SHP-1 in the regulation of LFA-1-mediated cell adhesion, we used in vitro-generated and ex vivo-expanded T cells. Their generation is based on the priming of naive T cells from B10.A mice (responder) with dendritic cells from C57BL/6 mice (allogeneic stimulator) or from B10.A mice (syngeneic stimulator). We have previously used this system to dissect the effect of in vitro-generated cytotoxic T cells (CTLs) on the graft-versus-leukemia effect or GVHD after adoptive T cell transfer across MHC barriers (12, 13).
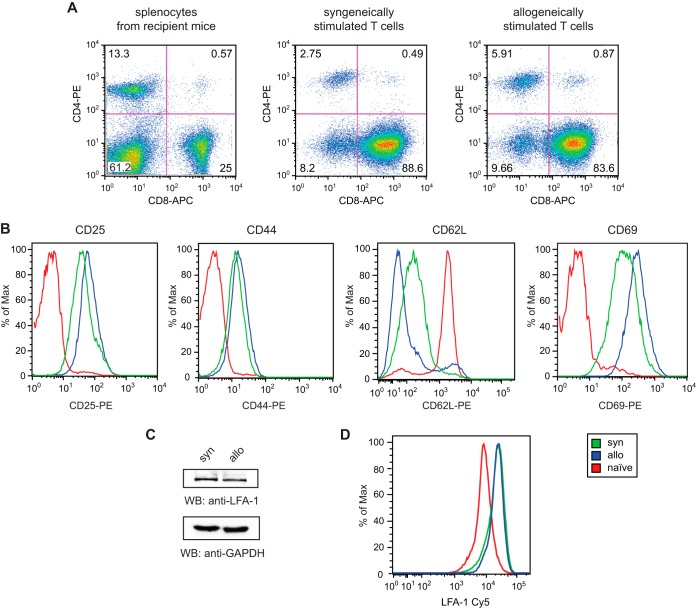
Since adhesion of CTLs to APCs is crucial for the development of alloresponses (14), we first analyzed the expression of the CTL-specific coreceptor CD8. FACS analysis showed that the majority of both syngeneically and allogeneically stimulated T cells expressed CD8 (Fig. 1A). CD8+ T cells were further examined for the expression of the T cell activation markers CD25, CD44, CD62L, and CD69. Syngeneically activated cells showed less CD25 and CD69 and much higher levels of CD62L than alloactivated cells, consistent with a more effective stimulation of alloactivated cells. Both T cell populations differed significantly from the phenotype of CD8+ naive T cells, which were used as a control (Fig. 1B).
FIG 1.
Characterization of in vitro-generated and expanded T cells by flow cytometry and Western blotting. (A) CD4/CD8 analysis of syngeneically or allogeneically stimulated T cells in comparison to splenocytes from B10.A mice. (B) Phenotype analysis of syngeneically (syn) or allogeneically (allo) stimulated CD8+ T cells compared to CD8+ naive T cells with antibodies as indicated. Naive T cells were recovered from the spleens of B10.A mice. (C) A total of 50 μg lysate from syngeneically (syn) or allogeneically (allo) stimulated T cells was analyzed by Western blotting (WB) using the antibodies indicated. GAPDH was used as a loading control. (D) Expression of LFA-1 on syngeneically (syn) or allogeneically (allo) stimulated CD8+ T cells and CD8+ naive T cells from the spleens of B10.A mice.
To further rule out that adhesion is mediated by unequal LFA-1 expression, we analyzed LFA-1 expression in stimulated T cells by Western blotting. LFA-1 was equally expressed in both T cell populations (Fig. 1C). In addition, we examined LFA-1 expression by flow cytometry. Syngeneically and allogeneically stimulated CD8+ cells showed identical levels of LFA-1, which were significantly elevated compared to those in CD8+ naive T cells from B10.A mice (Fig. 1D).
Alloactivation is associated with enhanced LFA-1-mediated adhesion and interaction of ADAP with LFA-1 and SLP-76.
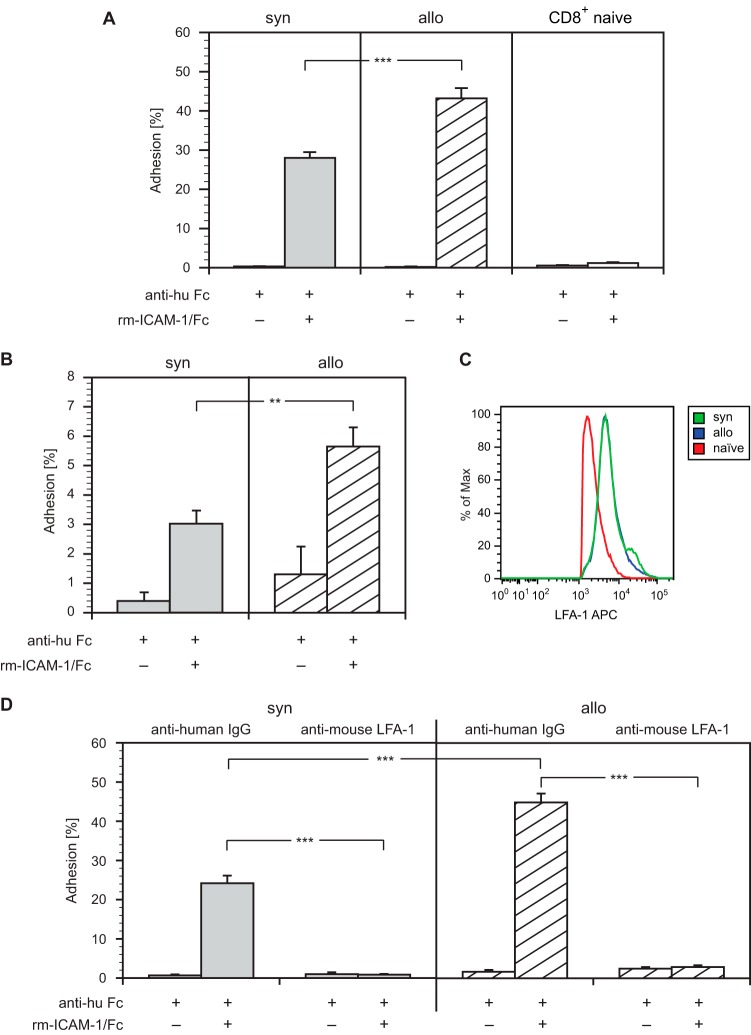
Besides TCR engagement, the interaction between LFA-1 and its ligand ICAM-1 is essential for mediating alloresponses of primed T cells (15). Consequently, LFA-1-mediated adhesion was used as a readout in our studies. First, we performed adhesion assays with recombinant ICAM-1 by incubating stimulated T cells or CD8+ naive T cells from the spleens of B10.A mice on plates coated with anti-human Fc antibody that had been reincubated with recombinant ICAM-1/Fc. As shown in Fig. 2A, the adhesion of allogeneically stimulated T cells was significantly higher than that of syngeneically stimulated cells. As expected, the adhesion of CD8+ naive T cells was negligible.
FIG 2.
Alloactivation enhances LFA-1-mediated adhesion to recombinant ICAM-1. (A) Syngeneically (syn) or allogeneically (allo) stimulated T cells and CD8+ naive T cells from the spleens of B10.A mice were incubated on plates coated with anti-human Fc antibody and then reincubated with recombinant mouse ICAM-1/Fc or buffer. After washing, bound cells were counted, and the adhesion was calculated by the ratio of bound cells to incubated cells. Error bars indicate standard error of the mean (SEM) (n = 10). (B) ICAM-1 adhesion of syngeneically (syn) or allogeneically (allo) stimulated CD8+ T cells after MLR was analyzed as described for panel A. Error bars indicate SEM (n = 10). (C) Expression of LFA-1 on syngeneically (syn) or allogeneically (allo) stimulated CD8+ T cells after MLR by flow cytometry. (D) Syngeneically (syn) or allogeneically (allo) stimulated T cells were incubated with anti-human IgG (negative control) or anti-mouse LFA-1 antibody (each at 20 μg/ml) for 30 min at 37°C, and then their adhesion to recombinant ICAM-1 was analyzed as described for panel A. Error bars indicate SEM (n = 20). ***, P < 0.001; **, 0.001 < P < 0.01.
To underscore the physiological relevance of our findings, which are based on long-term-cultured T cells, we performed a mixed-leukocyte reaction (MLR). Thus, B10.A responder splenocytes were cocultured with irradiated C57BL/6 (allogeneic) or B10.A (syngeneic) stimulator splenocytes. After 48 h, we harvested the responders, isolated the CD8+ cells, and analyzed them for ICAM-1-mediated adhesion. Alloreactive CD8+ T cells adhered significantly more strongly to recombinant ICAM-1 than syngeneic controls (Fig. 2B). To rule out that differences in adhesion were mediated by unequal LFA-1 expression, we analyzed LFA-1 by flow cytometry. Syngeneically and allogeneically stimulated CD8+ cells showed identical levels of LFA-1, which were significantly elevated compared to those in naive T cells from B10.A mice (Fig. 2C).
To exclude that adhesion to recombinant ICAM-1 was mediated by receptors other than LFA-1, stimulated T cells were preincubated with either anti-mouse LFA-1 antibody or anti-human IgG antibody (negative control) before seeding them on ICAM/Fc-coated plates. As expected, the adhesion of both T cell populations was virtually abolished after incubation with the anti-LFA-1 antibody, whereas the anti-human IgG antibody used as a negative control showed little, if any, effect (Fig. 2D).
To reinforce these findings, the adhesion of stimulated T cells to murine fibroblasts was examined using fibroblasts generated from the skin of syngeneic B10.A mice that were transduced with a lentiviral vector containing the sequence of eGFP. Their purity was confirmed by FACS analysis with an antibody specific for mouse embryonic fibroblasts (Fig. 3A). For adhesion assays, stimulated T cells were seeded on plates that had been coated with eGFP-expressing fibroblasts. The mixed cultures were incubated with anti-mouse LFA-1 or with anti-human IgG (negative control) antibody for 2 h. After washing and fixing, the adherent T cells were probed with antibody to CD8. Fluorescence microscopy showed increased adhesion of allogeneically stimulated CD8+ cells when incubated with control antibody compared to that of syngeneically stimulated cells under identical conditions. As a control, anti-LFA-1 antibody reduced the numbers of adhered CD8+ T cells, both syngeneically and allogeneically stimulated (Fig. 3B). Counting of the adhering CD8+ cells corroborated our findings (Table 1).
TABLE 1.
Detection of adherent CD8+ cells by fluorescence microscopy
The adaptor proteins SLP-76 and ADAP are implicated in the activation of LFA-1-mediated adhesion; after TCR ligation, the SH2 domain of SLP-76 interacts with ADAP, which plays a crucial role in LFA-1 clustering (8, 16). To examine the role of both adaptor proteins in T cell-mediated alloresponses, we performed anti-ADAP immunoprecipitations from total lysates of syngeneically or allogeneically stimulated T cells. Western blots probed with antibodies to LFA-1 or SLP-76 showed an enhanced coprecipitation of LFA-1 and SLP-76 with ADAP in allogeneically stimulated cells compared to syngeneically stimulated cells (Fig. 3C and D).
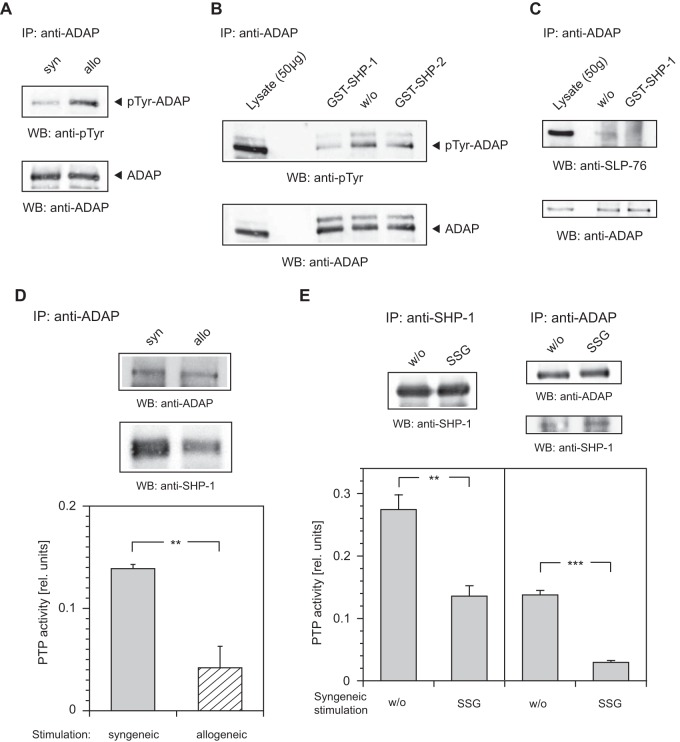
PTP activity and binding of SHP-1 are modulated upon alloactivation.
It is widely accepted that SHP-1 is a negative regulator of TCR-mediated signaling. In contrast, SHP-2, which shares the same general structure with SHP-1, has been reported to have both positive and negative effects on cells (17). To investigate the impact of alloactivation on the activity of either protein tyrosine phosphatase (PTP), we performed in vitro PTP activity assays. We immunoprecipitated SHP-1 or SHP-2 from the lysates of syngeneically or allogeneically stimulated cells. Enzymatic activity of immunoprecipitated PTPs was then quantified by conversion of the artificial substrate para-nitrophenyl-phosphate. Specific activity levels were corrected for the amount of precipitated protein detected by blotting. Remarkably, syngeneic stimulation of the cells resulted in strong activation of SHP-1, whereas alloactivation yielded only 50% of this level. SHP-2 activity was low in both syngeneic and allogeneic cells, with no significant difference (Fig. 4A).
FIG 4.
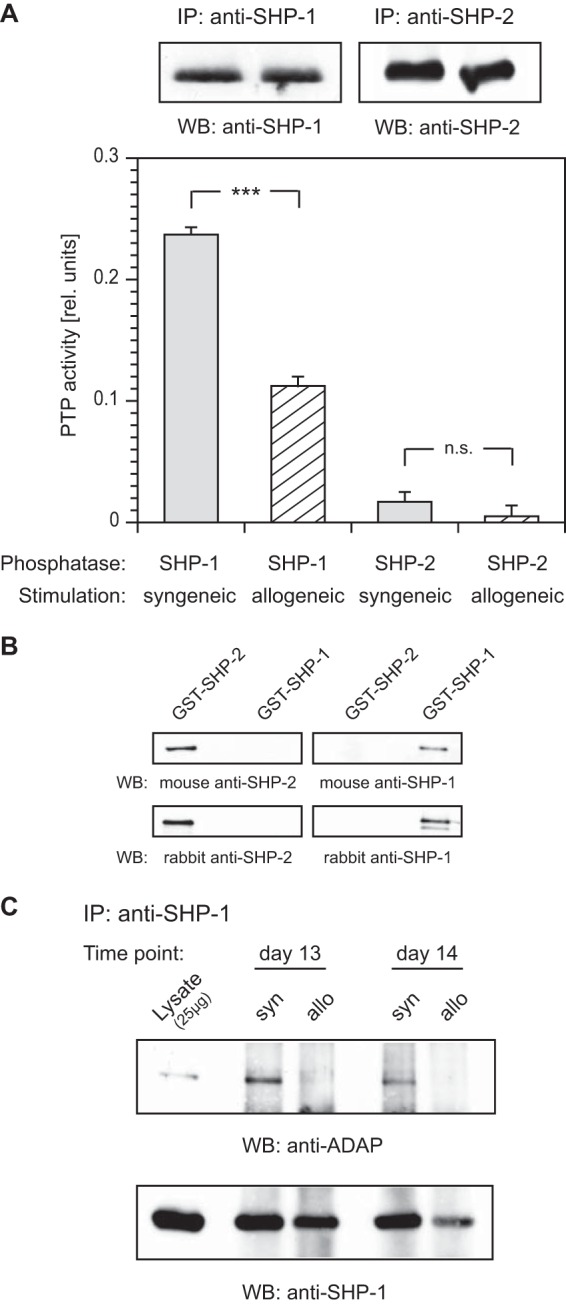
PTP activity and binding of SHP-1 are modulated upon alloactivation. (A) For PTP activity assays, stimulated T cells were lysed, and SHP-1 or SHP-2 was immunoprecipitated (IP). The assays were initiated by addition of the artificial substrate para-nitrophenyl-phosphate (pNPP), and PTP activity was detected by measuring the conversion of pNPP at 405 nm. The PTP activity was corrected for the amount of precipitated protein detected by blotting. Error bars indicate standard deviation (SD) (n = 3). ***, P < 0.001; n.s., not significant (P > 0.05). (B) Analysis of antibody specificity to SHP-1 and SHP-2. GST–SHP-1 or GST–SHP-2 fusion protein (0.5 μg) was probed by Western blotting (WB) with antibodies as indicated. (C) Lysates from syngeneically (syn) or allogeneically (allo) stimulated T cells harvested on two consecutive days (days 13 and 14) were precipitated (IP) and probed (WB) with antibodies as indicated.
GST–SHP-1 and GST–SHP-2 fusion proteins were used to confirm the specificity of our SHP-1 and SHP-2 antibodies by Western blotting. As expected, all antibodies tested were highly specific and exhibited no cross-reactivity (Fig. 4B). Next, we investigated the involvement of SHP-1 in LFA-1-mediated signaling. Thus, anti-SHP-1 immunoprecipitations were performed from the lysates of syngeneically or allogeneically stimulated T cells on two consecutive days (days 13 and 14). The 24-hour data point was introduced to serve as an additional control experiment. Western blot analysis with antibody to ADAP clearly demonstrated that SHP-1 was strongly associated with ADAP upon syngeneic stimulation of the T cells but not after allogeneic stimulation (Fig. 4C). These data showed a difference in both the level and the activity of ADAP-associated SHP-1 in syngeneically versus allogeneically activated T cells.
Knockdown of SHP-1 strongly enhances LFA-mediated adhesion.
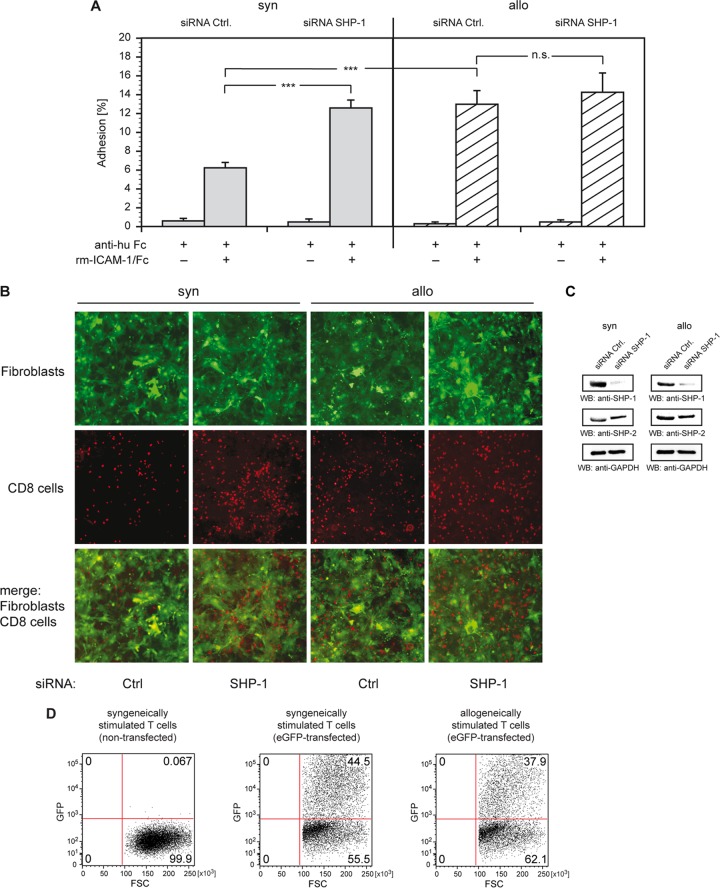
We examined the influence of an siRNA-based knockdown of SHP-1 on the adhesion of syngeneically and allogeneically stimulated T cells. Stimulated T cells were transiently transfected with either SHP-1-specific siRNA or nontargeting control siRNA. Adhesion assays with recombinant mouse ICAM-1 were set up as before (Fig. 2A). Allostimulated T cells transfected with control siRNA showed 2-fold-higher adhesion than syngeneically stimulated cells (Fig. 5A). The ratio of syngeneic and allogeneic levels was similar to that in nontransfected cells (compare Fig. 2D). In contrast, transfection of syngeneically stimulated cells with SHP-1-specific siRNA resulted in a 2-fold increase in adhesion compared to that for cells transfected with control siRNA under identical conditions. In contrast, SHP-1-specific siRNA transfection revealed only a marginal increase in adhesion of allostimulated cells compared to that of identically stimulated cells that were transfected with control siRNA. These data indicated that syngeneic and allogeneic modes of stimulation had surprising potent effects in determining the activity and involvement in LFA-1-mediated adhesion.
FIG 5.
Knockdown of SHP-1 strongly enhances LFA-1-mediated adhesion. (A) Syngeneically (syn) or allogeneically (allo) stimulated T cells were transfected with nontargeting control siRNA (siRNA Ctrl.) or SHP-1 siRNA (siRNA SHP-1). At 48 h after transfection, the cells were incubated on plates coated with anti-human Fc antibody loaded with recombinant mouse ICAM-1/Fc or buffer. After washing, bound cells were counted, and the adhesion was calculated by the ratio of bound cells to incubated cells. Error bars indicate SEM (n = 20). ***, P < 0.001; n.s., not significant (P > 0.05). (B) Stimulated T cells were transfected with siRNA and were incubated on plates seeded with syngeneic eGFP-expressing fibroblasts 48 h after transfection. After incubation for 2 h at 37°C, washing, and fixing, the adherent T cells were probed with antibody against CD8, and adhesion was visualized by fluorescence microscopy. (C) Lysates of stimulated T cells, transfected with siRNA, were probed by Western blotting (WB) with antibodies as indicated. GAPDH was used as a loading control. (D) Transfection efficiencies of stimulated T cells were evaluated by nucleofection of control vector pmaxGFP. Expression of GFP was examined by FACS analysis. The panel shows results from a representative experiment; displayed are equal cell numbers.
To further support these findings, adhesion of stimulated T cells to eGFP-expressing fibroblasts generated from the skin of syngeneic B10.A mice was examined. Syngeneically and allogeneically stimulated T cells were transiently transfected with SHP-1-specific siRNA or with control siRNA and seeded on plates coated with fibroblasts. After washing and fixing of the mixed cultures, the adherent T cells were probed with antibody to CD8 and visualized by fluorescence microscopy (Fig. 5B). In additional controls, the specific knockdown of SHP-1 was confirmed by Western blotting (Fig. 5C), and the transfection efficiencies were analyzed using the positive-control vector pmaxGFP (Fig. 5D). When transfected with control siRNA, as expected, significantly more allostimulated T cells adhered to fibroblasts than syngeneically stimulated T cells. Transfection of the latter with SHP-1-specific siRNA, however, strongly enhanced adhesion. In contrast, transfection of allostimulated T cells with SHP-1-specific siRNA did not result in significantly increased adhesion. Manual counting of the adhering CD8+ cells underscored these findings (Table 1).
GVHD is associated with enhanced LFA-1-mediated adhesion and decreased activity of SHP-1.
Graft-versus-host disease (GVHD) is caused mainly by the engagement of alloreactive T cells (2). To investigate the role of alloactivated T cells in ICAM-1 adhesion and SHP-1 activity in mice with GVHD, we performed fully MHC-mismatched allogeneic hematopoietic stem cell transplantations (HCTs). We used C57BL/6 mice as recipients and B10.A (allogeneic) or C57BL/6 (syngeneic control) mice as donors. After receiving total body irradiation at 10.5 Gy, recipients were cotransplanted with T cell-depleted bone marrow cells and splenocytes.
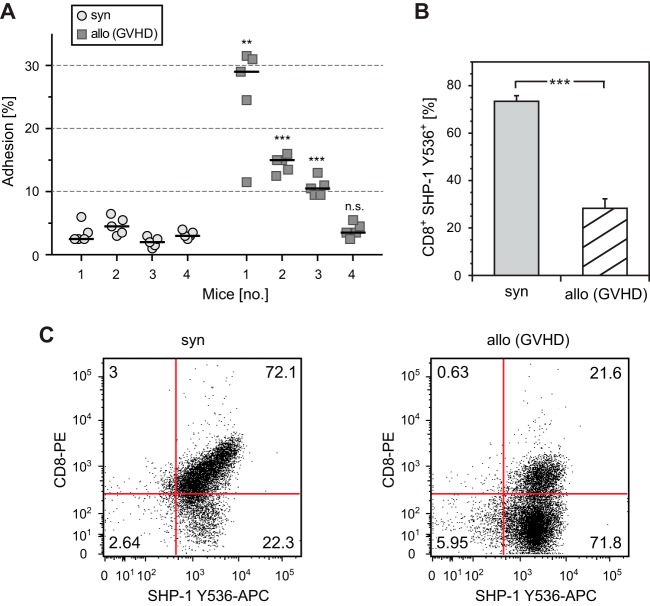
First, we examined the adhesion of CD8+ splenic T cells to recombinant ICAM-1. As shown in Fig. 6A, adhesion of CD8+ T cells from three of a total of four mice after allogeneic HCT was significantly higher than those from four mice after syngeneic HCT. Next, we analyzed SHP-1 activity in thymocytes. Since analysis of SHP-1 activity by conventional PTP activity assays was technically not feasible due to the low number of CD8+ cells early after transplantation, we performed flow cytometry using an antibody that detects SHP-1 activity by staining the phosphorylated tyrosine residue 536 (Y536); phosphorylation of Y536 reflects strongly increased SHP-1 activity (17).
FIG 6.
Graft-versus-host disease is associated with enhanced LFA-1-mediated adhesion and decreased activity of SHP-1. (A) CD8+ splenic T cells from 4 mice each after syngeneic HCT or allogeneic HCT (GVHD) were incubated on plates coated with anti-human Fc antibody that had been reincubated with recombinant mouse ICAM-1/Fc or buffer. After a washing, bound cells were counted, and adhesion was calculated by the ratio of bound to incubated cells. Five independent ICAM-1 adhesion assays were performed per mouse, and results are displayed as scatter plot including the arithmetic mean. (B) Thymocytes from mice after syngeneic HCT or allogeneic HCT (GVHD) were analyzed for coexpression of CD8 and SHP-1 Y536 by flow cytometry. Four mice were analyzed per group. Error bars indicate SEM (n = 4). ***, P < 0.001. (C) Representative flow cytometry dot plot analysis of CD8+ SHP-1 Y536+ thymocytes as described above.
Remarkably, CD8+ T cells of mice after syngeneic HCT showed strong phosphorylation of Y536, whereas allogeneic HCT resulted in less than 50% of this level (Fig. 6B and C). These data confirm in an in vivo GVHD model using syngeneically versus allogeneically activated T cells a difference in both the adhesion level and SHP-1 activity.
ADAP is a substrate of SHP-1.
Several phosphotyrosine residues of ADAP have been reported to interact with the SLP-76 SH2 domain (18–23). We therefore next examined the effect of SHP-1 on the phosphorylation of ADAP by using an anti-ADAP antibody to precipitate the adaptor protein followed by blotting with a monoclonal antibody to phosphotyrosine. Intriguingly, the blots revealed stronger tyrosine phosphorylation of ADAP in allogeneically stimulated cells but not in syngeneically stimulated cells (Fig. 7A).
FIG 7.
PTP activity of SHP-1 is associated with ADAP. (A) Lysates from syngeneically (syn) or allogeneically (allo) stimulated T cells were immunoprecipitated (IP) and probed (WB) with antibodies as indicated. (B) Dephosphorylation assays were performed with GST–SHP-1 and GST–SHP-2 fusion protein. Splenocytes were lysed and immunoprecipitated (IP) with antibody to ADAP. The precipitates were incubated in dephosphorylation assay buffer alone (w/o) or supplemented with 1 μg of GST–SHP-1 or GST–SHP-2 fusion protein for 5 min at 30°C. Western blots (WB) were detected as indicated. (C) Dephosphorylation assay with GST–SHP-1 fusion protein was carried out as described above; the Western blot (WB) was detected as indicated. (D and E) Lysates from syngeneically (syn) or allogeneically (allo) stimulated T cells were immunoprecipitated (IP) with antibody to ADAP or SHP-1, and PTP activity assays were performed as described in the text. PTP activity assay mixtures were supplemented with sodium stibogluconate (SSG) at 200 μM or incubated in assay buffer alone (w/o) as indicated. Error bars indicate SD (n = 3). The Western blots (WB) were detected as indicated. ***, P < 0.001; **, 0.001 < P < 0.01.
To further examine these findings, in vitro dephosphorylation assays with recombinant GST–SHP-1 and GST–SHP-2 fusion proteins were performed. ADAP immune complexes gathered from the lysate of splenocytes (B10.A mice) were incubated with equivalent amounts of GST–SHP-1 and GST–SHP-2 fusion proteins, the enzymatic activity and specificity of which has been described in detail previously (24). Within 5 min, treatment of an anti-ADAP immune complex with GST–SHP-1 resulted in significant tyrosine dephosphorylation of ADAP. In contrast, addition of GST–SHP-2 had only a marginal effect (Fig. 7B). When an anti-ADAP immune complex was incubated with GST–SHP-1 fusion protein for 5 min, no binding of SLP-76 to ADAP was seen. As expected, when the ADAP immune complex was incubated without fusion protein, SLP-76 interaction with ADAP was clearly detectable (Fig. 7C).
To establish the involvement of SHP-1 in dephosphorylation of ADAP, we carried out PTP activity assays with ADAP-associated tyrosine phosphatase. Anti-ADAP antibody was used to precipitate from the lysates of syngeneically or allogeneically stimulated T cells. PTP activity in the immunoprecipitates was quantified by conversion of the artificial substrate para-nitrophenyl-phosphate. As depicted in Fig. 7D, PTP activity associated with the anti-ADAP immune complex decreased more than 3-fold upon alloactivation of T cells, compared to the PTP activity of syngeneically stimulated T cells. Western blotting also revealed more binding of SHP-1 to ADAP in syngeneically stimulated cells.
To verify the involvement of SHP-1 in ADAP-associated PTP activity, we performed PTP activity assays with anti-SHP-1 or anti-ADAP immunoprecipitates from the lysate of syngeneically stimulated T cells and supplemented them with sodium stibogluconate (SSG) to inhibit SHP-1 activity (25). Treatment of SHP-1 immunoprecipitate (positive control) with SSG resulted in a significant decrease of PTP activity compared to that of untreated immunoprecipitate. A similar decrease of PTP activity occurred, as expected, when ADAP immunoprecipitate was treated with SSG (Fig. 7E).
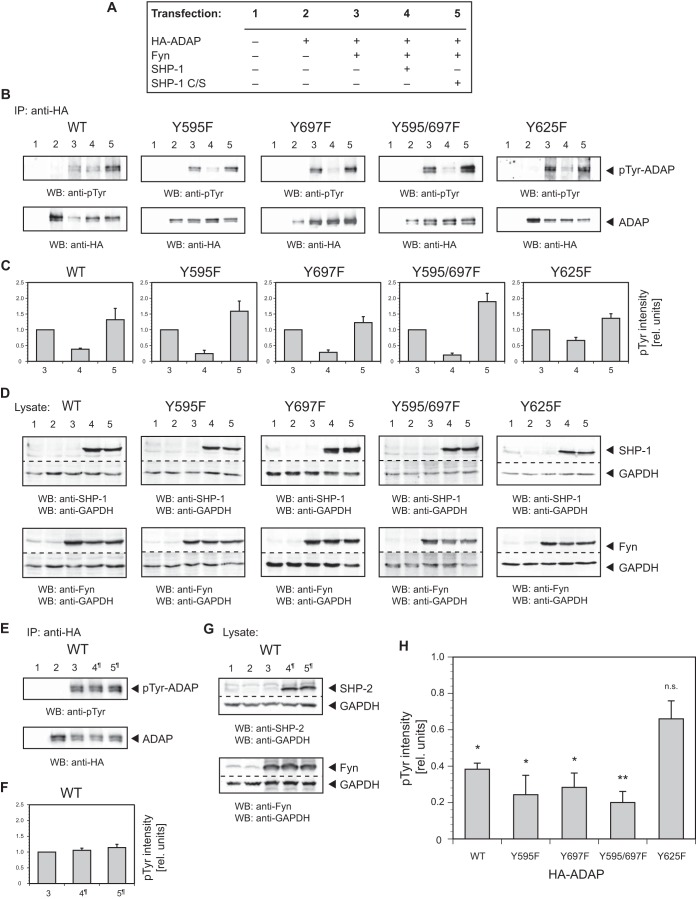
Published data favor either YDDV tyrosine motif of ADAP (Y595, Y651 [isoform ADAP-120] or Y595, Y697 [isoform ADAP-130]) as a major binding site for the SLP-76 SH2 domain and suggest a requirement of both sites for optimal SLP-76 binding (18–23). Moreover, the YDGI tyrosine motif (Y625) plays an additional role in binding to SLP-76. Phosphorylated YDGI interacts with the SH2 domain of the Src family kinase Fyn, which we showed previously phosphorylates both YDDV motifs, which are essential for ADAP binding to SLP-76 (18, 26).
To identify the tyrosine residue(s) being dephosphorylated by SHP-1, we transiently transfected NIH 3T3 fibroblasts with plasmids expressing HA-tagged wild-type ADAP-130 or ADAP-130 containing various tyrosine-to-phenylalanine point mutations (Y595F, Y625F, Y697F, and Y595/697F). Since our studies are focused on the extension of earlier findings that have been obtained with these ADAP point mutations (18–23), we did not investigate other combinations of point mutations. In order to ensure tyrosine phosphorylation of transient ADAP, the cells were cotransfected with Fyn. In addition, for analysis of tyrosine dephosphorylation, a plasmid was cotransfected with wild-type SHP-1 or a catalytically inactive form of SHP-1 (SHP-1 C/S), used as control (Fig. 8A).
FIG 8.
The ADAP YDGI motif with tyrosine residue Y625 acts as a major dephosphorylation site for SHP-1. NIH 3T3 fibroblasts were cotransfected with plasmids expressing HA-tagged wild-type ADAP-130 (WT) or ADAP-130 containing tyrosine-to-phenylalanine point mutations (Y595F, Y625F, Y697F, and Y595/697F), Fyn, wild-type SHP-1, and SHP-1 C/S. (A) Experimental setup for transfection of NIH 3T3 fibroblasts with plasmids as above. Numbers refer to the lane or bar numbers in panels B, C, and D. (B and C) Transfected fibroblasts were lysed and immunoprecipitated (IP) with antibody to HA. Western blots (WB) were detected as indicated, and the intensity of tyrosine phosphorylation was evaluated by densitometric quantification. The ratio of densitometric intensities of immunoprecipitated ADAP (B, lower panel) and corresponding phosphorylation (B, upper panel) in lane 3 were set to 1.0, and the actual ratio was used as a correction factor for the intensity levels (relative units) reported for lanes 4 and 5. Error bars indicate SEM (n = 3). (D) Lysates of transfected NIH 3T3 fibroblasts were probed by Western blotting (WB) with antibodies as indicated. GAPDH was used as a loading control. (E and F) Experimental setup and analysis as laid out in panel A, with the exception that wild-type SHP-1 and SHP-1 C/S were replaced by wild-type SHP-2 and SHP-2 C/S, respectively. Accordingly, lanes 4 and 5 are labeled 4¶ and 5¶, respectively. (G) Western blotting (WB) with antibodies as indicated. GAPDH was used as a loading control. (H) Phosphorylation intensities of wild-type ADAP (WT) and ADAP point mutants (Y595F, Y697F, Y595/697F, and Y625F) after coexpression with Fyn and wild-type SHP-1 (see panel C, bars 4). **, 0.001 < P < 0.01; *, P < 0.05; n.s., not significant (P > 0.05).
We then performed anti-HA immunoprecipitations from the lysates of transfected cells and probed the Western blots with an antibody to phosphotyrosine. To quantify the phosphorylation of ADAP, the ratios of densitometric intensities of immunoprecipitated ADAP (Fig. 8B, lower panels) and corresponding phosphorylation (Fig. 8B, upper panels) in lanes 3 were set to 1.0, and the actual ratio was being used as a correction factor for the intensity levels (relative units) reported for lanes 4 and 5. As expected, transiently expressed ADAP was tyrosine phosphorylated when coexpressed with Fyn but not in the absence of Fyn. Additional cotransfection of wild-type SHP-1 revealed a dramatic reduction in tyrosine phosphorylation of wild-type ADAP and ADAP mutants Y595F, Y697F, and Y595/697F compared to that of cells without added SHP-1. In contrast, the ADAP mutant Y625F was dephosphorylated to a lesser degree (i.e., half as much). Coexpression of control SHP-1 C/S, as expected, had no effect on dephosphorylation (Fig. 8B and C). Transient expression of SHP-1 (wild type or catalytically inactive form) and Fyn was verified by Western blotting (Fig. 8D). As a further control, we examined the role of SHP-2 in this setting. Thus, we cotransfected a plasmid with wild-type SHP-2 or a catalytically inactive form of SHP-2 (SHP-2 C/S) instead of SHP-1 (wild-type or catalytically inactive form). As expected, cotransfection of both SHP-2 variants had no effect on tyrosine phosphorylation of wild-type ADAP (Fig. 8E and F). In addition, expression of transient SHP-2 and Fyn was verified by Western blotting (8G).
Taken together, the consistent and strong dephosphorylation of both wild-type ADAP and ADAP mutants Y595F, Y697F, and Y595/697F under coexpression of SHP-1 as opposed to a rather low dephosphorylation of the ADAP mutant Y625F provides the first evidence that the YDGI tyrosine motif (Y625) acts as a major dephosphorylation site for SHP-1. Figure 8H provides a summary overview for the resulting normalized dephosphorylation efficiencies of the different ADAP variants used (Fig. 8C, bars 4), thus clearly pointing to Y625 as a target for SHP-1.
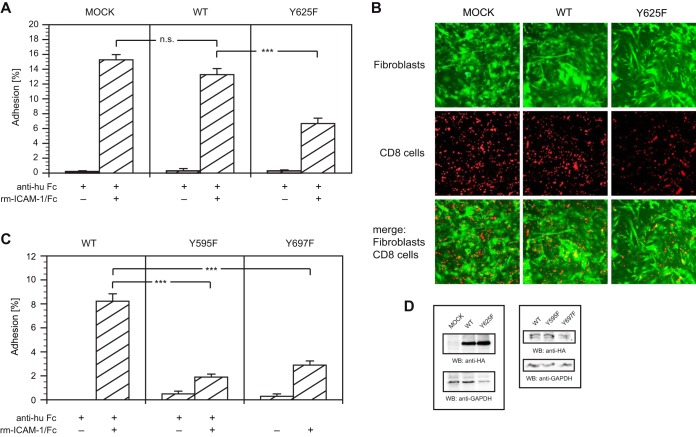
ADAP mutant Y625F downregulates LFA-1-mediated adhesion.
Since our experiments have confirmed the YDGI tyrosine motif of ADAP (Y625) as a major dephosphorylation site for SHP-1, we next investigated its influence on adhesion to ICAM-1 on plates (Fig. 9A). Allogeneically stimulated T cells were transiently transfected with plasmids expressing HA-tagged wild-type ADAP-130 or ADAP-130 mutant Y625F. Adhesion assays with recombinant mouse ICAM-1 were set up as described above. In our assay, the transfection of wild-type ADAP resulted in adhesion that did not differ significantly from the adhesion of mock-transfected cells. However, the ADAP mutant Y625F-transfected cells exhibited reduced adhesion (i.e., >50%) relative to that of wild-type ADAP-transfected cells (Fig. 9A).
FIG 9.
ADAP mutant Y625F downregulates LFA-1-mediated adhesion. (A) Allogeneically stimulated T cells were transiently transfected with HA-tagged wild-type ADAP-130 (WT) or ADAP-130 mutant Y625F (Y625). At 24 h after transfection, the cells were incubated on plates coated with anti-human Fc antibody that had been reincubated with recombinant mouse ICAM-1/Fc or buffer. After washing, bound cells were counted, and the adhesion was calculated as the ratio of bound cells to incubated cells. Error bars indicate SEM (n = 15). (B) Allogeneically stimulated T cells transfected with HA-tagged ADAP-130 (WT or Y625F) were incubated on plates seeded with syngeneic eGFP-expressing fibroblasts 24 h after transfection. After incubation for 2 h at 37°C, washing, and fixing, the adherent T cells were probed with antibody against CD8, and adhesion was visualized by fluorescence microscopy. (C) Allogeneically stimulated T cells were transiently transfected with HA-tagged ADAP-130 mutant Y595F or Y697F. At 24 h after transfection, adhesion to ICAM-1 was analyzed as described for panel A. (D) Lysates of allogeneically stimulated T cells transfected with HA-tagged variants of ADAP-130 were probed by Western blotting (WB) with antibodies as indicated. GAPDH was used as a loading control. ***, P < 0.001; n.s., not significant (P > 0.05).
To support these findings, the adhesion of transfected T cells to eGFP-expressing fibroblasts generated from the skin of syngeneic B10.A mice was investigated. T cells transiently transfected with wild-type ADAP or ADAP mutant Y625F were seeded on fibroblast-coated plates. After washing and fixing of the mixed cultures, the adherent T cells were probed with antibody to CD8 and visualized by fluorescence microscopy (Fig. 9B). As expected, significantly fewer cells transfected with ADAP mutant Y625F adhered to fibroblasts compared to cells transfected with wild-type ADAP or mock-transfected cells. Manual counting of the adhering CD8+ cells substantiated our findings (Table 1).
Published data favor either YDDV tyrosine motif of ADAP (Y595, Y651 [isoform ADAP-120] or Y595, Y697 [isoform ADAP-130]) as a major binding site for the SLP-76 SH2 domain (18–23), both impacting LFA-1-mediated adhesion. We thus transiently transfected allogeneically stimulated T cells with plasmids expressing HA-tagged wild-type ADAP-130 or ADAP-130 mutants Y595F and Y697F, respectively. As expected, transfection with ADAP mutant Y595F or Y697F resulted in strong decrease of ICAM-1 adhesion compared to transfection with wild-type ADAP (Fig. 9C).
In addition, transient expression of HA-tagged variants of ADAP-130 was confirmed by Western blotting (Fig. 9D). These results indicate that the target site of SHP-1 on ADAP when mutated showed a significant decrease in ICAM-1 binding.
DISCUSSION
Alloactivation of donor T cells profoundly relies on the presence of recipient-derived APCs (1, 27). These priming processes can be in part recapitulated in vitro (13). Based on the stronger interaction between a TCR and allogeneic MHC than between a TCR and syngeneic MHC, we hypothesized that allostimulation would trigger a signaling profile distinctive from that observed after syngeneic stimulation. Allogeneic stimulation can be severalfold stronger than that seen in the syngeneic context (28, 29). In this regard, the duration of a stable T cell-APC interaction is critical for determining whether a T cell is to be activated or deleted (30, 31). SHP-1-deficient T cells have been shown to be more capable of forming stable conjugates with APCs than normal T cells (32). However, the role of SHP-1 in the regulation of T cell adhesion remains unclear.
Here, we identified SHP-1 as a key regulator of ICAM-1 adhesion, where it plays a preferential role in affecting allogeneically stimulated primary murine T cells (Fig. 10). We found that SHP-1 activity is significantly reduced upon alloactivation, resulting in an increased LFA-1-mediated adhesion of alloactivated T cells. The activity of SHP-1 in allostimulated cells was reproducibly found to be approximately 50% of the activity detected in syngeneically stimulated cells. Our findings introduce the possibility that SHP-1 is an attractive target in an allotransplantation setting.
FIG 10.
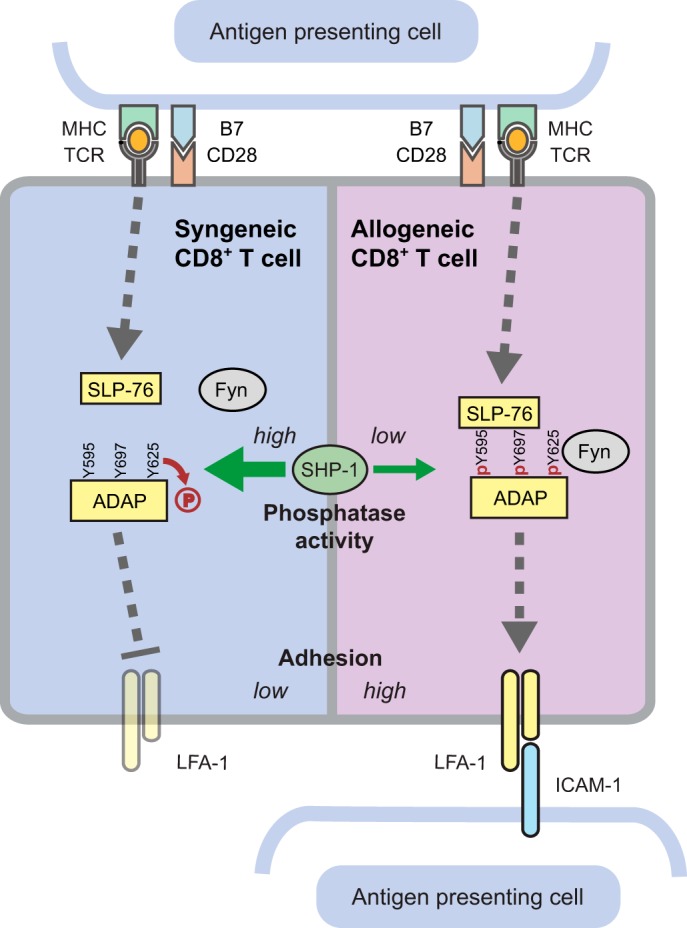
Summary cartoon. Syngeneic versus allogeneic T cell activation reveals SHP-1 as a key regulator in LFA-1-mediated adhesion.
Interestingly, we also noted a major difference in the involvement of SHP-1 relative to SHP-2 in allostimulation. However, these opposing activities of SHP-1 and SHP-2 measured in our assays were not unexpected. Despite close similarities in both sequence and structure, it is widely accepted that SHP-1 is a negative regulator of TCR-mediated signaling, while SHP-2 can act both as a signaling promoter and as a negative regulator (17, 33). In this context, it has been shown that receptor-stimulated oxidation of SHP-2 promotes T cell adhesion through binding of SLP-76 to ADAP (34).
In T cells, although several binding partners for SHP-1 have been proposed, the complete range of direct substrates remains to be identified. Previously published studies were done with various tumor-derived cell lines, thus producing controversial results depending on the cell line used (17). These drawbacks may be overcome using modern technologies that allow for the expansion of polyclonal T cells ex vivo, thus enabling studies on primary cells. Here, we showed that SHP-1 interacts significantly more strongly with the adaptor protein ADAP upon syngeneic stimulation of T cells than upon allogeneic stimulation. Slight differences in binding intensities as visualized by Western blotting might be attributed to the polyclonality of the T cell populations. To our knowledge, this observation establishes ADAP as a binding partner for SHP-1 for the first time. ADAP was also a substrate of SHP-1, and consistent with this, ADAP was more tyrosine phosphorylated in allogeneically stimulated T cells than in T cells activated by syngeneic APCs, thus providing for a decisive physiological role of SHP-1 in this model.
It is worth noting that naive T cells when cultured with cytokines sharing the common γ chain in the absence of TCR stimulation may become anergic to TCR stimulation. However, we could demonstrate that our final T cell product responds to the respective antigen, lyses its target specifically, and can undergo further rounds of proliferation, both in vitro and in vivo (data not shown). This has been confirmed by others and does rule out an anergic state (35).
Previous studies have shown that ADAP can interact via its YDDV motif with the SH2 domain of SLP-76 (18–23). Based on this, we designed a number of control experiments using GST–SHP-1/-2 fusion proteins in dephosphorylation assays, and we observed that ADAP-associated PTP activity was also stronger upon syngeneic stimulation than after allostimulation. Published studies provide additional evidence that aside from the YDDV motifs, the YDGI motif plays an indirect role in ADAP binding to SLP-76. In this context, phosphorylated YDGI interacts with the SH2 domain of the Src family kinase Fyn (18, 36), which phosphorylates both YDDV motifs, which are essential for ADAP binding to SLP-76. We thus analyzed tyrosine phosphorylation of both wild-type ADAP and ADAP mutants with tyrosine-to-phenylalanine point mutations within the YDDV and YDGI motifs under the influence of SHP-1. For our studies, the isoform ADAP-130 was used, which differs from the isoform ADAP-120 by the addition of 46 amino acids between the YDGI motif and the C-terminal YDDV motif (36). Our results clearly pointed to the YDGI motif as a major target site for SHP-1. In this regard, Fyn has also been implicated in T cell-APC interaction (37). The low dephosphorylation levels of the ADAP YDGI mutant suggest dephosphorylation of residues other than Y625. ADAP contains six further tyrosine residues partially associated with binding to other proteins besides the tyrosine motifs focused in this study (22). If so, to what degree these residues are dephosphorylated by transient SHP-1 or endogenous PTP remains elusive. However, it is tempting to speculate that the slight phosphorylation shift of ADAP when cotransfected with enzymatically inactive SHP-1 C/S is due to repressed ADAP interaction with endogenous PTP that is induced by overexpressed SHP-1 C/S.
In summary, we identified a novel role for SHP-1 in the regulation of LFA-1-mediated adhesion. The inhibitory function of active SHP-1 that has been shown in this paper may be useful to modulate harmful alloresponses and provides clues to the development of novel immunosuppressive strategies.
ACKNOWLEDGMENTS
We thank Martin R. Hadam for helpful discussions and critical reading of the manuscript.
This project was financed from funds provided by the Deutsche Forschungsgemeinschaft, the IFB-TX (grants SFB-738 and CBT_6 to M.G.S.), the Hannover Medical School, and the Deutsche José Carreras Leukämie-Stiftung (grants HiLF and DJCLS R 12/21 to C.K.).
M.G.S. introduced T cell priming and expansion concepts as well as technologies, provided generous support with the animal facilities, discussed the data, and wrote and edited parts of the manuscript. J.H. performed FACS analyses and assisted with experiments. U.D. generated the fibroblasts. C.E.R. reviewed and discussed the data and edited parts of the manuscript. C.K. designed, supervised, and performed all experiments, analyzed the data, and wrote and edited the manuscript.
We declare no competing financial interests.
REFERENCES
- 1.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. 1999. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 2.Newton-Nash DK. 1994. The molecular basis of allorecognition. Assessment of the involvement of peptide. Hum Immunol 41:105–111. [DOI] [PubMed] [Google Scholar]
- 3.von Andrian UH, Mempel TR. 2003. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 4.Sims TN, Dustin ML. 2002. The immunological synapse: integrins take the stage. Immunol Rev 186:100–117. doi: 10.1034/j.1600-065X.2002.18610.x. [DOI] [PubMed] [Google Scholar]
- 5.Dustin ML, Bivona TG, Philips MR. 2004. Membranes as messengers in T cell adhesion signaling. Nat Immunol 5:363–372. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- 6.Giblin PA, Lemieux RM. 2006. LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics. Curr Pharm Des 12:2771–2795. doi: 10.2174/138161206777947731. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Rudd CE. 2008. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol 18:486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudd CE, Wang H. 2003. Hematopoietic adaptors in T-cell signaling: potential applications to transplantation. Am J Transplant 3:1204–1210. doi: 10.1046/j.1600-6143.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 9.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. 1997. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol 159:5921–5930. [PubMed] [Google Scholar]
- 10.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte JJ, Crawford JM, Ferrara JL. 1996. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation. I. The roles of minor H antigens and endotoxin. Blood 88:3230–3239. [PubMed] [Google Scholar]
- 11.Gu H, Griffin JD, Neel BG. 1997. Characterization of two SHP-2-associated binding proteins and potential substrates in hematopoietic cells. J Biol Chem 272:16421–16430. doi: 10.1074/jbc.272.26.16421. [DOI] [PubMed] [Google Scholar]
- 12.Sauer MG, Ericson ME, Weigel BJ, Herron MJ, Panoskaltsis-Mortari A, Kren BT, Levine BL, Serody JS, June CH, Taylor PA, Blazar BR. 2004. A novel system for simultaneous in vivo tracking and biological assessment of leukemia cells and ex vivo generated leukemia-reactive cytotoxic T cells. Cancer Res 64:3914–3921. doi: 10.1158/0008-5472.CAN-03-3991. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh A, Koestner W, Hapke M, Schlaphoff V, Langer F, Baumann R, Koenecke C, Cornberg M, Welte K, Blazar BR, Sauer MG. 2009. Donor T cells primed on leukemia lysate-pulsed recipient APCs mediate strong graft-versus-leukemia effects across MHC barriers in full chimeras. Blood 113:4440–4448. doi: 10.1182/blood-2008-09-181677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraverty R, Flutter B, Fallah-Arani F, Eom HS, Means T, Andreola G, Schwarte S, Buchli J, Cotter P, Zhao G, Sykes M. 2008. The host environment regulates the function of CD8+ graft-versus-host-reactive effector cells. J Immunol 181:6820–6828. doi: 10.4049/jimmunol.181.10.6820. [DOI] [PubMed] [Google Scholar]
- 15.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Vallera DA. 1995. Coblockade of the LFA1:ICAM and CD28/CTLA4:B7 pathways is a highly effective means of preventing acute lethal graft-versus-host disease induced by fully major histocompatibility complex-disparate donor grafts. Blood 85:2607–2618. [PubMed] [Google Scholar]
- 16.Wilkinson B, Wang H, Rudd CE. 2004. Positive and negative adaptors in T-cell signalling. Immunology 111:368–374. doi: 10.1111/j.0019-2805.2004.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz U. 2009. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev 228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raab M, Kang H, da Silva A, Zhu X, Rudd CE. 1999. FYN-T-FYB-SLP-76 interactions define a T-cell receptor zeta/CD3-mediated tyrosine phosphorylation pathway that up-regulates interleukin 2 transcription in T-cells. J Biol Chem 274:21170–21179. doi: 10.1074/jbc.274.30.21170. [DOI] [PubMed] [Google Scholar]
- 19.Geng L, Raab M, Rudd CE. 1999. Cutting edge: SLP-76 cooperativity with FYB/FYN-T in the Up-regulation of TCR-driven IL-2 transcription requires SLP-76 binding to FYB at Tyr595 and Tyr651. J Immunol 163:5753–5757. [PubMed] [Google Scholar]
- 20.Boerth NJ, Judd BA, Koretzky GA. 2000. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J Biol Chem 275:5143–5152. doi: 10.1074/jbc.275.7.5143. [DOI] [PubMed] [Google Scholar]
- 21.Lange S, Sylvester M, Schumann M, Freund C, Krause E. 2010. Identification of phosphorylation-dependent interaction partners of the adapter protein ADAP using quantitative mass spectrometry: SILAC vs (18)O-labeling. J Proteome Res 9:4113–4122. doi: 10.1021/pr1003054. [DOI] [PubMed] [Google Scholar]
- 22.Sylvester M, Kliche S, Lange S, Geithner S, Klemm C, Schlosser A, Grossmann A, Stelzl U, Schraven B, Krause E, Freund C. 2010. Adhesion and degranulation promoting adapter protein (ADAP) is a central hub for phosphotyrosine-mediated interactions in T cells. PLoS One 5:e11708. doi: 10.1371/journal.pone.0011708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coussens NP, Hayashi R, Brown PH, Balagopalan L, Balbo A, Akpan I, Houtman JC, Barr VA, Schuck P, Appella E, Samelson LE. 2013. Multipoint binding of the SLP-76 SH2 domain to ADAP is critical for oligomerization of SLP-76 signaling complexes in stimulated T cells. Mol Cell Biol 33:4140–4151. doi: 10.1128/MCB.00410-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tossidou I, Dangers M, Koch A, Brandt DT, Schiffer M, Kardinal C. 2008. Tyrosine phosphatase SHP-2 is a regulator of p27(Kip1) tyrosine phosphorylation. Cell Cycle 7:3858–3868. doi: 10.4161/cc.7.24.7260. [DOI] [PubMed] [Google Scholar]
- 25.Pathak MK, Yi T. 2001. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol 167:3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 26.da Silva AJ, Li Z, de Vera C, Canto E, Findell P, Rudd CE. 1997. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc Natl Acad Sci U S A 94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. 2005. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med 11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald WA, Chen Z, Gras S, Archbold JK, Tynan FE, Clements CS, Bharadwaj M, Kjer-Nielsen L, Saunders PM, Wilce MC, Crawford F, Stadinsky B, Jackson D, Brooks AG, Purcell AW, Kappler JW, Burrows SR, Rossjohn J, McCluskey J. 2009. T cell allorecognition via molecular mimicry. Immunity 31:897–908. doi: 10.1016/j.immuni.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Yin L, Huseby E, Scott-Browne J, Rubtsova K, Pinilla C, Crawford F, Marrack P, Dai S, Kappler JW. 2011. A single T cell receptor bound to major histocompatibility complex class I and class II glycoproteins reveals switchable TCR conformers. Immunity 35:23–33. doi: 10.1016/j.immuni.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iezzi G, Karjalainen K, Lanzavecchia A. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8:89–95. doi: 10.1016/S1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 31.Mempel TR, Henrickson SE, Von Andrian UH. 2004. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 32.Sathish JG, Dolton G, Leroy FG, Matthews RJ. 2007. Loss of Src homology region 2 domain-containing protein tyrosine phosphatase-1 increases CD8+ T cell-APC conjugate formation and is associated with enhanced in vivo CTL function. J Immunol 178:330–337. doi: 10.4049/jimmunol.178.1.330. [DOI] [PubMed] [Google Scholar]
- 33.Poole AW, Jones ML. 2005. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal 17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Kwon J, Qu CK, Maeng JS, Falahati R, Lee C, Williams MS. 2005. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J 24:2331–2341. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong P, Pamer EG. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol 166:5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 36.Veale M, Raab M, Li Z, da Silva AJ, Kraeft SK, Weremowicz S, Morton CC, Rudd CE. 1999. Novel isoform of lymphoid adaptor FYN-T-binding protein (FYB-130) interacts with SLP-76 and up-regulates interleukin 2 production. J Biol Chem 274:28427–28435. doi: 10.1074/jbc.274.40.28427. [DOI] [PubMed] [Google Scholar]
- 37.Filby A, Seddon B, Kleczkowska J, Salmond R, Tomlinson P, Smida M, Lindquist JA, Schraven B, Zamoyska R. 2007. Fyn regulates the duration of TCR engagement needed for commitment to effector function. J Immunol 179:4635–4644. doi: 10.4049/jimmunol.179.7.4635. [DOI] [PubMed] [Google Scholar]