Abstract
Objective: This study aimed to investigate the protective effects of curcumin on the homocysteine (HCY) induced injury to the endothelial cells. Methods: Endothelial cells were treated with HCY at different concentrations, and MTT assay was employed to determine an optimal concentration of HCY. Cells were divided into 3 groups: normal control group, HCY group and HCY + curcumin group. In curcumin group, cells were pretreated with 2.5 mmol/L HCY for 2 h and then incubated with curcumin at different concentrations. MTT assay was employed to detect the cell viability. ELISA was performed to detect the content of IL-8 in the supernatant. Western blotting was used to detect NF-κB expression in cells. Results: (1) Endothelial cells were polygonal or stone-like, or aggregated to form masses, and then gradually became long spindle shaped, cell body enlarged, cells were rich in cytoplasm, and immunohistochemistry for factor VIII showed positive. (2) MTT assay showed HCY at ≥2.5 mmol/L caused significant damage to endothelial cells as compared to control group. Thus, 2.5 mmol/L HCY was used in following experiments. (3) ELISA showed IL-8 in the supernatant increased significantly in a time dependent manner after HCY treatment (P<0.01), but curcumin could significantly inhibit the IL-8 secretion in endothelial cells after HCY treatment. (4) Western blotting showed HCY was able to markedly increase NF-κB expression, which, however, was significantly inhibited by curcumin. Conclusion: Curcumin is able to protect the endothelial cells against HCY induced injury through inhibiting NF-κB activation and down-regulating IL-8 expression.
Keywords: Vascular endothelial cells, homocysteine, nuclear factor-κB, pro-inflammatory cytokine
Introduction
A recent Chinese report about the cardiovascular diseases indicates that there are about 230 million patients with cardiovascular diseases. Cardiovascular diseases have significantly threatened human health and increased economic losses and mental anguish due to their high morbidity, high mortality and high disability [1]. Thus, to explore the etiology of cardiovascular diseases and investigate the effective strategies for the treatment and prevention of these diseases have been one of focuses in biological and medical studies.
In past several decades, some factors have been confirmed as being closely related to the pathogenesis of cardiovascular diseases. They include advanced age, dyslipidemia, hypertension, diabetes, smoking and obesity. Although great effort has been conducted to interfere with these factors and treat these diseases, the morbidity and disability of cardiovascular diseases remain at a high level, or even tend to increase, and patients diagnosed with cardiovascular diseases tend to be younger.
Homocysteine (HCY) is a sulfur-containing amino acid and was first identified by Devgnesud in 1932. In 1969, investigators found that homocystinuria caused by congenital cystathionine β synthase defect is related to cerebrovascular diseases and for the first time proposed that hyperhomocystinemia was one of risk factors of atherosclerotic vascular diseases, which confirms the relationship between HCY and cardiovascular diseases [2]. In recent years, a variety of clinical trials and epidemiological studies have confirmed that increased plasma HCY is an independent risk factor of ischemic cardiovascular disease [3-5]. Animal studies also reveal that HCY may promote the vascular endothelial inflammation, cause damage to the endothelial cells of major vessels such as carotid artery and aorta and induce the formation of atherosclerotic plaques and thrombi. However, the mechanism underlying the HCY induced endothelial injury is still unclear.
Curcumin is the principal curcuminoid of turmeric, which is a member of the ginger family. It has anti-inflammatory, anti-oxidative and anti-tumor activities [6]. However, the mechanism of its vascular protection is poorly understood. In this study, we investigated the protective effects of curcumin on HCY induced endothelial injury and explore the potential mechanism, aiming to provide a new way for the prevention and treatment of cardiovascular diseases.
Materials and methods
Materials
Following materials were used in this study: human umbilical vein endothelial cell (UVEC) line, RPMI1640, fetal bovine serum (HyClone, USA), dimethyl sulfoxide (Sigma, USA), trypsin (Sigma, USA), L-glutamine (Sigma, USA), EDTA (Sigma, USA), penicillin, streptomycin (Harbin Pharmaceutical Group) and PBS (HyClone, USA).
Methods
Cell culture and passaging
UVECs were thaw at 37°C for 2 min and then transferred into a tube containing 5 ml of RPMI1640 followed by centrifugation at 1500 r/min for 5 min. The supernatant was removed, and cells were re-suspended in RPMI1640 containing 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS and then transferred into a dish, followed by incubation at 37°C in an environment with 5% CO2. The medium was refreshed once every 2 days. When the cell confluence reached 100%, cells were washed with PBS twice, and then 0.25% trypsin (1 ml) was added. Cells were observed under a light microscope. When the intercellular space widened and cells retracted and became round, RPMI1640 containing 10% FBS was added to stop the digestion. The cells were pipetted to form single cell suspension. Cells were counted under a light microscope and then passaged at a ratio of 1:2.
MTT assay
At pre-designed time points, MTT solution was added to each well (20 μl/well), followed by incubation at 37°C for 4 h. The supernatant was removed, and 150 μl DMSO was added to each well, followed by incubation for 10 min. When the crystals resolved, optical density (OD) was measured at 490 nm with a microplate reader (BioRad, USA).
Grouping
Cells in logarithmic growth phase were seeded into 96-well plates at a density of 2.0×104/mL (100 μL/well). Cells were divided into 3 groups: (1) control group: cells were maintained at 37°C for 48 h without any treatment; (2) HCY group: cells were incubated with HCY at different concentrations (0.1, 0.5, 1, 2.5, 5, 10 and 20 mmol/L) at 37°C for 48 h; (3) Curcumin + HCY group: to investigate the protective effects of curcumin on HCY induced endothelial injury, hUVECs were pre-treated with 2.5 mmol/L HCY for 2 h, and then incubated with curcumin at different concentrations (6.25, 12.5, 25, 50 or 100 μg/m L) at 37°C for 48 h. Cell viability was measured with MTT assay.
Factor VIII immunofluorescence staining
Cells on coverslips were placed in 35-mm or 60-mm dish and washed thrice with PBS. Cells were then fixed in pre-cold 4% paraformaldehyde for 20 min, followed by washing in PBS thrice. Cells were treated with 0.2% Triton X-100 for 10 min, followed by washing in PBS thrice. After blocking in normal serum for 30 min, cells were washed thrice with PBS. Then, cells were incubated with primary antibody at 4°C over night and washed in PBS thrice. After incubation with secondary antibody at room temperature for 2 h in dark, cells were washed in PBS thrice. The nucleus was stained with DAPI, and cells were photographed under a fluorescence microscope. Distilled water was used to remove PBS, followed by mounting.
Western blot
hUVECs suspension was treated with protein lysis buffer according to manufacturer’s instructions, followed by centrifugation at 12000 r/min for 20 min at 4°C. After quantification of protein concentration, proteins of equal amount were loaded for SDS-PAGE and then transferred onto PVDF membrane. The membrane was blocked for 1 h and then treated with primary antibody for 90 min. After washing, the membrane was treated with horseradish peroxidase (HRP) conjugated secondary antibody for 45 min and then with ECL solution of equal volume for 1 min. The membrane was exposed with a film, and protein bands were scanned and analyzed with Smartscape image analysis system.
Detection of plasma NF-κB by ELISA
ELISA was employed for the detection of plasma NF-κB of rats. Samples or standards were independently added to 96-well plate (100 μL/well), followed by incubation for 2.5 h at room temperature. After washing in PBS 4 times, 100 μL of 1× detection antibody was added, followed by incubation at room temperature for 1 h. Then, 100 μL of 1× HRP conjugated streptavidin was added, followed by incubation at room temperature for 45 min. After washing 4 times, 100 μL of tetramethylbenzidine was added. After addition of 50 μL of stop solution, the OD was detected at 450 nm with a microplate reader. Experiment was performed thrice.
Statistical analysis
Statistical analysis was performed with SPSS version 16.0. Quantitative data are expressed as mean ± standard deviation. Data were compared with one way analysis of variance or t test. A value of P<0.05 was considered statistically significant.
Results
Identification of endothelial cells
Cells were observed under an inverted microscope. Vascular endothelial cells were adherent under a microscope, and triangular or spindle-shaped. The cytoplasm was transparent, the nucleus was round, and the confluent cells were paving-stone like (Figure 1). These cells were positive for factor VIII which was mainly found in the cytoplasm (Figure 2).
Figure 1.
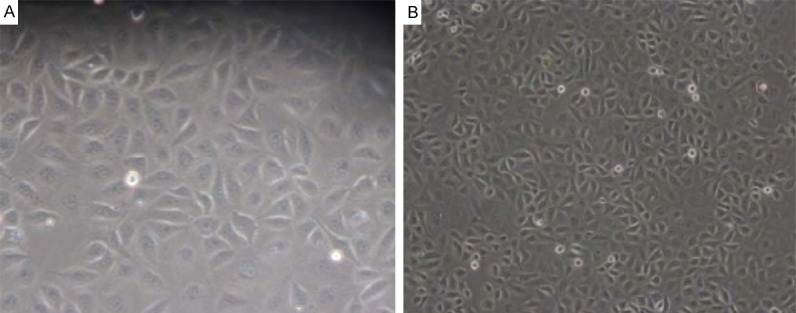
Morphology of vascular endothelial cells. A: Cells were triangular or spindle-shaped (200×). B: Cells were paving-stone like (100×).
Figure 2.
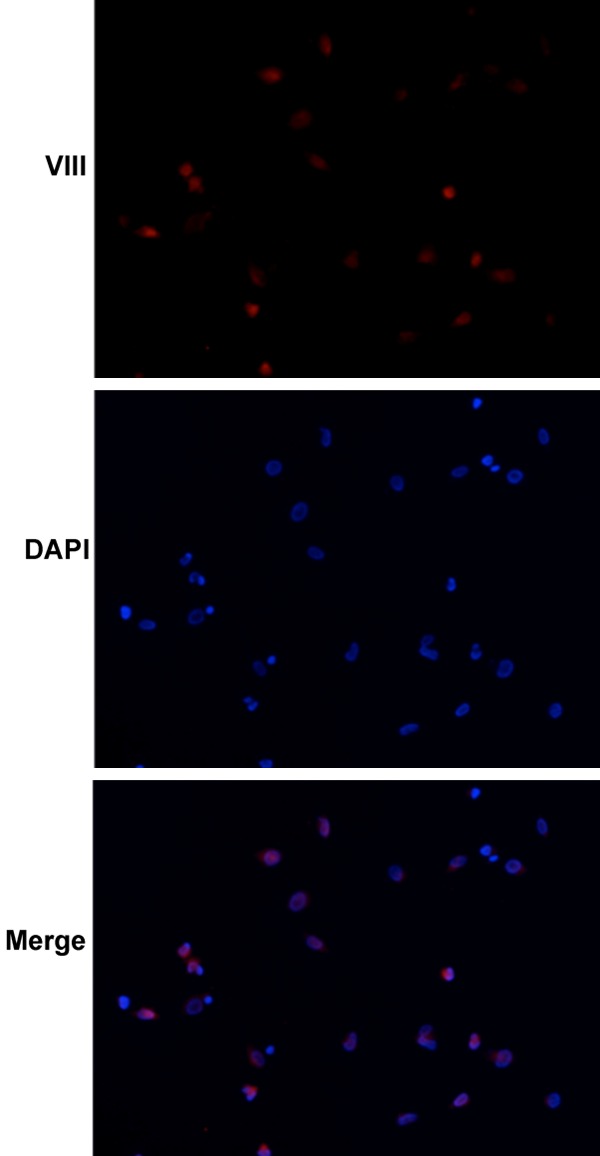
Factor VIII expression in vascular endothelial cells. The vascular endothelial cells had a high factor VIII expression. DAPI was used to stain nucleus. After merging, factor VIII was mainly found in the cytoplasm.
HCY induced vascular endothelial injury
MTT assay showed the OD of endothelial cells decreased with the increase in HCY concentration (Table 1). When the HCY concentration was 0.1, 0.5 and 1 mmol/L, the OD of HCY treated cells was similar to that in control group. When its concentration was 2.5 mmol/L, the OD of HCY treated cells reduced significantly when compared with control group (P<0.05), and the higher the HCY concentration, the lower the OD was (Figure 3).
Table 1.
Effect of HCY at different concentrations on the survival rate of vascular endothelial cells
| HCY (mmol/L) | Cells (1×104/ml) | Survival rate |
|---|---|---|
| 0 | 100 μL | 98% |
| 0.1 | 100 μL | 88% |
| 0.5 | 100 μL | 82% |
| 1 | 100 μL | 72% |
| 2.5 | 100 μL | 53% |
| 5 | 100 μL | 42% |
| 10 | 100 μL | 33% |
| 20 | 100 μL | 17% |
Figure 3.
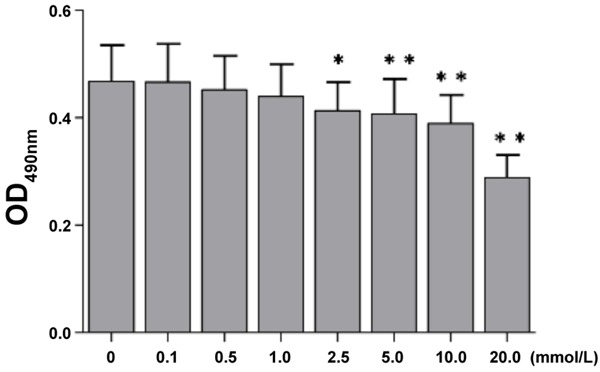
Effect of HCY on viability of vascular endothelial cells. The OD of vascular endothelial cells reduced with the increase in HCY concentration. When the HCY concentration was 2.5 mmol/L or higher, the OD of HCY treated cells was significantly lower than that in control group (P<0.05 or 0.01).
After treatment with HCY at different concentrations for 48 h, cells were counted. When the HCY concentration was 2.5 mmol/L, the survival rate was near to that at LD50. According to the findings from MTT assay, the cell viability reduced significantly when the HCY concentration was 2.5 mmol/L as compared to control group (P<0.05). Thus, HCY at 2.5 mmol/L was used in following experiments.
Effects of HCY on the expression of IL-8 and NF-κB in vascular endothelial cells
2.5 mmol/L HCY could promote the secretion of IL-8 in a time dependent manner. The IL-8 content after 2-h HCY treatment was significantly higher than in control group (P<0.05), thereafter further increased and peaked at 12 h. However, the IL-8 content reduced thereafter, but it at 24 h was still higher than in control group (P<0.05). At 48 h, the IL-8 content returned to normal level (Figure 4).
Figure 4.
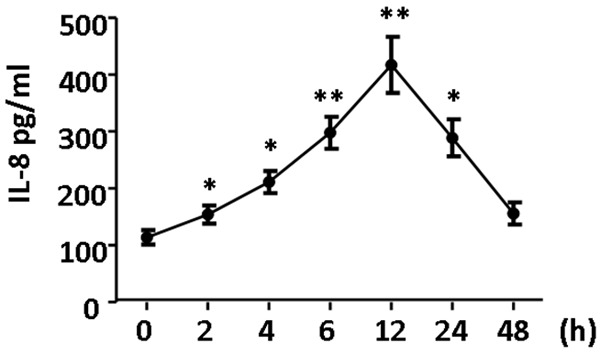
Effect of HCY on IL-8 expression in vascular endothelial cells. 2.5 mmol/L HCY increased IL-8 secretion in hUVECs in a time dependent manner. After 2-h HCY treatment, IL-8 content was significantly higher than in control group, which was also observed at 4 h and 6. The IL-8 content peaked at 12 h. However, IL-8 content reduced thereafter and returned to normal level at 48 h (P<0.05 or 0.01).
Effects of HCY on NF-κB expression
Western blotting showed, after treatment with 2.5 mmol/L HCY, NF-κB P65 protein expression increased significantly and peaked at 0.5 h (P<0.01), but thereafter reduced and returned to baseline level at 4 h. This indicates that HCY treatment activates NF-κB (Figure 5).
Figure 5.
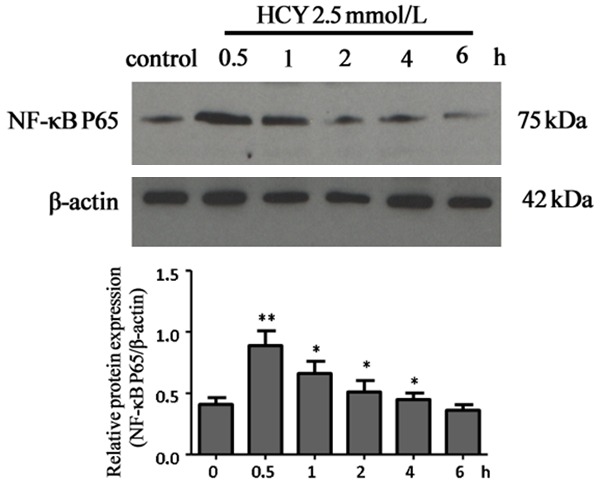
Effect of HCY on NF-κB expression in vascular endothelial cells. After treatment with HCY at 2.5 mmol/L, the NF-κB P65 protein expression increased significantly, peaked at 0.5 h, thereafter reduced and returned to baseline level at 4 h (P<0.05, P<0.01).
Protective effects of curcumin on HCY induced endothelial injury
MTT assay showed curcumin at different concentration was able to significantly increase cell viability (P<0.05). In the following experiment, curcumin at 12.5 ng/ml was used (Figure 6).
Figure 6.
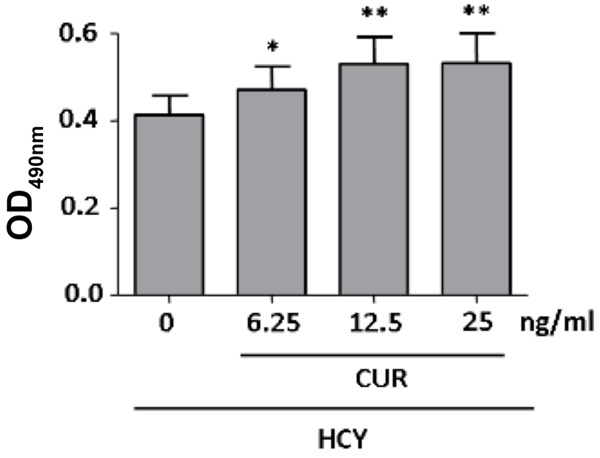
Protective effects of curcumin on HCY induced endothelial injury. HCY 2.5 mmol/L significantly reduced the proliferation of vascular endothelial cells. When additional curcumin (6.25 ng/ml or higher) was added, the cell viability increased significantly. Significant increase in viability was observed when the concentration of curcumin was 6.25 ng/ml or higher, and thus curcumin at 6.25 ng/ml was used in following experiments.
In HCY treated hUVECs, curcumin at 6.25 ng/ml was able to significantly reduce IL-8 in the supernatant of cells as compared to curcumin free cells (Figure 7).
Figure 7.
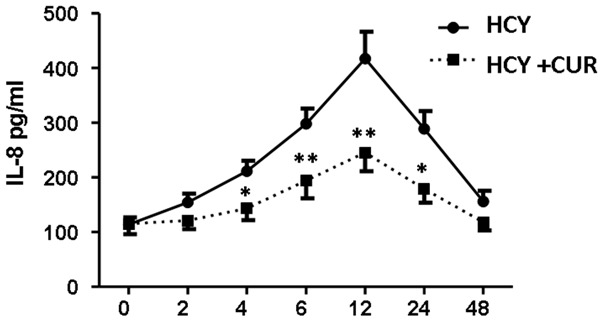
Effect of curcumin on HCY induced IL-8 secretion in endothelial cells (*P<0.05, **P<0.01).
Western blotting showed curcumin at 6.25 ng/ml was able to significantly reduce NF-κB P65 protein expression at 0.5, 1, 2 and 4 h, but there was no significant difference at 6 h between crucumin treated cells and curcumin free cells (Figure 8).
Figure 8.
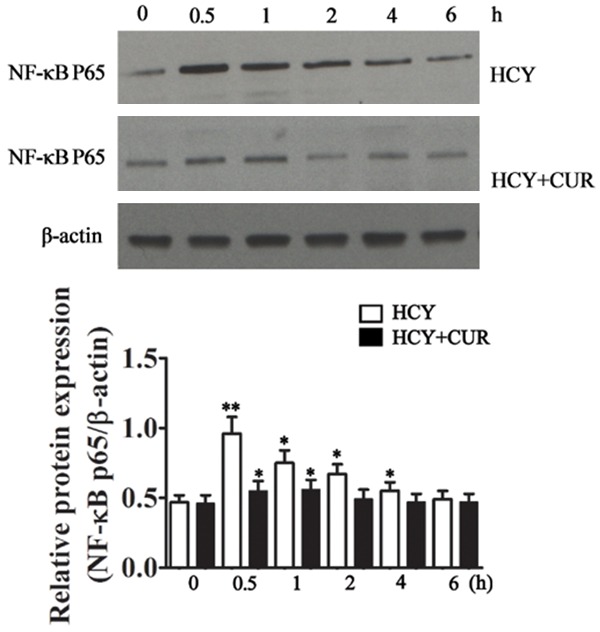
Effects of curcumin on NF-κB expression in HCY treated endothelial cells. Curcumin treatment for 0.5 h significantly reduced NF-κB p65 expression, which was also observed at 1, 2, and 4 h, but there was no significant difference at 6 h between crucumin treated cells and curcumin free cells.
Discussion
Studies have confirmed that hyperhomocystinemia is an independent risk factor of cardiovascular diseases, and increment of 5 μmol/L plasma HCY may increase the risk for arteriosclerosis as an increase of 0.52 mmol/L cholesterol [7]. The mechanism of hyperhomocystinemia related cardiovascular diseases is still poorly understood and might be related to the endothelial injury, proliferation of smooth muscle cells, increased lipid peroxidation and thrombosis [8,9].
IL-8 is a CXC chemokine. It may induce the chemotaxis and activation of neutrophils and T lymphocytes, but stimulate the proliferation and migration of smooth muscle cells and induce the chemotaxis and adhesion of monocytes to vascular endothelial cells, which play important roles in the pathogenesis of arteriosclerosis [7]. HCY induced IL-8 expression has been found to be associated with increased intracellular oxidative stress and activation of NF-κB [10-12]. Our results showed HCY at 2.5 mmol/L could induce the synthesis and secretion of IL-8 in hUVECs at 6 h and 12 h, and peaked at 12 h, its high expression remained for more than 24 h, and thereafter it returned gradually to baseline level. In cell culture, HCY treatment is transient, but in human body, hyperhomocystinemia is persistent and elevated HCY may persistently induce the secretion of IL-8 in endothelial cells, making it remain at a high level. This may unavoidably cause damage to vascular endothelial cells, promote the adhesion of leukocytes to vascular endothelial cells and then induce the arteriosclerosis. This indicates that hyperhomocystinemia may induce vascular inflammation, which may be one of mechanisms underlying the hyperhomocystinemia induced arteriosclerosis.
Inflammation plays important roles in the initiation and progression of arteriosclerosis as well as the occurrence of arteriosclerosis related complications. NF-κB is a key transcription factor in the production of pro-inflammatory cytokines. NF-κB has been detected in the atherosclerotic plaques, and specific silencing or inhibition of NF-κB pathway may reduce the area of atherosclerotic plaques and decrease atherosclerosis related complications [12]. NF-κB is a member of Rel protein family. Kranzhofer et al found the activation of NF-κB in vascular smooth muscle cells may induce the expression of adhesion molecule, inflammatory mediators and growth factors in vascular endothelial cells and smooth muscle cells. In the presence of adhesion molecules, monocytes are adherent to endothelial cells and migrate subendothelially. Moreover, inflammatory mediators and growth factors mediate the chronic inflammation in blood vessel wall as well as the proliferation of smooth muscle cells and their migration into intima, resulting in atherosclerosis [13,14]. Our results also indicated HCY significantly increased NF-κB P65 protein expression in hUVECs, suggesting that HCY induced release of inflammatory cytokines in vascular endothelial cells is related to the activation of NF-κB signaling pathway.
Currently, cardiovascular studies focus on the capability of curcumin to reduce serum triglycerides, cholesterol and low density lipoprotein, increase high-density lipoprotein, elevate cell proliferation and induce cell apoptosis. However, little is known about the protective effects of curcumin on HCY induced endothelial injury. Our results showed curcumin could significantly inhibit NF-κB activation in HCY treated cells and reduce IL-8 secretion. Thus, we speculate that curcumin is able to directly or indirectly block NF-κB signaling pathway to inhibit the release of inflammatory factors by endothelial cells, which protects endothelial cells against inflammation induced injury. Other drugs such as insulin, thiazolidinedione derivatives, metformin, angiotensin transferase inhibitors, angiotensin receptor blockers and statins have also been confirmed to inhibit inflammatory cytokines in cells, but curcumin has little adverse effects and thus is safer as compared to these drugs.
Taken together, HCY may induce the expression and release of inflammatory cytokines by vascular endothelial cells, which is mediated by NF-κB signaling pathway. However, curcumin is able to significantly inhibit these effects of HCY, exerting protective effects on endothelial cells. Our findings provide a new strategy for the prevention of cardiovascular diseases in patients with hyperhomocystinemia.
Acknowledgements
The study was supported by Shanghai Municipal Commission of Health and Family Planning (2015ZB0502). Thanks very much for the kind help from Xiaoyun Xie and Li Cai in Tongji Hospital Affiliated to Tongji University, and Zhaolin Wang in Shanxi Medical University.
Disclosure of conflict of interest
None.
References
- 1.Hu DS, Gu DF. Epidemiological studies for cardiovascular diseases in China from 1980 to 2010. Chin J Epidemiol. 2011;32:1059–1064. [PubMed] [Google Scholar]
- 2.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 3.Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346:1395–1398. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- 4.Selhub J, D’Angelo A. Relationship between homocysteine and thrombotic disease. Am J Med Sci. 1998;316:129–141. doi: 10.1097/00000441-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choy PC, Mymin D, Zhu Q, Dakshinamurti K, O K. Atherosclerosis risk factors: the possible role of homocysteine. Mol Cell Biochem. 2000;207:143–148. doi: 10.1023/a:1017286006708. [DOI] [PubMed] [Google Scholar]
- 10.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 11.Outinen PA, Sood SK, Pfeifer SI, Pamidi S, Podor TJ, Li J, Weitz JI, Austin RC. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94:959–967. [PubMed] [Google Scholar]
- 12.Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 13.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 14.Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994;341:19–22. doi: 10.1016/0014-5793(94)80232-7. [DOI] [PubMed] [Google Scholar]


