Abstract
Besides its effect on high blood pressure, T-type calcium channel blocker is renoprotective in experimental models of renal fibrosis. However, the exact mechanism of T-type calcium channel blocker on tubulointerstitial fibrosis is unclear. We investigated whether the renoprotective effect of T-type calcium channel blocker is associated with modulation of the signaling of oxidative stress-induced renal fibrosis. Treatment with a non-hypotensive dose of efonidipine, a T-type calcium channel blocker, or nifedipine, an L-type channel blocker, was initiated one day before unilateral ureteral obstruction (UUO) in C57BL6/J mice, and was continued until 3 and 7 days after UUO. In the obstructed kidneys, treatment with efonidipine significantly attenuated interstitial fibrosis, collagen deposition and inflammation increased by UUO creation compared with treatment with nifedipine. Additionally, efonidipine significantly increased the expression of the antioxidant enzymes heme oxygenase-1, NAD(P)H: quinone oxidoreductase 1, catalase and superoxide dismutase 1. Increased apoptotic cell death and decreased B-cell lymphoma 2 expression were also significantly ameliorated by efonidipine. The expression of the histone acetyltransferase p300/CBP-associated factor, a regulator of inflammatory molecules, was significantly inhibited by efonidipine. These beneficial effects of efonipidine were attributed to the increased nuclear expression of nuclear factor-erythroid-2-related factor 2 (Nrf2) on UUO day 3 and the increased expressions of both total and nuclear Nrf2 with elevated Kelch-like ECH-associated protein 1 on UUO day 7. The data indicate that T-type calcium channel blocker exerts beneficial effects in renal interstitial fibrosis by activating Nrf2 and subsequent antioxidant enzymes.
Keywords: Calcium channel blocker, renal fibrosis, oxidative stress, nuclear factor-erythroid-2-related factor 2, antioxidant
Introduction
Voltage-dependent calcium (Ca) channels are widely distributed throughout the body and play roles in the maintenance of vascular tone [1]. Ca channels are typically classified into several subtypes that include L-type, T-type, N-type, P/Q-type and R-type Ca based on their electrophysiological properties. These Ca channels subtypes are present in the kidney [2]. L-type Ca channels are predominantly distributed in the afferent arterioles of vessels but are sparsely expressed in the efferent arteriole [3]. For several decades, calcium channel blockers (CCBs) targeting L-type Ca channels, such as nifedipine (NFP) and amlodipine, have been widely used for cardiovascular disorders [1,2]. However, the main disadvantage of the L-type CCBs is that it might accelerate glomerular hypertension by preferentially dilating the afferent arteriole [1,3-5].
In contrast, T-type Ca channels are expressed in both afferent and efferent arterioles [3]. This unique distribution may allow T-type CCBs to exert a beneficial action on kidney function by protecting glomeruli from systemic hypertension [1,3]. Efonidipine (EFP), a CCB that acts on both L-type and T-type Ca channels, decreases proteinuria in spontaneously hypertensive rats [1,6]. This renoprotection is almost unaffected by the hypotensive effect, suggesting that non-hemodynamic factors may contribute to the renoprotective mechanisms of T-type CCBs [3].
In the early days of their use, it was reported that a low concentration of EFP, but not NFP or verapamil, showed a significant inhibitory effect on nuclear factor kappa B (NF-κB) in human mesangial cells [7]. Given that Rho kinase activation participates in renal injury, the inhibition of Rho kinase activity by EFP would be another explanation of renoprotective effects of EFP [1,3]. More recently, EFP was reported to attenuate tubulointerstitial damage more favorably than NFP, an L-type CCB, suppressed transforming growth factor (TGF)-β1 and connective tissue growth factor (CTGF) and decreased NADPH oxidase (Nox) subunits in a study using a rat model of unilateral ureteral obstruction (UUO) [8].
Tubulointerstitial fibrosis is the final common pathological feature that eventually progresses to end-stage renal disease [9,10], whose incidence and prevalence have not decreased and have even increased in numerous countries over the last two decades [11,12]. To circumvent this problem, several strategies have been being developed to halt renal fibrosis. However, clinical trials of new drugs that were expected to be effective yielded unsatisfactory results [13,14].
The exact mechanism by which EFP inhibits oxidative stress irrespective of a hypotensive effect would be useful to know. In this study of UUO-induced renal damage, the intracellular pathways of EFP modulation of oxidative stress and subsequent attenuation of renal fibrosis were investigated.
Materials and methods
Animals
Male C57BL/6 mice weighing 20-25 g (OrientBio, Inc., Seoul, Republic of Korea) received UUO or sham surgery as described previously [9,15]. Briefly, the left ureter in the UUO group was ligated using 4-0 silk under general anesthesia and the sham operation was performed in a similar manner except for the absence of ureteral ligation. To assess the effect of T-type Ca channel blocking on the inhibition of renal interstitial fibrosis associated with oxidative stress, we compared the renoprotective effects of EFP, a dual T/L-type CCB, with NFP, a more L-type-selective CCB, in the obstructed kidneys. EFP (50 mg/kg; Sigma-Aldrich Co., St. Louis, MO, USA) or NFP (20 mg/kg; Sigma-Aldrich) was used. The doses chosen have been demonstrated to be non-hypotensive [8]. EFP and NFP were administered by oral gavage one day before UUO and the use of each was continued for 3 or 7 days after surgery. Mice were divided into nine groups (n=8 per group): sham control (sham Cont; sham-operated mice), sham NFP (NFP-treated sham), sham EFP (EFP-treated sham), UUO D3 Cont (mice sacrificed on day 3 after UUO surgery), UUO D3 NFP (NFP-treated UUO D3), UUO D3 EFP (EFP-treated UUO D3), UUO D7 Cont (mice sacrificed on day 7 after UUO surgery), UUO D7 NFP (NFP-treated UUO D7) and UUO D7 EFP (EFP-treated UUO D7). Blood pressure was assessed after UUO surgery using the tail-cuff method with an electrosphygnomanometer (IITC Life Science, Woodland Hills, CA, USA) as described previously [16], confirming that systolic blood pressure was similar among groups on day 6 after UUO (data not shown). All experiments were conducted with the approval of The Institutional Animal Care and Use Committee (IACUC) of The Catholic University of Korea Incheon St. Mary’s Hospital.
Quantitative assessment of kidney collagen content
Total collagen in the obstructed or control kidneys was measured by hydroxyproline assay as described previously [9,17]. Briefly, each accurately weighed sample of the kidney was hydrolyzed in 6 N HCL for 18 hours at 110°C and dried thoroughly at 73°C. After neutralization, the released hydroxyproline was oxidized with chloramine-T solution (1.4% chloramine-T and 10% n-propanol in citric acid buffer). To start the color reaction, 100 μL of Ehrlich’s reagent (15% 4-dimethylamino-benzaldehyde, 62% n-propanol and 18% perchloric acid) was added. Sample absorbance was measured at 550 nm. Total collagen in the kidney tissue was calculated on the assumption that collagen contains 12.7% hydroxyproline by weight.
Histology and immunohistochemistry
Kidney tissues embedded in paraffin wax were used for histologic analyses as previously described [9,15]. Tubulointerstitial fibrosis was assessed semiquantitatively on Masson trichrome-stained paraffin sections. The fibrotic area was quantified using MetaMorph imaging software (Molecular Devices, Sunnyvale, CA, USA) in more than 10 randomly selected fields of each kidney; calculation of the ratio of the fibrotic area to the total selected field indicated the severity of tubulointerstitial fibrosis. The prevalence of interstitial myofibroblasts and degree of apoptosis were determined by immunohistochemistry for α-smooth muscle actin (SMA; Abcam, Cambridge, MA, USA) and TdT-mediated dUTP nick end labelling (TUNEL) assay. The kidney sections were incubated with 3% bovine serum albumin for 30 min at room temperature and incubated with α-SMA at 4°C overnight, followed by peroxidase-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) as secondary antibody. The proportional areas of α-SMA staining were quantified using MetaMorph software. The TUNEL assay was performed on paraffin sections with 4% paraformaldehyde according to the manufacturer’s protocol (Millipore, Billerica, MA, USA). TUNEL-positive cells were evaluated in 20 randomly selected tubulointerstitial fields per each kidney sample. Another slides were reacted with anti-p300/CBP-associated factor (PCAF) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), which was followed by incubation with a biotinylated anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA). Quantification of PCAF-positive stained areas was performed using ImageJ 1.49 software (National Institutes of Health, Bethesda, MD, USA) across 10 randomly selected fields per specimen. All slides were analyzed in a blinded manner.
Western blot analysis
The total cellular protein content of the renal tissues was extracted as previously described [9,15]. Equal amounts of proteins were separated by 15% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Nuclear extracts for nuclear factor-erythroid-2-related factor 2 (Nrf2) immunoblotting were prepared with the NE-PER nuclear kit (Pierce Biotechnology, Rockford, IL, USA). After electroblotting, nonspecific binding was blocked with 5% skim milk. Primary antibodies against the following proteins were used: Nox1 and Nox4 (Santa Cruz Biotechnology), superoxide dismutase (SOD) 1 and 2 (Abcam), heme oxygenase (HO)-1 (BD Biosciences, San Jose, CA, USA), NAD(P)H: quinone oxidoreductase-1 (NQO-1; Santa Cruz Biotechnology), catalase (Abcam), B-cell lymphoma 2 (Bcl-2; Santa Cruz Biotechnology), Kelch-like ECH-associated protein 1 (Keap1; Abcam), Nrf2 (Abcam), and p300/CBP-associated factor (PCAF; Santa Cruz Biotechnology). The blots were washed and incubated with a secondary antibody conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The protein detection was performed by the Image-Quant LAS 4000 mini chemiluminescence detection system (GE Healthcare, Piscataway, NJ, USA). The density of the bands was analyzed using ImageJ software.
Statistics
Data depicted in graphs are expressed as mean ± SE. Statistical differences among groups were calculated using one-way analysis of variance (ANOVA) with Bonferroni correction. In all analyses, P<0.05 was considered statistically significant.
Results
Renal morphology and collagen content
When the kidneys were harvested after the end of study, the kidneys subjected to obstruction developed a conspicuous tubulointerstitial fibrosis, tubular dilatation and atrophy (Figure 1A). Analysis of interstitium by the Masson trichrome staining showed that EFP attenuated interstitial fibrosis more significantly than NFP (Figure 1B). Total collagen content in kidneys was increased after UUO (Figure 2). In contrast, EFP significantly decreased the amount of total collagen in the obstructed kidneys on day 7. In the obstructed kidneys, there was a substantial amount of α-SMA staining within the renal interstitium (Figure 3A). However, UUO D7 EFP exhibited less interstitial α-SMA, with a significant reduction compared to UUO D7 Cont and UUO D7 NFP (Figure 3B). These data showed that although both EFP and NFP treatment could affect renal morphology the administration of EFP resulted in more significant improvement of renal tubulointerstitial injury. According to these morphological results, sham NFP and EFP groups were omitted from further analysis.
Figure 1.

Effect of EFP or NFP on tubulointerstitial fibrosis in UUO kidney. A. Representative images of trichrome staining kidney sections (×200 magnification). B. Histopathologic scores of fibrosis. *P=0.040 vs. UUO D3 NFP and **P=0.029 vs. UUO D7 NFP. EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction.
Figure 2.
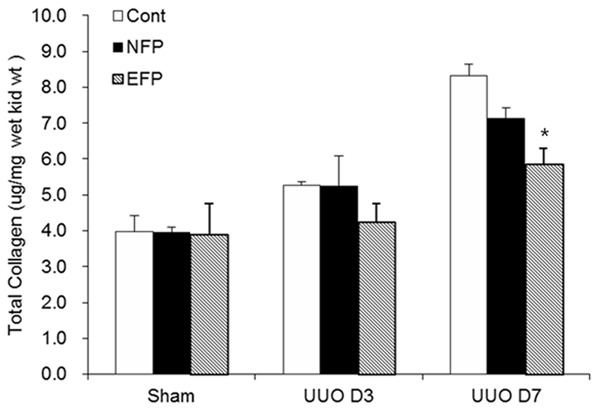
Change in total collagen content with EFP or NFP in the obstructed kidney after UUO. *P<0.05 vs. UUO D7 NFP. EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction.
Figure 3.
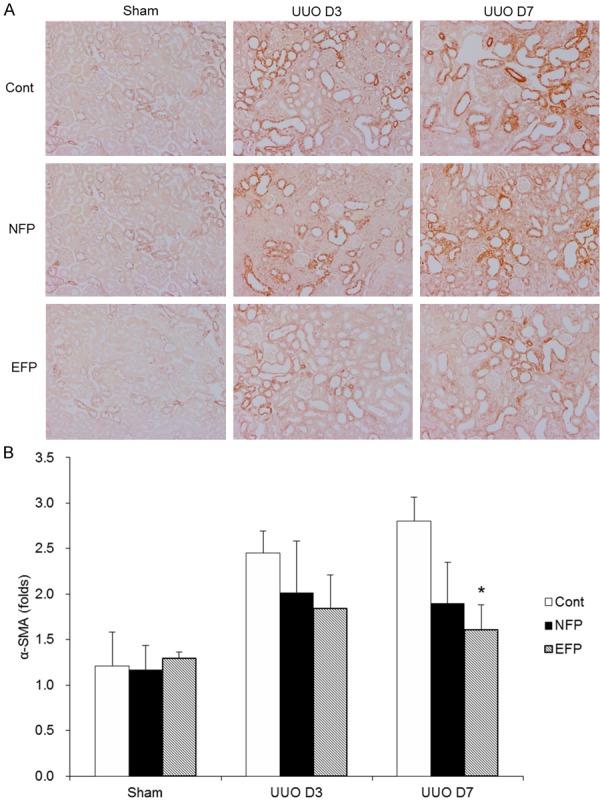
Effect of EFP or NFP on myofibroblast expression in UUO kidney. A. Representative images of α-SMA staining kidney sections (×200 magnification). B. Histopathologic scores of α-SMA. *P<0.001 vs. UUO D7 Cont and P=0.039 vs. UUO D7 NFP. EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction; Cont, control.
Renal oxidative stress and antioxidant enzymes
The expression of Nox1 in the obstructed kidneys was similar among groups (Figure 4A). Renal Nox4 expression, which was elevated in the kidneys of UUO controls, was more significantly decreased in EFP-treated mice compared with in NFP-treated mice on day 3 after UUO, and was similarly decreased in EFP- or NFP-treated mice on day 7 after UUO (Figure 4B). By analyzing the expression of the antioxidants SOD1 and SOD2, we also examined the development of oxidative stress. SOD1 expression was decreased in the obstructed kidneys of UUO mice. There was a significant elevation in renal SOD1 expression with the administration of EFP (Figure 5A). NFP treatment also increased SOD1 expression in UUO kidneys, but not as much as EFP treatment did. In contrast, SOD2 expression was not changed among groups (Figure 5B). Next, renal levels of HO-1, NQO-1 and catalase were determined. EFP significantly enhanced HO-1 at both days 3 and 7 after UUO compared with NFP (Figure 5C). NFP also increased the expression of HO-1 at UUO day 3, but did not affect HO-1 at UUO day 7. EFP also increased NQO-1 protein at UUO day 3 when compared with NFP (Figure 5D). At day 7 after UUO, both EFP and NFP enhanced NQO-1 in the obstructed kidneys. Kidney tissue catalase was increased in UUO D3 EFP compared with UUO D3 Cont and UUO D3 NFP (Figure 5E), but was not changed among groups at day 7 after obstruction. Taken together, antioxidant enzymes in the obstructed kidneys were clearly increased with EFP treatment more than NFP treatment. Furthermore, it is suggested that a major change of cytosolic antioxidant enzymes would be controlled by the center of the cell.
Figure 4.
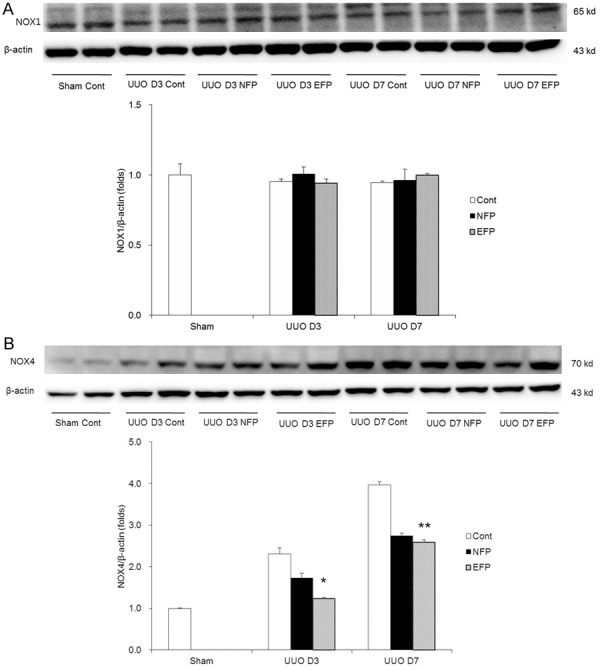
Change in expression of oxidative stress with EFP or NFP in UUO kidney. A. Expression of Nox1. B. Expression of Nox4. *P=0.017 vs. UUO D3 NFP and **P=0.002 vs. UUO D7 Cont. EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction; Nox1, NADPH oxidase 1; Nox2, NADPH oxidase 2; Cont, control.
Figure 5.
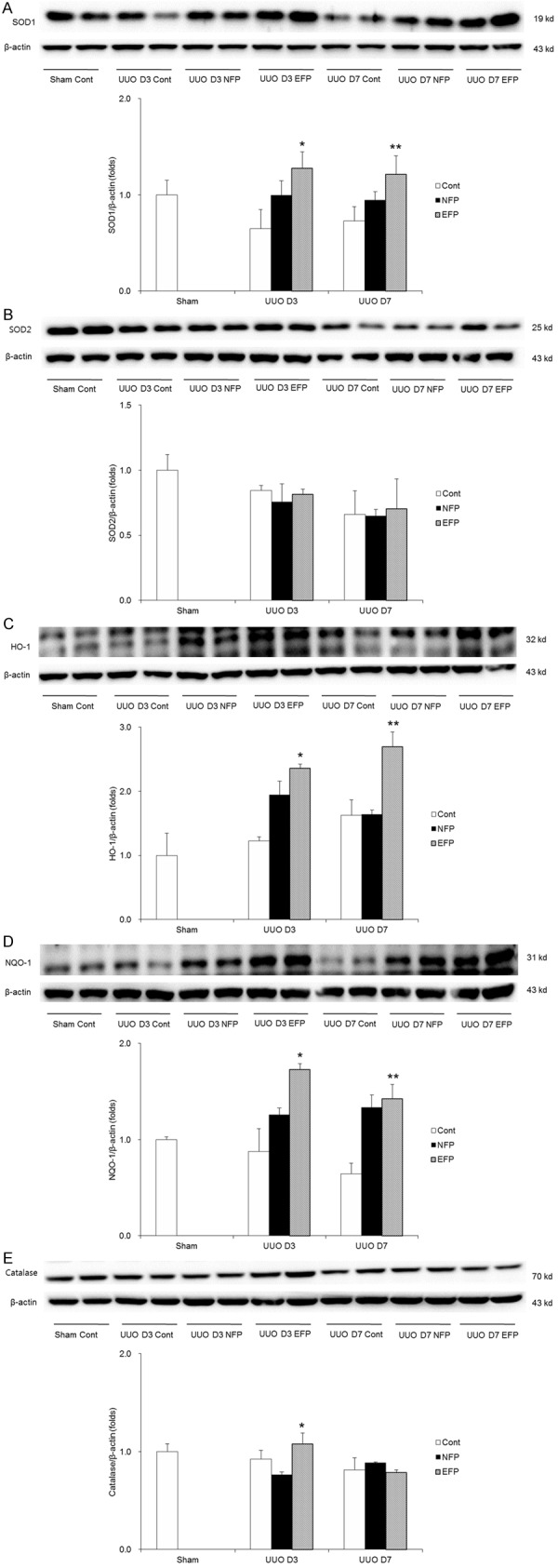
Change in expression of antioxidant enzymes with EFP or NFP in UUO kidney. A. Expression of SOD1. *P=0.017 vs. UUO D3 NFP and **P=0.043 vs. UUO D7 NFP. B. Expression of SOD2. C. Expression of HO-1. *P=0.011 vs. UUO D3 Cont and P=0.028 vs. UUO D3 NFP, and **P<0.001 vs. UUO D7 NFP. D. Expression of NQO-1. *P=0.048 vs. UUO D3 NFP and **P=0.015 vs. UUO D7 Cont. E. Expression of catalase. *P=0.013 vs. UUO D3 Cont and P=0.006 vs. UUO D3 NFP. EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; HO-1, heme oxygenase-1; Cont, control; NQO-1, NAD(P)H: quinone oxidoreductase-1.
Nrf2 signaling pathway
Given that HO-1, NQO-1, catalase and SOD1 are major target genes of Nrf2, we hypothesized that EFP influences the Nrf2 signaling pathway. Renal expressions of total Nrf2 and Keap1 were similar among the UUO groups at day 3, but were significantly increased with EFP treatment at UUO day 7 (Figure 6A and 6B). In addition, nuclear Nrf2 in the kidneys of EFP-treated UUO mice was enhanced at both days 3 and 7 after UUO (Figure 6C). EFP contributed to the increased nuclear expression of Nrf2 at UUO day 3 and the increased expressions of both total and nuclear Nrf2 at UUO day 7. These results indicate that EFP promotes activation of Nrf2 differently depending time course after UUO.
Figure 6.
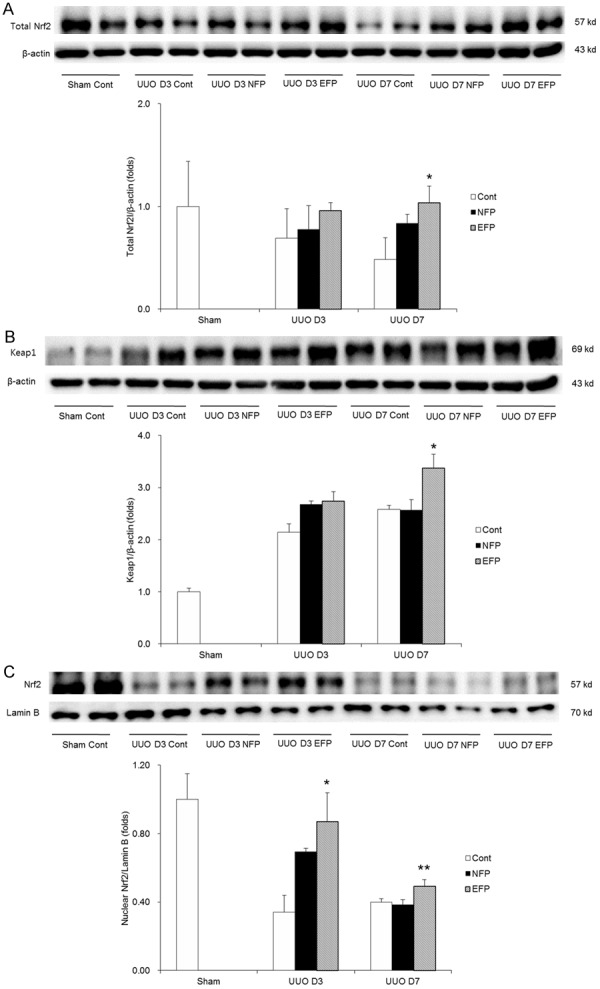
Nrf2 expression in total amount and nuclear fraction and Keap1 expression of renal tissue with EFP or NFP after UUO. A. Expression of total Nrf2. *P=0.006 vs. UUO D7 Cont and P=0.047 vs. UUO D7 NFP. B. Expression of Keap1. *P=0.006 vs. UUO D7 Cont and UUO D7 NFP. C. Expression of nuclear Nrf2. *P<0.001 vs. UUO D3 Cont and P=0.018 vs. UUO D3 NFP and **P=0.034 vs. UUO D7 Cont and UUO D7 NFP. Nrf2, nuclear factor-erythroid-2-related factor 2; Keap1, Kelch-like ECH-associated protein 1; EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction; Cont, control.
Renal apoptosis
To evaluate whether NFP or EFP treatment affected the intrarenal apoptotic networks, TUNEL assay and Western blot of anti-apoptotic Bcl-2 were performed. When TUNEL staining in the obstructed kidney tissue from each treatment group was compared with the UUO controls, EFP treatment groups significantly reduced TUNEL-positive cells (Figure 7A and 7B). The renal expression of Bcl-2 was decreased at 3 or 7 day after obstruction (Figure 7C). However, NFP or EFP treatment had a tendency to increase the level of Bcl-2 in the obstructed kidneys at day 3 after UUO, whereas EFP treatment significantly increased the renal level of Bcl-2 more than NFP treatment did at day 7 after UUO. This result confirmed the previous finding that increased Bcl-2 levels as a result of HO-1 induction protected from renal damage by UUO [18].
Figure 7.
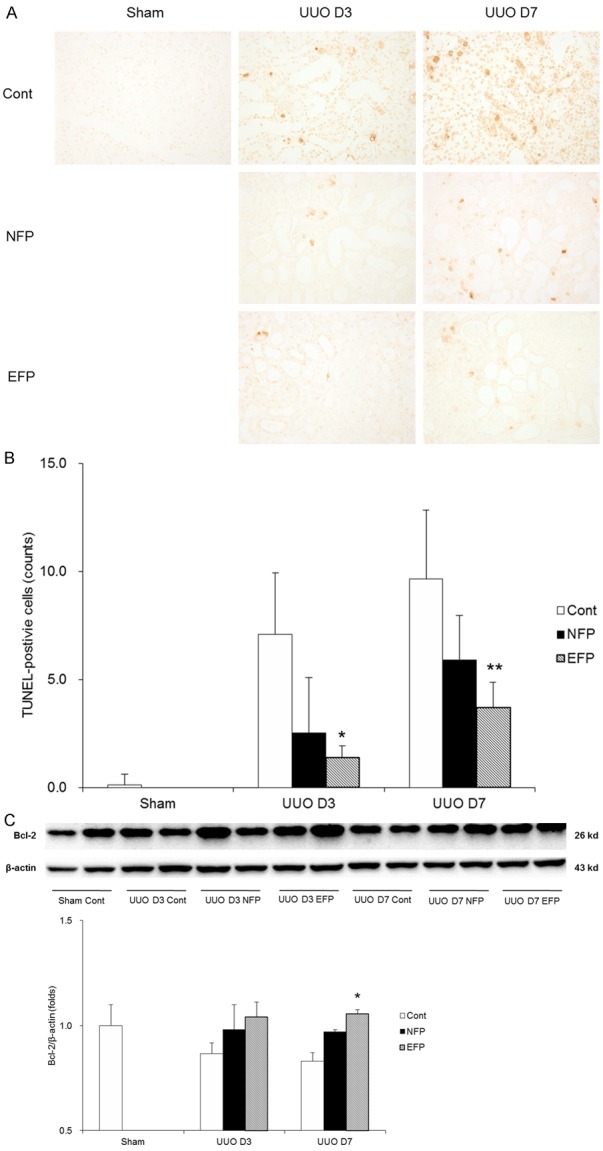
Effect of EFP or NFP on apoptosis in UUO kidney. A. Representative images of TUNEL assay (×200 magnification). B. Quantification of TUNEL-positive cells. *P<0.001 vs. UUO D3 Cont and P=0.032 vs. UUO D3 NFP, and **P<0.001 vs. UUO D7 Cont and P=0.041 vs. UUO D7 NFP. C. Western blot for Bcl-2. *P=0.046 vs. UUO D7 NFP. EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction; TUNEL, TdT-mediated dUTP nick end labelling; Cont, control; Bcl-2, B-cell lymphoma 2.
PCAF expression
The expression of a histone acetyltransferase PCAF, which is an inflammatory regulator [19], was examined. Interestingly, immunostaining with PCAF was reduced by administration of EFP in UUO mice (Figure 8A and 8B). Similarly, there was a significantly increase in protein level of PCAF in the obstructed kidneys of UUO controls but treatment with either EFP or NFP resulted in reduction of its level (Figure 8C). Especially, EFP administration showed the marked reduction in PCAF expression at UUO day 7 when compared with NFP.
Figure 8.
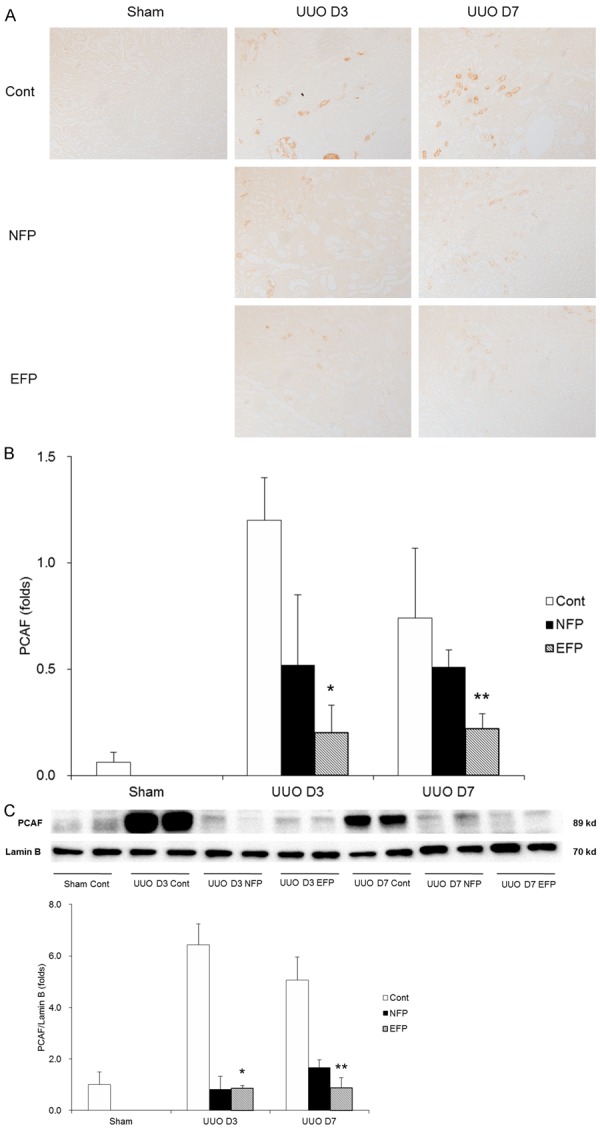
PCAF expression of renal tissue with EFP or NFP after UUO. A. Representative images of PCAF ×200 magnification). B. Quantification of PCAF-positive area. *P=0.002 vs. UUO D3 Cont and P=0.045 vs. UUO D3 NFP, and **P=0.004 vs. UUO D7 Cont and P=0.047 vs. UUO D7 NFP. C. Western blot for PCAF. *P<0.001 vs. UUO D3 Cont and **P<0.001 vs. UUO D7 Cont and P=0.016 vs. UUO D7 NFP. PCAF, p300/CBP-associated factor; EFP, efonidipine; NFP, nifedipine; UUO, unilateral ureteral obstruction; Cont, control.
Discussion
In this study, treatment with a non-hypotensive dose of EFP significantly inhibited renal tubulointerstitial fibrosis induced by UUO. Concomitantly, decreased levels of antioxidant enzymes induced by UUO were significantly restored by EFP. EFP attenuated renal fibrosis by activating the Nrf2 signaling pathway, which lead to the elevation of antioxidant enzymes. In addition, EFP modulated apoptotic and inflammatory responses in UUO-induced renal injury. In contrast, NFP had weak and inconsistent effects on renal fibrotic change, antioxidant enzymes, anti-apoptotic index, inflammatory molecule regulator and Nrf2 signaling.
Among the insults involved in the kidney fibrosis, oxidative stress has been implicated in the pathogenesis of kidney fibrosis [20-22]. Oxidative stress causes tubulointerstitial damage by lipid peroxidation, elevated level of hydrogen peroxide, DNA breakdown and protein modification [9], and also stimulates interstitial fibroblast proliferation, myofibroblast activation and extracellular matrix accumulation [22,23]. Considering that increased concentrations of reactive oxygen species (ROS) are observed in fibrotic kidneys as well as decreased activities of antioxidant enzymes [24,25], a novel therapeutic strategy would be to correct the imbalance and simultaneously increasing the levels of antioxidant enzymes. The best approach could involve Nrf2.
Nrf2 is one of several transcription factors that helps boost cell defenses. It is a master regulator of the antioxidant response element (ARE) that modulates expression of several genes including phase 2 and antioxidant enzymes that participate in detoxification of ROS and electrophilic species, such as HO-1, NQO-1, glutathione-S-transferase, glutamate cysteine ligase catalytic subunit, thioredoxin reductase-1 and SOD [26-28]. Under normal conditions, Nrf2 binds Keap1 and is constitutively degraded through proteasomes located in the cytoplasm [27,28]. When ROS is detected by Keap1, Nrf2 dissociates from Keap1 and translocates from the cytoplasm to the nucleus [9,27,28]. Within the nucleus, Nrf2 binds ARE in the promoter regions of a set of genes encoding several phase 2 detoxifying and antioxidant enzymes, resulting in decreasing oxidative stress and its related injury [27,28]. Presently, treatment with EFP effectively suppressed oxidative stress and increased levels of antioxidants enzymes regardless of its original effect of lowering blood pressure. Given that antioxidant enzymes such as HO-1, NQO-1, SOD1 and catalase were increased with EFP, it is conceivable that EFP could influence a common factor involved in antioxidant regulation. We hypothesized, investigated and proved that the transcription factor Nrf2 has a central role in shaping the antioxidant activities of EFP. This is a novel protective mechanism for EFP in renal fibrosis.
Among antioxidant enzymes upregulated by Nrf2, HO-1 in particular seems to play a major role in maintaining antioxidant homeostasis during oxidative stress [20]. In the current study, HO-1 showed a more significant difference than other Nrf2 downstream gene products. This finding was similar to observations from previous studies of the marked difference in expression of HO-1 after pharmacological intervention compared with other Nrf2 downstream effectors [9,27,29,30]. In a recent study, sustained overexpression of HO-1 protected tubular epithelial cells and peritubular capillaries and suppressed renal inflammation and fibrosis induced by UUO [31]. The mechanism by which HO-1 protects against UUO-induced renal injury appears attributable, in part, to reducing macrophage infiltration and preventing the activation of Wnt/β-catenin signaling [31].
Another unique result in this study is that PCAF was increased in kidneys of UUO mice and EFP and NFP decreased PCAF. This suggests that PCAF would promote signaling molecule associated with regulators of inflammation or oxidative stress, bringing them to the target gene promoter and subsequently affecting target gene transcription. PCAF has been shown to acetylase p53 in response to DNA damage, resulting in the increased transcription of p53-regulated genes [32]. Nrf2 can enhance its transcription capacity and downstream target expression through its acetylation [33]. In addition, Nrf2 may have a role in recruiting the basic transcriptional control machinery to efficiently transcribe the target genes including the various co-activators such as PCAF [34]. Taken together, it is suggested that increased level of PCAF in the obstructed kidneys would lead to an alteration of gene expression involved in cell survival and protection. The current study showed that T-type CCB could discourage possible inflammatory process associated with PCAF and might allow injured cells to survive from their predators. The association between histone acetyltransferase or histone deacetylase and the signaling pathway, such as Nrf2, are largely unknown. More studies are needed to determine the molecular mechanisms underlying the action of PCAF in relation with Nrf2.
There are some points related to current results that we have to address. First, it is necessary to look at both the total and nuclear levels of Nrf2 in the obstructed kidneys without treatment. In response to oxidative stress or inflammation induced by UUO, Nrf2 in UUO kidneys should have been dissociated with its cytosolic inhibitor Keap1 and translocated to the nucleus. However, Nrf2 expression in UUO kidneys decreased rather than increased. Indeed, a higher burden of oxidative stress and a lower level of expression of Nrf2 and its target genes were observed in CKD models [9,27,35]. Second, although NFP showed somewhat weaker renoprotective effect compared with EFP, NFP may have antioxidant activity in renal fibrosis. However, the effect L-type CCB on renal fibrosis remains controversial: one study showed that NFP can suppress perivascular fibrosis, but not interstitial fibrosis in the kidney of renin transgenic hypertensive rats, while another study reported that amlodipine markedly improved renal interstitial fibrosis in a high-salt diet-fed Dahl sati-sensitive rats [8]. Further research is needed to find answers to these unanswered questions. Finally, besides Nrf2, other possible transcriptional factors or signaling pathways that might affect UUO kidneys by EFP treatment. Nrf2 activation would be just one of numerous pathways that are responsible for the cytoprotective activities of EFP. A previous study demonstrated that T-type CCB affects Rho-kinase activity and participates in epithelial-mesenchymal transition [3]. In the present study, to find out the renoprotective mechanism of T-type CCB, its influence on antioxidant pathway was examined though immunoblotting and immunohistochemical studies of antioxidant enzymes and their regulator Nrf2. The results indicate another positive attribute of T-type CCB in providing the protective efficacy towards renal tubulointerstitial fibrosis.
In conclusion, this study is the first to show that T-type CCB attenuates UUO-induced renal tubulointerstitial fibrosis by activating Nrf2 and upregulating its subsequent antioxidants. T-type CCB may be the basis of a novel and effective therapeutic approach.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (NRF-2015R1C1A1A02037258). We thank Jong Hee Chung (Department of Statistics, The Graduate School of Ewha Womans University, Seoul, Republic of Korea) for her statistical advice.
Disclosure of conflict of interest
None.
Authors’ contribution
Sungjin Chung and Seok Joon Shin designed the study. Sungjin Chung, Soojeong Kim, Minyoung Kim, Eun Sil Koh, Hye Eun Yoon and Seok Joon Shin carried out experiments. Sungjin Chung, Ho-Shik Kim, Cheol Whee Park, Yoon Sik Chang and Seok Joon Shin analyzed the data. Sungjin Chung and Seok Joon Shin prepared the manuscript. All authors read and approved the manuscript.
References
- 1.Hayashi K, Homma K, Wakino S, Tokuyama H, Sugano N, Saruta T, Itoh H. T-type Ca channel blockade as a determinant of kidney protection. Keio J Med. 2010;59:84–95. doi: 10.2302/kjm.59.84. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda J, Matsubara M, Yao K. Effects of benidipine in a rat model for experimental angina. Yakugaku Zasshi. 2006;126:1377–1381. doi: 10.1248/yakushi.126.1377. [DOI] [PubMed] [Google Scholar]
- 3.Sugano N, Wakino S, Kanda T, Tatematsu S, Homma K, Yoshioka K, Hasegawa K, Hara Y, Suetsugu Y, Yoshizawa T, Hara Y, Utsunomiya Y, Tokudome G, Hosoya T, Saruta T, Hayashi K. T-type calcium channel blockade as a therapeutic strategy against renal injury in rats with subtotal nephrectomy. Kidney Int. 2008;73:826–834. doi: 10.1038/sj.ki.5002793. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- 5.Chapter 2: Lifestyle and pharmacological treatments for lowering blood pressure in CKD ND patients. Kidney Int Suppl (2011) 2012;2:347–356. doi: 10.1038/kisup.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shudo C, Masuda Y, Sugita H, Tamura T, Furukawa S, Hayashi K, Hirata H, Shikada K, Tanaka S, Tomita K. Effects of efonidipine, nicardipine and captopril on proteinuria in aged spontaneously hypertensive rats. Arzneimittelforschung. 1996;46:852–854. [PubMed] [Google Scholar]
- 7.Hayashi M, Yamaji Y, Nakazato Y, Saruta T. The effects of calcium channel blockers on nuclear factor kappa B activation in the mesangium cells. Hypertens Res. 2000;23:521–525. doi: 10.1291/hypres.23.521. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda H, Mori T, Kurumazuka D, Kitada K, Hayashi T, Nagatoya K, Inoue T, Ukimura A, Matsumura Y, Ishizaka N, Kitaura Y. Inhibitory effects of T/L-type calcium channel blockers on tubulointerstitial fibrosis in obstructed kidneys in rats. Urology. 2011;77:249, e9–e15. doi: 10.1016/j.urology.2010.07.496. [DOI] [PubMed] [Google Scholar]
- 9.Chung S, Yoon HE, Kim SJ, Kim SJ, Koh ES, Hong YA, Park CW, Chang YS, Shin SJ. Oleanolic acid attenuates renal fibrosis in mice with unilateral ureteral obstruction via facilitating nuclear translocation of Nrf2. Nutr Metab (Lond) 2014;11:2. doi: 10.1186/1743-7075-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 11.Chung S, Chung JH, Kim SJ, Koh ES, Yoon HE, Park CW, Chang YS, Shin SJ. Blood lead and cadmium levels and renal function in Korean adults. Clin Exp Nephrol. 2014;18:726–734. doi: 10.1007/s10157-013-0913-6. [DOI] [PubMed] [Google Scholar]
- 12.Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J, Kim YK. Lessons from 30 years’ data of Korean end-stage renal disease registry, 1985-2015. Kidney Res Clin Pract. 2015;34:132–139. doi: 10.1016/j.krcp.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin MP, Reisman SA, Bakris GL, O’Grady M, Linde PG, McCullough PA, Packham D, Vaziri ND, Ward KW, Warnock DG, Meyer CJ. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol. 2014;39:499–508. doi: 10.1159/000362906. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Kim SJ, Yoon HE, Chung S, Choi BS, Park CW, Shin SJ. Fimasartan, a novel angiotensin-receptor blocker, protects against renal inflammation and fibrosis in mice with unilateral ureteral obstruction: the possible role of Nrf2. Int J Med Sci. 2015;12:891–904. doi: 10.7150/ijms.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung S, Park CW, Shin SJ, Lim JH, Chung HW, Youn DY, Kim HW, Kim BS, Lee JH, Kim GH, Chang YS. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant. 2010;25:389–399. doi: 10.1093/ndt/gfp472. [DOI] [PubMed] [Google Scholar]
- 17.Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am J Pathol. 2008;173:631–642. doi: 10.2353/ajpath.2008.080025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Yang JI, Jung MH, Hwa JS, Kang KR, Park DJ, Roh GS, Cho GJ, Choi WS, Chang SH. Heme oxygenase-1 protects rat kidney from ureteral obstruction via an antiapoptotic pathway. J Am Soc Nephrol. 2006;17:1373–1381. doi: 10.1681/ASN.2005091001. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Wan D, Li J, Chen H, Huang K, Zheng L. Histone acetyltransferase PCAF regulates inflammatory molecules in the development of renal injury. Epigenetics. 2015;10:62–72. doi: 10.4161/15592294.2014.990780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Moon SY, Kim JS, Baek CH, Kim M, Min JY, Lee SK. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol. 2015;308:F226–F236. doi: 10.1152/ajprenal.00495.2014. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Yoon SP, Toews ML, Imig JD, Hwang SH, Hammock BD, Padanilam BJ. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;308:F131–F139. doi: 10.1152/ajprenal.00531.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang ST, Kuo YH, Su MJ. KS370G, a caffeamide derivative, attenuates unilateral ureteral obstruction-induced renal fibrosis by the reduction of inflammation and oxidative stress in mice. Eur J Pharmacol. 2015;750:1–7. doi: 10.1016/j.ejphar.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Kim JI, Noh MR, Kim KY, Jang HS, Kim HY, Park KM. Methionine sulfoxide reductase A deficiency exacerbates progression of kidney fibrosis induced by unilateral ureteral obstruction. Free Radic Biol Med. 2015;89:201–8. doi: 10.1016/j.freeradbiomed.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Chan GC, Yiu WH, Wu HJ, Wong DW, Lin M, Huang XR, Lan HY, Tang SC. N-acetyl-seryl-aspartyl-lysyl-proline alleviates renal fibrosis induced by unilateral ureteric obstruction in BALB/C mice. Mediators Inflamm. 2015;2015:283123. doi: 10.1155/2015/283123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang B, Du F, Su X, Sun G, Zhou G, Bian X, Liu N. Epigallocatechin-3-gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic Clin Pharmacol Toxicol. 2015;117:164–172. doi: 10.1111/bcpt.12383. [DOI] [PubMed] [Google Scholar]
- 26.Shokeir AA, Barakat N, Hussein AM, Awadalla A, Harraz AM, Khater S, Hemmaid K, Kamal AI. Activation of Nrf2 by ischemic preconditioning and sulforaphane in renal ischemia/reperfusion injury: a comparative experimental study. Physiol Res. 2015;64:313–323. doi: 10.33549/physiolres.932834. [DOI] [PubMed] [Google Scholar]
- 27.Hong YA, Lim JH, Kim MY, Kim EN, Koh ES, Shin SJ, Choi BS, Park CW, Chang YS, Chung S. Delayed treatment with oleanolic acid attenuates tubulointerstitial fibrosis in chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J Transl Med. 2014;12:50. doi: 10.1186/1479-5876-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng J, Zhao T, Yuan Y, Hu N, Tang X. Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-κB pathways. Chem Biol Interact. 2015;242:353–362. doi: 10.1016/j.cbi.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Basu A, Singha Roy S, Bhattacharjee A, Bhuniya A, Baral R, Biswas J, Bhattacharya S. Vanadium(III)-L-cysteine protects cisplatin-induced nephropathy through activation of Nrf2/HO-1 pathway. Free Radic Res. 2016;50:39–55. doi: 10.3109/10715762.2015.1102908. [DOI] [PubMed] [Google Scholar]
- 30.Dai C, Tang S, Deng S, Zhang S, Zhou Y, Velkov T, Li J, Xiao X. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the Nrf2/HO-1 pathway. Antimicrob Agents Chemother. 2015;59:579–585. doi: 10.1128/AAC.03925-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Wei SY, Li JS, Zhang QF, Wang YX, Zhao SL, Yu J, Wang C, Qin Y, Wei QJ, Lv GX, Li B. Overexpression of heme oxygenase-1 prevents renal interstitial inflammation and fibrosis induced by unilateral ureter obstruction. PLoS One. 2016;11:e0147084. doi: 10.1371/journal.pone.0147084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HL, Seo YR. Molecular and genomic approach for understanding the gene-environment interaction between Nrf2 deficiency and carcinogenic nickel-induced DNA damage. Oncol Rep. 2012;28:1959–1967. doi: 10.3892/or.2012.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai D, Yin S, Yang J, Jiang Q, Cao W. Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis. Arthritis Res Ther. 2015;17:269. doi: 10.1186/s13075-015-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W, Shen G, Yuan X, Jain MR, Yu S, Zhang A, Chen JD, Kong AN. Regulation of Nrf2 transactivation domain activity by p160 RAC3/SRC3 and other nuclear co-regulators. J Biochem Mol Biol. 2006;39:304–310. doi: 10.5483/bmbrep.2006.39.3.304. [DOI] [PubMed] [Google Scholar]
- 35.Shelton LM, Lister A, Walsh J, Jenkins RE, Wong MH, Rowe C, Ricci E, Ressel L, Fang Y, Demougin P, Vukojevic V, O’Neill PM, Goldring CE, Kitteringham NR, Park BK, Odermatt A, Copple IM. Integrated transcriptomic and proteomic analyses uncover regulatory roles of Nrf2 in the kidney. Kidney Int. 2015;88:1261–1273. doi: 10.1038/ki.2015.286. [DOI] [PMC free article] [PubMed] [Google Scholar]


