Abstract
Background: A meta-analysis was conducted to evaluate the accuracy of MRI, CT and FDG PET/CT in TNM stage of nasopharyngeal carcinoma patients (NPC). Methods: Through a search of studies from 1996 to April 2015, pooled estimated sensitivity, specificity, pooled diagnostic odds ratio (DOR), summary receiver operating characteristic (SROC) curves and Q*-index were calculated. Results: Totally 23 studies were included for analysis. In T stage, the pooled sensitivity, specificity, DOR and SROC of MRI were 0.95 (95% CI 0.93-0.97), 0.76 (95% CI 0.71-0.80), 86.85 (16.36-461.06) and 0.9213 (SE 0.0372) respectively. The pooled sensitivity, specificity, DOR and SROC of CT were 0.84 (95% CI 0.79 to 0.88), 0.80 (95% CI 0.71 to 0.88), 6.32 (1.17 to 34.02) and 0.7215 (SE 0.054) respectively. The pooled sensitivity, specificity, DOR and SROC of FDG PET/CT were 0.85 (95% CI 0.76 to 0.91), 0.91 (95% CI 0.84 to 0.96) and 0.8673 (SE 0.0311). In N stage, the pooled sensitivity, specificity, DOR and SROC of MRI were 0.88 (95% CI 0.85-0.90), 0.95 (95% CI 0.93-0.97), 93.68 (23.21-379.69) and 0.9153 (SE 0.099) respectively. The pooled sensitivity, specificity, DOR and SROC of CT were 0.92 (95% CI 0.88-0.95), 0.93 (0.76-0.99), 93.81 (22.39-393.03) and 0.8872 (SE 0.0520) respectively. The pooled sensitivity, specificity, DOR and SROC of FDG PET/CT were 0.88 (95% CI 0.85-0.90), 0.95 (95% CI 0.93-0.97), 93.88 (23.21-379.69) and 0.9153 (SE 0.0299) respectively. In M stage, the pooled sensitivity and specificity of MRI were 0.53 (95% CI 0.35-0.70) and 0.99 (95% 0.95-1.00). The pooled sensitivity and specificity of CT were 0.80 (95% CI 0.44-0.97) and 0.93 (95% CI 0.86-0.97) respectively. The pooled sensitivity, specificity and SROC of FDG PET/CT were 0.82 (95% 0.74-0.88), 0.98 (95% CI 0.96-0.99) and 0.9002 (SE 0.075) respectively. Conclusion: The analysis suggested that MRI had good accuracy in diagnosis of T stage. Whereas CT is currently a good performance in diagnosis of N stage, FDG PET/CT shows good accuracy in diagnosis of M stage.
Keywords: Meta-analysis, MRI, CT, FDG PET/CT, nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is globally an uncommon cancer with approximately 80.000 new cases reported per year and accounting 0.7% of all cancer. Even though the incidence rate is less than 1 case per 100,000 population in North American and Europe, In endemic areas like Southern China (e.g. Hong Kong) and Southeast Asia, the annual age-standardized incidence rates are as high as 20 to 30 cases per 100,000 population in men and 8 to 15 cases per 100,000 populations in women [1]. NPC is an aggressive head and neck cancer with a high incidence of loco-regional spread and of distant metastasis at presentation. NPC may spread locally to involve the parapharyngeal soft tissue base of skull or intracranial structures. The nasopharynx has a rich lymphatic plexus; 75% of patients present with enlarged cervical nodes, 80% of whom have bilateral involvement. NPC has a relative high incidence of systemic metastasis (up to 41%) when compared with the other head and neck tumor (5-24%). The most common sites of metastasis are bone (20%), lung (13%), and liver (9%) [2].
Owing to the specific anatomic location and highly responsive to radiation, the main treatment of NPC is radiotherapy and chemotherapy. Staging of patients affected with NPC represents the basic step to successful treatment. The accurate diagnosis of the tumor extension and the delineation of target volume mostly depend on imaging.
The American Joint Committee on Cancer (AJCC) T (primary tumor) N (Regional lymph nodes) M (Distant Metastasis) system is one of the most widely used staging system internationally [3]. The National Comprehensive Cancer Network (NCCN) guidelines recommend MRI of the nasopharynx and neck as well as CT scan for T and N classification. For patients with N and M classifications, it suggests that FDG PET/CT scan may be considered [4]. The aim of this study was to analyze the sensitivity, specificity and accuracy of MRI, CT and FDG PET/CT in staging NPC patients.
Materials and methods
Search strategy and study selection
We searched MEDLINE, EMBASE and Chinese national knowledge infrastructure (CNKI) from 1996 to April 2015. The search strategy was based on the combination of the following keywords: 1) MRI or magnetic resonance; 2) CT or computed tomography; 3) FDG PET/CT or FDG positron emission tomography; 4) nasopharyngeal carcinoma or metastasis of nasopharynx or lymph node; 5) detection or staging or accuracy. Conference abstracts and letters to the journal editors were excluded because they contained limited data. Two reviewers independently judged study eligibility and disagreements were resolved by discussion and if necessary by a third reviewer.
The included criteria were: 1) histopathology analysis or clinical and imaging follow-up or compared with reference standard were used as reference standard; 2) only studies which a 2X2 table could be constructed for true-positive, true-negative, false-positive and false-negative values were included; 4) the studies were based on per patient statistics; 5) when data or subsets of data were presented in more than one articles, the article with the most detail or the most recent article was chosen; 6) the studies including at least 10 patients were selected for inclusion in the study since very small studies may be vulnerable to selection bias.
Data extraction
Two reviewers extracted data from each eligible study independently using standardized data extraction form and any disagreement were resolved by discussion or by appeal to a third reviewer.
Reviewer were not blinded with regard to information about the journal name, the authors, country of origin or the year of publication; as this has been shown to the unnecessary [5]. In addition, more information (sample size, age, gender distribution, stage of patients and reference test used to define the stage of the disease. Publications investigating more than one aspect of classification were analyzed independently. The number of cases was only extracted with true positive, true negative, false negative and false positive.
We assess the methodological quality of the studies using the quality assessment for studies of diagnostic accuracy (QUADAS) tool [6]. Fourteen items in QUADAS tool examined potential sources of bias in diagnostic studies in a systematic evidence-based manner. Higher scores suggest lower risk of bias in the study’s methodology.
Statistical analysis
The accuracy of 4 modality in 3 staging of NPC patients was determined by combined estimate of sensitivity and specificity, pooled diagnostic odds ratio (DOR), summary receiver operating characteristic (SROC) curves and Q*-index. The degree of heterogeneity in included studies was analyzed by Cochran chi-square statistic. A random effect model was applied while significant heterogeneity was observed (p<0.05). A random effects meta-regression model was used to compare subgroup estimates. SROC graph gives us a globe estimate of diagnostic test’s performance and illustrates the tradeoff between sensitivity and specificity [7]. Q*-index reflects the diagnostic value and is the best statistical summary method. Moreover, the diagnostic odds ratio (DOR) indicates the test accuracy that transfers the sensitivity and specificity into a number. The higher DOR value indicates better accuracy which is better discriminatory test performance. A value of 1.0 indicates that the test does not discriminate between patients between with and without malignance in each classification.
All these analysis were performed using META-DISC version 1.4 (XI Conchrane Colloquium, Barcelona, Spain) and level of significance set at 5%. The paired and inter-related comparison showed by sensitivity and specificity estimated the accuracy, both are reported simultaneously. Diagnositc odd ratio (DOR) is very useful in procedures like meta-regression. If heterogeneity is found to present from analysis, Meta-regression is used to explore the reason for such heterogeneity by relating study level co-variates to an accuracy measure. DOR is used to globally compare the overall diagnostic accuracy of different tests. The overall DOR is estimated by combining individual DORs through Mantel-haenszel or the DerSimonian Laird methods and then fits an SROC curve. The estimation of AUC and the Q* index, along with their standard errors can summarize measure of global accuracy and aids inter-test comparisons.
Results
Study selection and description
We identified 23 studies including 2413 patients using search strategy summarized in Figure 1. Ten studies addressed the T stage (local extent of the primary tumor) including 8 studies with MRI, 4 studies with CT and 6 studies with FDG PET/CT. Twelve studies were included in the analysis of N stage (lymph node metastasis). Among 12 studies, 10 studies of MRI test cases, 4 studies of CT test and 12 studies of FDG PET/CT test cases. Seven studies were analyzed in M stage (distant metastasis), which has 2 studies about whole body MRI, 2 studies about CT and 8 studies about FDG PET/CT.
Figure 1.

Flow diagram of study selection.
Eleven studies were published in the English language [8-18] while twelve studies in Chinese language [18-30]. The characteristics of the 23 studies are summarized in Table 1. In T classification, 884 patients were included in MRI test, 335 patients in CT test and 257 patients in FDG PET/CT test. In N classification, 1216 patients were included in MRI, while 290 patients in CT test and 1229 patients in FDG PET/CT. In M classification, 261 patients were included in MRI test and 98 patients in CT test while 1009 patients in FDG PET/CT test. The mean age of the included patients was 48.2 years and approximately 69.9% were male. Nine studies included patients of T stage [9,11-13,15,18,21,25,30], twelve studies included patients of N stage [9,11,19,22,24,26-30], and eight studies included patients of M stage [8-10,14,16,17,19,20].
Table 1.
Characteristics of included studies
| Study | Patient number | Median age | Male (%) | Language | Classification | QUADAS | Reference standard |
|---|---|---|---|---|---|---|---|
| Chen 2006 [9] | 20 | 46.3 | 70 | En | T, N, M | 10 | Nasoscope and CT/MR and clinical follow-up |
| Chua 2009 [10] | 78 | 50 | 76.9 | En | M | 9 | Histological proof, Clinical follow-up for 6 month |
| Comoretto 2008 [11] | 63 | 52 | 69.8 | En | T, N | 8 | Pathologic evaluation and follow up for at least 6 month |
| Gao 2014 [12] | 150 | 48 | 66 | En | T | 10 | Sonography or endoscopic biopsy |
| King 2011 [13] | 246 | 50 | 59.8 | En | T | 12 | Endoscope biopsy |
| Tang 2013 [14] | 583 | 46 | 81.3 | En | M | 13 | Conventional work-up (CWU) |
| Lim 2012 [15] | 78 | 51 | 76.9 | En | T | 10 | Histological proof |
| Ng 2009 [16] | 111 | 48.9 | 75.7 | En | M | 10 | Histological analysis or close clinical and imaging follow-up for 12 month |
| Ng 2009 [8] | 150 | 48.17 | 74 | En | M | 11 | Histological analysis or close clinical and imaging follow-up for 12 month |
| Iagaru 2011 [17] | 26 | 47.3 | 69.2 | En | M | 9 | Clinical follow-up |
| Ma 2009 [18] | 57 | 46 | 82.4 | En | T | 12 | Clinical symptom or MRI or CT |
| Shen 2007 [20] | 23 | 50 | 69.7 | Ch | M | 9 | Endoscope biopsy and follow-up |
| Zhang 2010 [21] | 13 | 46.7 | 61.9 | Ch | T, N | 11 | Histology, biopsy and follow-up |
| Wang 2007 [22] | 18 | 52 | 60.5 | Ch | N | 8 | CT and MRI |
| Wang 2014 [23] | 60 | 52 | 60.5 | Ch | N | 9 | Histology biopsy |
| Huang 2013 [24] | 80 | 49.2 | 70 | Ch | N | 11 | Histology proof and follow up |
| Cai 2011 [25] | 25 | 50 | 64 | CH | T | 12 | Clinical findings, MRI or CT |
| Hu 2005 [26] | 105 | 43 | 78.1 | Ch | N | 10 | Histology and follow-up |
| Zhang 2006 [27] | 116 | 51 | 79.3 | Ch | N | 10 | Follow up |
| Lin 2008 [28] | 68 | 41 | 58.8 | Ch | N | 11 | MRI neck |
| Su 2006 [29] | 53 | 40 | 68 | Ch | N | 11 | MRI-looking at retropharyngeal LN |
| Sun 2005 [30] | 249 | 45 | 75 | Ch | T, N | 9 | Follow-up |
| Lin 2009 [19] | 41 | 52.3 | 60.9 | Ch | N, M | 6 | Clinical follow-up |
Quality assessment showed moderate quality scores of the included studies with a medium score (Table 1). Studies exploring more than one aspect of classification were assessed independently for quality. The methodological quality was high in Tang study (QUADAS score ≥13 [14], moderate in fourteen studies (QUADAS 10-12) [8,9,12,13,15,16,18,21,24-27,29] and low in eight studies (QUADAS<10) [10,11,17,19,20,22,23,30]. Most studies did not describe question 11 and question 12 clearly.
Accuracy
T classification
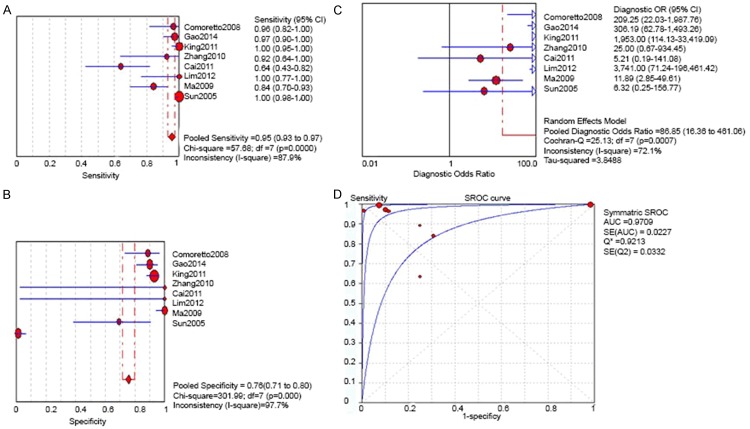
In MRI, the combined data from eight available studies revealed a sensitivity of 0.95 (95% CI 0.93-0.97) and specificity of 0.76 (95% CI 0.71-0.80). The pooled diagnostic odds ratio (DOR) was 86.85 (16.36-461.06). The Q*-index was 0.9213 (SE 0.0372) (Figure 2). As regard as CT, the four included studies were combined and evaluation of T classification showed sensitivity of 0.84 (95% CI 0.79 to 0.88) and specificity of 0.80 (95% CI 0.71 to 0.88). While the pooled DOR is 6.32 (1.17 to 34.02) and Q*-index was 0.7215 (SE 0.054) (Figure 3). In terms of FDG PET/CT, The combined four studies indicate a sensitivity of 0.85 (95% CI 0.76 to 0.91), and specificity of 0.91 (95% CI 0.84 to 0.96). The Q*-index was 0.8673 (SE 0.0311) (Figure 4). Compared to MRI, the sensitivity of CT and FDG PET/CT was lower (0.95 vs 0.84 and 0.85).
Figure 2.
For T Classification by MRI: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio; D. Summary receiver operating Characteristic (SROC) curve with Q*-index.
Figure 3.
For T Classification by CT: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio; D. Summary receiver operating Characteristic (SROC) curve with Q*-index.
Figure 4.
For T Classification by FDG PET/CT: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio; D. Summary receiver operating Characteristic (SROC) curve with Q*-index.
N classification
The combined sensitivity of MRI estimated for N classification in ten studies is 0.88 (95% CI 0.85-0.90) and specificity is 0.95 (95% CI 0.93-0.97). The pooled DOR of MRI was 93.68 (23.21-379.69) and Q*-index was 0.9153 (SE 0.099) (Figure 5). Whereas the combined four studies in CT detection revealed the sensitivity and specificity are 0.92 (95% CI 0.88-0.95) and 0.93 (0.76-0.99) separately. The pooled DOR of CT was 93.81 (22.39-393.03) and Q*-index was 0.8872 (SE 0.0520) (Figure 6). The combined ten studies of FDG PET/CT showed that sensitivity is 0.88 (95% CI 0.85-0.90) and specificity is 0.95 (95% CI 0.93-0.97). The pooled DOR was 93.88 (23.21-379.69) and Q*-index was 0.9153 (SE 0.0299) (Figure 7). The reference standard used among the studies varied. One study used MRI neck [28]. Six studies required Histology and Pathology biopsy or nasoscope [9,11,21,23,24,26]. Four studies relied on clinical follow-up [19,21,26,27,30].
Figure 5.
For N Classification by MRI: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio; D. Summary receiver operating Characteristic (SROC) curve with Q*-index.
Figure 6.
For N Classification by CT: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio; D. Summary receiver operating Characteristic (SROC) curve with Q*-index.
Figure 7.
For N Classification by FDG PET/CT: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio; D. Summary receiver operating Characteristic (SROC) curve with Q*-index.
There is no difference of sensitivity between individual studies on CT no matter what reference standard was used in the studies (p=0.0578). The effect on sensitivity was higher for studies on CT than that on MRI and FDG PET/CT (0.92 vs 0.82 and 0.88). Whereas, specificity showed significant difference among studies in FDG PET/CT and MRI, but did not show difference among studies in CT (p=0.9500).
M classification
The combined sensitivity estimate for MRI is 0.53 (95% CI 0.35-0.70) (Firgure 8), for CT is 0.80 (95% CI 0.44-0.97) (Figure 9), whereas the combined sensitivity for FDG PET/CT is 0.82 (95% 0.74-0.88) (Figure 10). The specificity of MRI, CT and FDG PET/CT is 0.99 (95% 0.95-1.00), 0.93 (95% CI 0.86-0.97) and 0.98 (95% CI 0.96-0.99) respectively. Since only two studies were included on MRI and CT test, no Q*-index was available. Q*-index of studies on FDG PET/CT is 0.9002 (SE 0.075) (Figure 10). All the studies relied on clinical follow-up. Sensitivity of FDG PET/CT is higher than that of MRI and CT (0.82 vs 0.53 and 0.80).
Figure 9.

For M Classification by CT: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio.
Figure 10.
For M Classification by FDG PET/CT: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio; D. Summary receiver operating Characteristic (SROC) curve with Q*-index.
Figure 8.

For M Classification by MRI: A. Pooled sensitivity; B. Pooled specitficity; C. Pooled diagnostic odds ratio.
Discussion
TNM stage is the major prognostic factor of patient survival in NPC [31-33]. In this analysis, MRI has high sensitivity (0.95 (95% CI 0.93-0.97)) than CT and FDG PET/CT in T stage, which indicated that MRI can provide a more accurate evaluation of the extent of the primary tumor. This result is consistent with Patriza study [34]. MRI can identify as retropharyngeal nodes finding previously misdiagnosed on CT as oropharyngeal or parapharngeal invasion. Since MRI has a good capacity to depict the detailed anatomic information, MRI has been widely used in the management of NPC.
In N stage, retropharyngeal node are involved in NPC, presented in 72% NPC patients with nodal disease [15]. The metastasis of cervical lymph nodes is frequently and presented in 60-88.1% NPC patients in regional node involvement [35,36]. This analysis showed that sensitivity of CT (0.92 (95% CI 0.88-0.95)) is higher than MRI (0.88 (95% CI 0.85-0.90)) and FDG PET/CT (0.88 (95% CI 0.85-0.90)). Moreover, sensitivity of MRI is equal to that of FDG PET/CT. Olmi study indicated that either CT or MRI can provide essential information in the staging of NPC. As regards to CT detection in N stage, only four studies were included while ten studies were included in MRI detection analysis and eleven studies included in FDG PET/CT analysis. Ng studied indicated that FDG PET/CT did not have adequate contrast resolution to identify the retropharyngeal nodes that merged with adjacent primary tumor or to discriminate direct tumor invasion from retropharyngeal metastasis. However for identifying cervical lymph node metastasis, FDG PET/CT may be more accurate than MRI [8]. The result of the analysis is keeping with King’s study that FDG PET/CT and MRI had a similar diagnostic accuracy for neck lymph node staging [37].
In distant metastasis stage (M stage), Approximately 15% of untreated NPC patients shows distant metastasis at initial diagnosis [38]. In NPC patients, distant metastasis is generally investigated by conventional imaging work-up (chest X-ray, abdominal ultrasound, and bone scan). In this analysis, two studies reported diagnosis in NPC patients by whole-body MRI. The sensitivity of FDG PET/CT (0.82 (95% 0.74-0.88)) is higher than that of MRI (0.53 (95% CI 0.35-0.70)) and CT (0.80 (95% CI 0.44-0.97)), which is keeping with Senft study [39].
This analysis addresses a pragmatic question, incorporates recently published data including Chinese language and had a standardized study quality assessment. Sensitivity analysis showed consistent results to published review and suggested robustness of the findings.
There are some limitations of this meta-analysis. First of all, this analysis excluded the abstract, letter of editor. This may have cause to publication bias. Second, the reference standard in T NM staging and follow up time in M staging in the included studies were heterogeneity. This may influence the generalizability of the result. Thirdly, all the patients in the included studies in this analysis were pre-treated NPC. In residence and recurrence diagnosis of post-treated NPC patients, the scar or injury in lesion influences the diagnosis accuracy of MRI. These studies were excluded in this analysis. Lastly, even though the majority of the studies were of low-moderate risk of bias based on the QUADAS assessment, the designs in included studies were varied.
In conclusion, for newly pre-treat NPCMRI provides good accuracy in T staging. In N staging, CT showed more accuracy compared to MRI and FDG PET/CT. Based on the different of lymph node metastasis, MRI and FDG PET/CT can potentially aid the delineation. For M stage NPC patients, FDG PET/CT is routine investigations. Further research should be needed to investigate the accuracy of FDG PET/CT together with MRI as a single staging modality in NPC patients [40,41].
Acknowledgements
This work was supported by the National Natural Science Foundation of China (#81360233, 81560282). The Hainan Key Science and Technology Project (#ZDXM20130069), the Hainan Natural Science Foundation-funded project (#812154), and the Hainan Health Department research project approval (#2011-23, #2012PT-19).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sham JS, Choy D, Choi PH. Nasopharyngeal carcinoma: the significance of neck node involvement in relation to the pattern of distant failure. Br J Radiol. 1990;63:108–13. doi: 10.1259/0007-1285-63-746-108. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti AM 3rd. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2009. [Google Scholar]
- 4.NCCN guideline for treatment of cancer by site. Head and neck cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- 5.Berlin JA. Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Meta-analysis Blinding Study Group. Lancet. 1997;350:85–6. doi: 10.1016/s0140-6736(05)62352-5. [DOI] [PubMed] [Google Scholar]
- 6.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;10:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moses LE, Shapiro D, Littenberg B. Combing independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches ans some additional considerations. Stat Med. 1993;12:1293–316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 8.Ng SH, Chan SC, Yen TC, Chang JT, Liao CT, Ko SF, Liu FY, Chin SC, Fan KH, Hsu CL. Staging of untreated nasopharyngeal carcinoma with PET/CT: comparison with conventional imaging work-up. Eur J Nucl Med Mol Imaging. 2009;36:12–22. doi: 10.1007/s00259-008-0918-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen YK, Su CT, Ding HJ, Chi KH, Liang JA, Shen YY, Chen LK, Yeh CL, Liao AC, Kao CH. Clinical usefulness of fused PET/CT compared with PET alone or CT alone in nasopharyngeal carcinoma patients. Anticancer Res. 2006;26:1471–7. [PubMed] [Google Scholar]
- 10.Chua ML, Ong SC, Wee JT, Ng DC, Gao F, Tan TW, Fong KW, Chua ET, Khoo JB, Low JS. Comparison of 4 modalities for distant metastasis staging in endemic nasopharyngeal carcinoma. Head Neck. 2009;31:346–54. doi: 10.1002/hed.20974. [DOI] [PubMed] [Google Scholar]
- 11.Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology. 2008;249:203–11. doi: 10.1148/radiol.2491071753. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Zhu SY, Dai Y, Lu BF, Lu L. Diagnostic accuracy of sonography versus magnetic resonance imaging for primary nasopharyngeal carcinoma. J Ultrasound Med. 2014;33:827–34. doi: 10.7863/ultra.33.5.827. [DOI] [PubMed] [Google Scholar]
- 13.King AD, Vlantis AC, Bhatia KS, Zee BC, Woo JK, Tse GM, Chan AT, Ahuja AT. Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology. 2011;258:531–7. doi: 10.1148/radiol.10101241. [DOI] [PubMed] [Google Scholar]
- 14.Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, Luo DH, Huang PY, Zhang X, Lin XP, Mo YX, Liu LZ, Mo HY, Li J, Zou RH, Cao Y, Xiang YQ, Qiu F, Sun R, Chen MY, Hua YJ, Lv X, Wang L, Zhao C, Guo X, Cao KJ, Qian CN, Zeng MS, Mai HQ. Prospective study of tailoring whole-body dual-modality [18F] fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J. Clin. Oncol. 2013;31:2861–9. doi: 10.1200/JCO.2012.46.0816. [DOI] [PubMed] [Google Scholar]
- 15.Lim TC, Chua ML, Chia GS, Ng DC, Ong SC, Wee JT, Khoo JB. Comparison of MRI, CT and 18F-FDG-PET/CT for the detection of intracranial disease extension in nasopharyngeal carcinoma. Head Neck Oncol. 2012;4:49–53. [Google Scholar]
- 16.Ng SH, Chan SC, Yen TC, Chang JT, Liao CT, Ko SF, Wang HM, Wai YY, Wang JJ, Chen MC. Pretreatment evaluation of distant-site status in patients with nasopharyngeal carcinoma: accuracy of whole-body MRI at 3-Tesla and FDG-PET-CT. Eur Radiol. 2009;19:2965–76. doi: 10.1007/s00330-009-1504-5. [DOI] [PubMed] [Google Scholar]
- 17.Iagaru A, Mittra ES, Gambhir SS. FDG-PET/CT in cancers of the head and neck: what is the definition of whole body scanning? Mol Imaging Biol. 2011;13:362–7. doi: 10.1007/s11307-010-0343-8. [DOI] [PubMed] [Google Scholar]
- 18.Ma XM, Ye M, Liu TF, Li L, Dai LY. Comparison of diagnostic value of PET using 18- fluoro-2-deoxyglucose, CT and MRI in detecting skull base invasion of nasopharyngeal carcinomas. Chin-German J Clin Oncol. 2009;8:456–9. [Google Scholar]
- 19.Lin QY, Z.H.-g. , Zhao JH, Lin CH. Comparison of diagnostic value between 18F-FDG PET/CT and MRI in nasopharyngeal carcinoma. J Jilin Univ (Med Ed) 2009;35:1163–7. [Google Scholar]
- 20.Shen C, L.C. , Yao SZ. Application of 18F-FDG PET-CT in follow-up of nasopharyngeal carcinoma after treatment. J Med Imaging. 2007;17:674–676. [Google Scholar]
- 21.Zhang H, Li GW, Xie AM, Liang ZY. Value of PET-CT and MRI in the diagnosis of recurrence cases after rediotherapy of nasopharyngeal carcinoma. Modern Oncol. 2010;18:2123–7. [Google Scholar]
- 22.Wang GH, Lau EW, Shakher R, Binns DS, Hogg A, Drummond E, Hicks RJ. [Clinical application of 18F-FDG PET/CT to staging and treatment effectiveness monitoring of nasopharyngeal carcinoma] . Chin J Cancer. 2007;26:638–642. [PubMed] [Google Scholar]
- 23.Wang F, L.Y. , Meng W, Li XL. Clinical assessment of 18F-FDG PET/CT in recurrance of nasopharyngeal carcinoma. Chin J Lab Diag. 2014;18:1009–11. [Google Scholar]
- 24.Huang JR, Zhang YL, Zhang C, Chen YM, Wu WY, Chen WJ, Zhao YH. The comparative analysis of MR and PET-CT detecting tetropharygeal lymph node metastasis in nasopharygeal carcinoma. J Med Imaging. 2013;23:846–849. [Google Scholar]
- 25.Cai L, Zhang W, Chen Y, Huang ZW. Value of 18F-FDG PET/CT and MRI for evaluating skull bone metastasis in nasonharyngeal cancer. Chongqing Med J. 2011;40:771–773. [Google Scholar]
- 26.Hu WH, Zhang GY, Liu LZ, Wu H, Li L, Gao YH, Wang QS. Comparison between PET-CT and MRI in Diagnosing Nodal Metastasis of Nasopharyngeal Carcinoma. Chin J Cancer. 2005;24:855–860. [PubMed] [Google Scholar]
- 27.Zhang GY, Hu WH, Liu LZ, Wu HB, Gao YH, Li L, Pan Y, Wang QS. Comparison between PET/CT and MRI in diagnosing lymph node metastatsis and N staging of nasopharyngeal carcinoma. Chin J Oncol. 2006;28:381–4. [PubMed] [Google Scholar]
- 28.Lin XP, Zhao C, Chen MY, Fan W, Zhang X, Zhi SF, Liang PY. [Role of 18F-FDG PET/CT in diagnosis and staging of nasopharyngeal carcinoma] . Chin J Cancer. 2008;27:974–8. [PubMed] [Google Scholar]
- 29.Su Y, Zhao C, Xie CM, Lu LX, Sun Y, Han F, Wu HB, Cui NJ, Zeng ZY, Lu TX. Evaluation of CT, MRI and PET-CT in Detecting Retropharyngeal Lymph Node Metastasis in Nasopharyngeal Carcinoma. Chin J Cancer. 2006;25:521–525. [PubMed] [Google Scholar]
- 30.Sun Y, Ma J, Huang Y. The Study of the Comparison of CT and MRI in Nasopharyngeal Carcinoma. Chin J Clin Oncol. 2005;32:788–91. [Google Scholar]
- 31.Razak AR, Siu LL, Liu FF, Ito E, O’Sullivan B, Chan K. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010;46:1967–78. doi: 10.1016/j.ejca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Rottey S, Madani I, Deron P, Van Belle S. Modern treatment for nasopharyngeal carcinoma: current status and prospects. Curr Opin Oncol. 2011;23:254–8. doi: 10.1097/CCO.0b013e328344f527. [DOI] [PubMed] [Google Scholar]
- 33.Chong VF, Ong CK. Nasopharyngeal carcinoma. Eur J Radiol. 2008;66:437–47. doi: 10.1016/j.ejrad.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Olmi P, Fallai C, Colagrande S, Giannardi G. Staging and follow-up of nasopharyngeal carcinoma: magnetic resonance imaging versus computerized tomography. Int J Radiat Oncol Biol Phys. 1995;32:795–800. doi: 10.1016/0360-3016(94)00535-S. [DOI] [PubMed] [Google Scholar]
- 35.Tang LL, Ma J, Chen Y, Zong JF, Sun Y, Wang Y, Wu HB, Cui NJ. The values of MRI, CT and PET/CT in detecting retropharyngeal lymph node metastasis of nasopharyngeal carcinoma. Ai Zheng. 2007;26:737–41. [PubMed] [Google Scholar]
- 36.King AD, Ahuja AT, Leung SF, Lam WW, Teo P, Chan YL, Metreweli C. Neck node matastases from nasopharyngeal carcinoma: MRI of patterns of disease. Head Neck. 2000;22:275–81. doi: 10.1002/(sici)1097-0347(200005)22:3<275::aid-hed10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 37.King AD, Ma BB, Yau YY, Zee B, Leung SF, Wong JK, Kam MK, Ahuja AT, Chan AT. The impact of 18F-FDG PET/CT on assessment of nasopharyngeal carcinoma at diagnosis. Br J Radiol. 2008;81:291–8. doi: 10.1259/bjr/73751469. [DOI] [PubMed] [Google Scholar]
- 38.Chang JT, Chan SC, Yen TC, Liao CT, Lin CY, Lin KJ, Chen IH, Wang HM, Chang YC, Chen TM, Kang CJ, Ng SH. Nasopharyngeal carcinoma staging by (18)F-fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2005;62:501–7. doi: 10.1016/j.ijrobp.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 39.Senft A, de Bree R, Hoekstra OS, Kuik DJ, Golding RP, Oyen WJ, Pruim J, van den Hoogen FJ, Roodenburg JL, Leemans CR. Screening for distant metastases in head and neck cancer patients by chest CT or whole body FDG-PET: a prospective multicenter trial. Radiother Oncol. 2008;87:221–9. doi: 10.1016/j.radonc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Zaidi H, Del Guerra A. An outlook on future design of hybrid PET/MRI systems. Med Phys. 2011;38:5667–89. doi: 10.1118/1.3633909. [DOI] [PubMed] [Google Scholar]
- 41.Loeffelbein DJ, Souvatzoglou M, Wankerl V, Martinez-Möller A, Dinges J, Schwaiger M, Beer AJ. PET-MRI fusion in head-and-neck oncology: current status and implications for hybrid PET/MRI. J Oral Maxillofac Surg. 2012;70:473–83. doi: 10.1016/j.joms.2011.02.120. [DOI] [PubMed] [Google Scholar]