Abstract
As a long noncoding RNA, HOX transcript antisense intergenic RNA (HOTAIR) is highly expressed in many types of tumors. However, its expression and function in oral squamous cell carcinoma (OSCC) cells and tissues remains largely unknown. We herein studied the biological functions of HOTAIR in OSCC Tca8113 cells. Real-time quantitative PCR showed that HOTAIR, p21 and p53 mRNA expressions in doxorubicin (DOX)-treated or γ-ray-irradiated Tca8113 cells were up-regulated. Knockdown of p53 expression inhibited DOX-induced HOTAIR up-regulation, suggesting that DNA damage-induced HOTAIR expression may be associated with p53. Transfection and CCK-8 assays showed that compared with the control group, overexpression of HOTAIR promoted the proliferation of Tca8113 cells, while interfering with its expression played an opposite role. Flow cytometry exhibited that HOTAIR overexpression decreased the rate of DOX-induced apoptosis. When HOTAIR expression was inhibited by siRNA, the proportions of cells in G2/M and S phases increased and decreased respectively. Meanwhile, the rate of DOX-induced apoptosis rose. DNA damage-induced HOTAIR expression facilitated the proliferation of Tca8113 cells and decreased their apoptosis. However, whether the up-regulation depends on p53 still needs in-depth studies.
Keywords: Long noncoding RNA, HOX transcript antisense intergenic RNA, oral squamous cell carcinoma, DNA damage
Introduction
Head and neck squamous cell carcinoma is one the most common malignant tumors, with 500 thousand new cases reported annually. As the most common type, oral squamous cell carcinoma (OSCC) severely affects patients’ prognosis due to high degree of malignancy as well as proneness to local invasion and cervical lymph node metastasis [1]. Although OSCC has been tentatively treated by surgery in combination with radiotherapy, neoadjuvant chemotherapy and targeted therapy, the 5-year survival rate is still lower than 50% [2]. Long noncoding RNAs (lncRNAs), which have the lengths of over 200 nt, lack complete open reading frames, barely or even without coding proteins. They can regulate gene expression on various levels [3,4]. Abnormally expressed in many types of tumor cells, lncRNAs play important roles in carcinogenic and tumor suppressor pathways. The molecular mechanisms for tumor-related lncRNAs remain unclear, but they allow early diagnosis and effective treatment.
Genomic stability is crucial to the survival and proliferation of all organisms. DNA damage mainly results from external environment and endogenous genotoxicity including ultraviolet light, ionizing radiation, chemotherapeutic agents and cellular metabolites. Particularly, reactive oxygen species lead to DNA double strand breaks as well as base missing and mismatch [5]. DNA damage response refers to a series of responses and resistances of eukaryotic cells to genetic toxic effects, which maintains genomic stability. Almost all DNA damage responses contain a series of closely related regulatory pathways, involving ataxia-telangiectasia mutated gene (ATM), histone H2AX, anti-oncogene p53 and its downstream gene p21. Upon DNA damage, ATM or related genes can recognize damaged molecules to phosphorylate checkpoint kinases, thereby activating p53 protein and inhibiting its ubiquitination and degradation to enhance the stability [6]. When DNA double strands are broken, activated ATM phosphorylates H2AX and recruits considerable DNA repair factors at the damage sites. The signal disappears after successful repair [7,8]. Besides, lncRNAs such as lincRNA-p21 [9], PANDA1 (p21 associated ncRNA DNA damage activated) [10] and CCND1 (cyclin D1) [11] are also involved in this process. To respond DNA damage stress, the expressions of many lncRNAs change and interact with downstream molecules through epigenetic regulation and/or transcriptional regulation. As a result, specific genes are regulated to affect biological processes such as cell cycle, proliferation and apoptosis.
HOX transcript antisense intergenic RNA (HOTAIR) is one of the seldom studied lncRNAs and has the length of 2158 bp, which exerts its effects through antisense silencing [12]. The effects of HOTAIR on breast, colon, liver and pancreatic cancers have been well documented, indicating that it may directly regulate cancer progression and be associated with the prognosis. Doxorubicin (DOX) causes DNA damage and induces up-regulation of HOTAIR in liver cancer cells [13]. Different cells respond to various damage factors differently. In this study, DNA damage in OSCC Tca8113 cells was triggered by using DOX or γ ray irradiation, after which changes in HOTAIR expression were detected to explore the relationship between this response and p53. The effects of HOTAIR overexpression or interference on the proliferation, apoptosis and cell cycle of these cells were evaluated, aiming to clarify the mechanism by which HOTAIR participated in cancer onset, progression and chemoradiotherapy.
Materials and methods
Materials
pcDNA3.0 HOTAIR plasmid was purchased from Biomoles (USA). OSCC Tca8113 cells were provided by China Center for Type Culture Collection. ImProm-II TM reverse transcriptase and jetPRIME® transfection kit were bought from Promega (USA). Trizol total RNA extraction kit, Lipofectamine2000 transfection kit and apoptosis detection kit were obtained from Life Technologies (USA). qPCR kit and SYBR premix ExTaq were purchased from TaKaRa (Japan). DOX was provided by Sigma (USA). CCK8 kit was bought from DOJINDO Laboratories. Color developing agent for Western blotting substrate was obtained from Sigma-Aldrich (USA). Nitrocellulose membrane was purchased from Amersham Biosciences (USA). p53 and γH2AX antibodies were provided by Abcam (USA). Interfering RNA sequences were synthesized by GenPharma (USA), and qRT-PCR primers were prepared by Life Technologies (USA). Double-strand p53 siRNA, HOTAIR siRNA and control sequences are summarized in Table 1. PCR primer sequences are listed in Table 2.
Table 1.
Primer sequences for siRNA
| Gene | Primer sequence (5’-3’) |
|---|---|
| NC | Sense: UUCUCCGAACGUGCACGUTT |
| Antisense: ACGUGACACGUUCGGAGAATT | |
| P53 siRNA (26) | Sense: GCAUGAACCGGAGGCCCAUTT |
| Antisense: AUGGGCCUCCGGUUCAUGCTT | |
| HOTAIR siRNA | Sense: AAAUCCAGAACCCUCUGACAUUUGC |
| Antisense: UUAAGUCUAGGAAGCACGAAGC |
Table 2.
Primer sequences for qRT-PCR
| Gene | Primer sequence (5’-3’) |
|---|---|
| HOTAIR | Sense: CAGTGGGGAACTCTGACTCG |
| Antisense: GTGCCTGGTGCTCTCTTACC | |
| P53 | Sense: ATCTACAAGCAGTCACAG |
| Antisense: TTTCTGTCATCCAAATACTC | |
| P21 | Sense: GTCACTGTCTTGTACCCTTG |
| Antisense: GAAATCTGTCATGCTGGTCT | |
| GAPDH | Sense: TCAGTGGTGGACCTGACCTG |
| Antisense: TGCTGTAGCCAAATTCGTTG |
Induction of cellular DNA damage
Tca8113 cells were inoculated in 25 mL flasks at the density of 8×105, cultured in 5 mL of high-glucose DMEM containing antibiotics (100 U penicillin and 100 μg streptomycin) and fetal bovine serum (10%), treated with DOX or Co 60 γ ray irradiation, and divided into a control group, a 0.2 μmol/L DOX group, a 0.5 μmol/L DOX group, a 2 Gy γ-ray group and an 8 Gy γ-ray group. After another 24 h of culture, total RNA was extracted with Trizol reagent.
Plasmid and siRNA transfection
Tca8113 cells were inoculated in 6-well plates at the density of 2×105, and 2 mL of high-glucose DMEM containing antibiotics and fetal bovine serum was added into each well. After 24 of culture, the cells were transfected with p53 siRNA to the final concentration of 50 pmol/L according to the instructions of Lipofectamine2000 transfection kit. Twenty-four hours after transfection, the cells were treated with 0.2 μmol/L DOX, divided into a +DOX group and a -DOX group and further cultured for 24 h, from which RNA was extracted. According to the instructions of jetPRIME® transfection kit, the cells were transfected with pcDNA3.0-HOTAIR plasmid (2 μg plasmids per well). According to the instructions of Lipofectamine2000 transfection kit, they were transfected with HOTAIR siRNA to the final concentration of 50 pmol/L.
Detection of HOTAIR expression by qRT-PCR
According to the instructions of Trizol reagent kit and reverse transcription kit, total RNA was extracted and cDNA was obtained by reverse transcription. PCR amplification was performed according to the instructions of qPCR kit through pre-denaturation at 95°C for 30 s, denaturation at 95°C for 10 s and at 60°C for 20 s, 40 cycles in total. GAPDH was used as the internal reference.
Immunofluorescence assay
Culture medium was discarded after cells were treated with DOX for 9 h or irradiated with γ ray for 1 h. Then the cells were fixed in 4% paraformaldehyde at room temperature for 20 min, left still in 0.5% detergent Triton-X-100 at room temperature for 5 min, blocked in goat serum working solution for 30 min, and incubated with diluted γH2AX antibody (1:100) at room temperature for 45 min and with goat anti-mouse secondary antibody (1:700) at room temperature for 45 min. Afterwards, cell nuclei were stained with diluted DAPI (1:1000), and the cells were dropped onto a coverslip, sealed with 50% glycerin and PBS, and observed under a fluorescence microscope.
Western blotting
Total protein was extracted from Tca8113 cells that had been transfected with siRNA for 48 h, subjected to electrophoresis (20 μg per well) and electronically transferred to a nitrocellulose membrane at 60 V for 1 h. The membrane was thereafter blocked in TBST containing 5% skimmed milk for 2 h, incubated with diluted p53 antibody (1:2000) and GAPDH antibody (1:5000) at room temperature for 5 h, washed three times in TBST on a shaker (10 min each time), incubated with goat anti-mouse secondary antibody (1:2000) at room temperature for 2 h, washed three times with the method mentioned above, color-developed and fixed.
Detection of cell proliferation by CCK8 assay
Tca8113 cells were transfected with plasmids and siRNA for 24 h (a control group transfected with pcDNA3.0, a group transfected with pcDNA3.0 HOTAIR, a control group transfected with interference and a group transfected with HOTAIR siRNA), and seeded onto 96-well plates at the density of 3×104. Subsequently, 100 μL of high-glucose DMEM containing antibiotics and fetal bovine serum was added to each well. Five replicate wells were set for each group. After 6 h of culture, 10 μL of CCK8 was added into each well. A well without cells was used as the zeroing well. The absorbance at 450 nm (A450) was measured after 2 h of incubation at the same time point for three consecutive days.
Detection of cell cycle by flow cytometry
Tca8113 cells transfected with pcDNA3.0 HOTAIR plasmid or HOTAIR siRNA for 48 h were digested with trypsin, centrifuged, washed twice with 5% BSA, resuspended in 300 μL of 10% BSA, fixed in 700 μL of absolute ethanol at -20°C for 24 h, washed twice with PBS, resuspended in 100 μL of PBS, incubated with 1 μL of RnaseA at 37°C for 30 min and reacted with 300 μL of 50 μg/mL PI in dark at room temperature for 20 min. Cell cycle was then detected by using flow cytometry.
Detection of cell apoptosis by flow cytometry
Tca8113 cells were transfected with plasmids or HOTAIR siRNA for 24 h. DOX at the final concentration of 0.2 μmol/L was added to each well. After being cultured for 24 h, the cells were digested with trypsin, centrifuged, washed twice with 2% BSA, resuspended with 500 μL of binding buffer and mixed with 5 μL of Annexin V-APC. Then cell apoptosis was detected by using flow cytometry.
Statistical analysis
All data were analyzed by SPSS software and compared by Student’s t test. P<0.05 was considered statistically significant. The data were plotted with GraphPad Prism5 software.
Results
DOX or γ ray irradiation induced cellular DNA damage
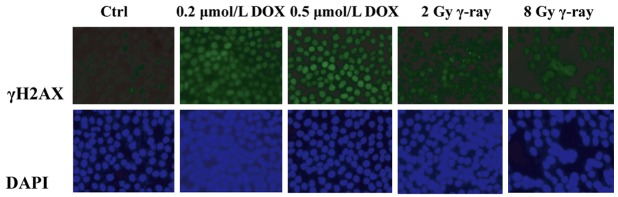
Tca8113 cells were treated with DOX for 9 h or irradiated with γ ray for 1 h, and γH2AX in the cell nucleus was detected by immunofluorescence assay to determine the degree of DNA damage. Figure 1 shows that cells in the blank control group are mildly stained and cell nuclei do not emit fluorescence. However, in DOX-treated and γ ray-irradiated cells, there were a large number of γH2AX foci, and cell nuclei were obviously stained. Compared with the irradiation groups, the DOX groups were stained more evidently in a dose-dependent manner. Therefore, DOX and γ ray irradiation managed to induce cellular DNA damage.
Figure 1.
Induction of HA2X phosphorylation (γ-H2AX) during DNA damage. Tca8113 cells were cultured and exposed to DOX (0.2 μmol/L and 0.5 μmol/L) for 9 h, or treated by γ-ray (2 Gy and 8 Gy) for 1 h. After harvest, the cells were fixed, permeabilized, and immunolabeled with anti-γ-H2AX antibody and secondary antibody (green). The nuclei were stained with DAPI (blue).
DNA damage induced up-regulation of HOTAIR mRNA expression
The effects of DNA damage on HOTAIR mRNA expressions in the cells were evaluated with qRT-PCR. Compared with the control group (Figure 2A), HOTAIR mRNA expression was up-regulated over 4-fold in the 0.2 μmol/L DOX group, over 13-fold in the 0.5 μmol/L DOX group, about 3-fold in the 2 Gy irradiation group and slightly in the 8 Gy irradiation group. As important stress response molecules for DNA damage, p21 and p53 were detected as positive controls. As shown in Figure 2B, p21 and p53 mRNA expressions in DOX-treated and γ ray-irradiated groups are up-regulated, suggesting that DNA damage induced up-regulation of HOTAIR expression in these cells.
Figure 2.
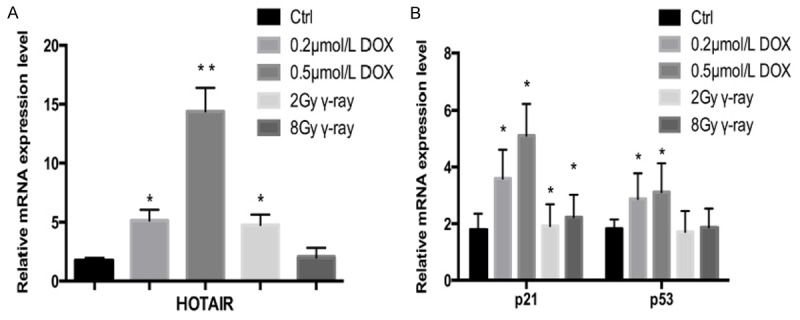
Expression levels of HOTAIR, p21 and p53 mRNA in OSCC Tca8113 cells after DNA damage. Different concentrations of DOX (0.2 μmol/L and 0.5 μmol/L) and γ-ray (2 Gy and 8 Gy) were used to induce DNA damage. Total RNA was isolated from Tca8113 cells 24 h later. The mRNA expressions of HOTAIR, p21 and p53 were examined by qRT-PCR. A. Expression level of HOTAIR in Tca8113 cells after DNA damage. B. Expression levels of p21 and p53 mRNA in Tca8113 cells after DNA damage. Results are mean ± SD of triplicate measurements from three separate experiments. *P<0.05, **P<0.01.
Inhibiting p53 expression weakened the up-regulatory effects of DOX on HOTAIR
p53 expressions in Tca8113 cells were inhibited through transfection of p53 siRNA, and then they were treated with DOX (+DOX group). Meanwhile, a -DOX group was set as control. The effects of p53 and DOX on HOTAIR RNA expression were assessed by using qRT-PCR. The p53 protein and mRNA expression levels were down-regulated in both +DOX and -DOX groups after transfection (Figure 3A), indicating that p53 interference was successful. In the -DOX group (Figure 3B), interfering with p53 hardly affected HOTAIR mRNA expression compared with that in the control group. Nevertheless, relative HOTAIR mRNA expression of the DOX-treated group decreased from 5.5 to 2.3 after p53 interference. In contrast, p21 mRNA expressions were down-regulated in +DOX and -DOX groups. Thus, p53 did not affect the background expression of HOTAIR mRNA in Tca8113 cells, but interfering with p53 gene expression inhibited the up-regulatory effects of DOX on HOTAIR.
Figure 3.
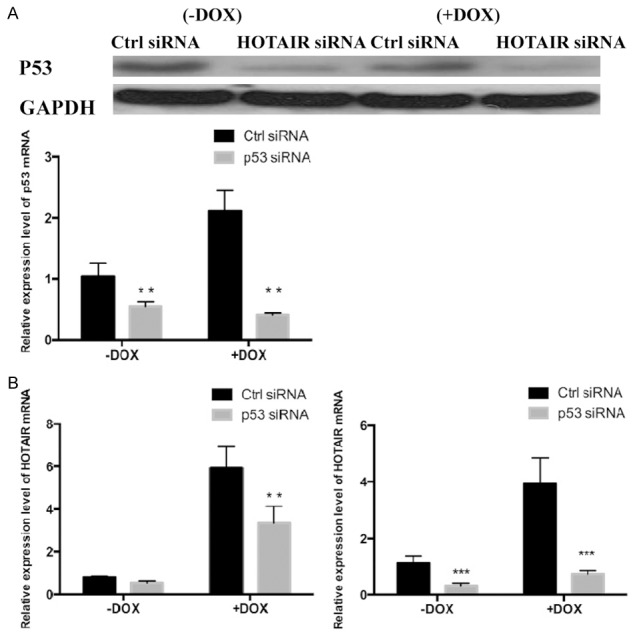
Expression levels of HOTAIR, p21 and p53 mRNA in Tca8113 cells transfected with p53 siRNA. Tca8113 cells were planted in 6-well plates at the density of 2×105 cells/well, transfected with control siRNA or p53 siRNA and treated with 0.2 μmol/L DOX for 24 h. Total RNA and protein were extracted after the cells were treated for 48 h. A. The expressions of p53 mRNA and protein in Tca8113 cells transfected with p53 siRNA were down-regulated, as detected by qRT-PCR and Western blotting. B. The mRNA expressions of HOTAIR and p21 in Tca8113 cells were detected by qRT-PCR. Results are mean ± SD of triplicate measurements from three separate experiments. **P<0.01, ***P<0.001.
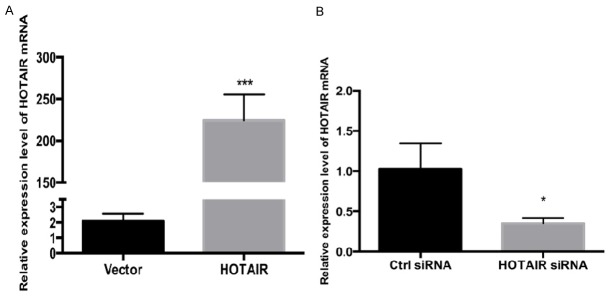
HOTAIR overexpression and inhibition of its expression
HOTAIR expressions in Tca8113 cells transfected with plasmids and HOTIAR siRNA were detected by qRT-PCR. Compared with the group transfected with pcDNA3.0 vector (Figure 4), HOTAIR expression level was up-regulated 300-fold in the group transfected with pcDNA3.0-HOTAIR. Compared with the control group, the relative expression of HOTAIR in the group transfected with HOTAIR siRNA reduced by 70%. Accordingly, HOTAIR overexpression and interference were both successful.
Figure 4.
Expressions of HOTAIR in Tca8113 cells transfected with HOTAIR or HOTAIR siRNA. Tca8113 cells were planted in 6-well plates at the density of 2×105 cells/well, and then transfected with HOTAIR expression vector or siRNA. Total RNA was extracted after the cells were treated for 48 h, and then qRT-PCR was used to detect the expression of HOTAIR. A. The expression of HOTAIR in Tca8113 cells increased after transfection with vector. B. The expression of HOTAIR decreased in Tca8113 cells after transfection with HOTAIR siRNA. Results are mean ± SD of triplicate measurements from three separate experiments. *P<0.05, ***P<0.001.
HOTAIR promoted the proliferation of Tca8113 cells
To evaluate the influence of HOTAIR on the proliferation of Tca8113 cells, CCK8 assay was conducted after transfection of pcDNA3.0 HOTAIR or HOTAIR-siRNA. As shown in Figure 5, the group transfected with pcDNA3.0 HOTAIR has a higher proliferation speed than that of the group transfected with pcDNA3.0. Compared with the control group, the group transfected with HOTAIR siRNA had a lower proliferation speed. Hence, HOTAIR was associated with regulation of the proliferation of Tca8113 cells.
Figure 5.
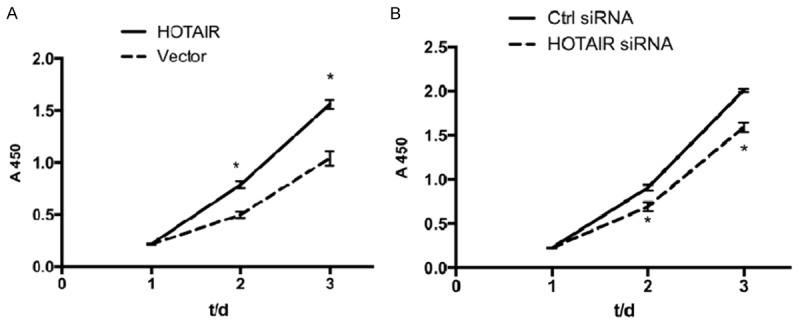
Effect of HOTAIR on proliferation of Tca8113 cells. Tca8113 cells were transfected with pcDNA3.0 vector, control siRNA or HOTAIR siRNA, and then planted in 96-well plates at the density of 3000 cells/well. The cells were incubated with 10 μl/well CCK-8 reagent for 2 h at 37°C. The absorbance at 450 nm was measured to calculate the numbers of viable cells in each well. They were measured for three consecutive days, and five replicate wells were set up in each group. A. Cell proliferation curve was plotted to observe the promotive effects of HOTAIR on Tca8113 cell proliferation. B. Cell proliferation curve was plotted to observe the inhibitory effects of HOTAIR siRNA on Tca8113 cell proliferation. Results are mean ± SD of triplicate measurements from three separate experiments. *P<0.05.
Inhibiting HOTAIR expression arrested Tca8113 cells in the G2/M phase
To assess the effects of HOTAIR on Tca8113 cell cycle, flow cytometry was carried out for all transfected groups. Figure 6 exhibits that after HOTAIR interference, the proportion of cells in the G2/M phase increases (siRNA control group: 43.57±4.75%, HOTAIR siRNA group: 54.75±5.52%), whereas that of cells in the S phase decreases (siRNA control group: 31.56±2.76%, HOTAIR siRNA group: 23.57±0.94%). The results suggested that HOTAIR suppressed the proliferation of cells by arresting them in the G2/M phase.
Figure 6.
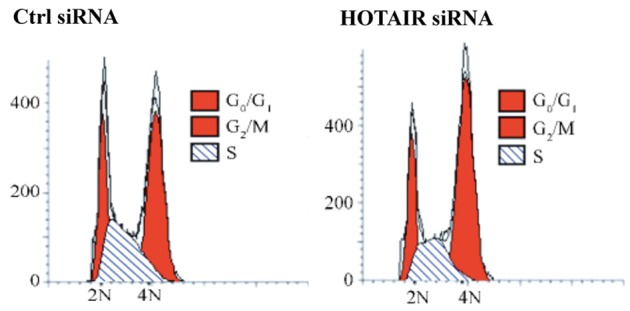
Repression of HOTAIR induced G2/M arrest in Tca8113 cells that were transfected with control or HOTAIR siRNA. After being transfected for 48 h, the cells were incubated with 1 μL of RNaseA (100 μg/mL) at 37°C for 30 min, stained with 300 μl of PI (50 μg/mL) and kept in dark for 20 min. Then cell cycle was analyzed by flow cytometry.
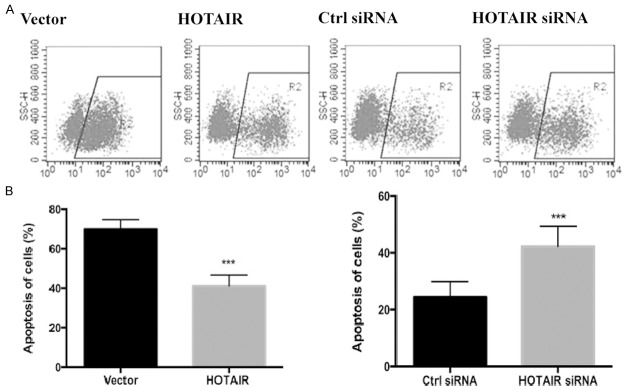
HOTAIR inhibited DOX-induced apoptosis of Tca8113 cells
Total apoptotic rates of all transfected groups were detected by flow cytometry. The apoptotic rate of cells in which HOTAIR was overexpressed decreased after DOX treatment (control group: 67.31±2.03%, HOTIAR group: 35.54±1.35%) (Figure 7). Contrarily, the apoptotic rate increased in DOX-induced cells after HOTAIR interference (siRNA control group: 24.23±2.34%, HOTIAR siRNA group: 45.22±1.52%). Therefore, increase of DOX-induced HOTAIR expression was able to inhibit the apoptosis of Tca8113 cells caused by DNA damage.
Figure 7.
Effect of HOTAIR on DNA damage-induced cell apoptosis. Tca8113 cells were transfected with pcDNA3.0 vector or HOTAIR, control siRNA or HOTAIR siRNA. Then all of transfected groups were treated with 0.2 μmol/L DOX for 24 h. After being transfected for 48 h, the cells were harvested, resuspended in 500 μl of binding buffer and mixed with 5 μl of Annexin V-APC. Then cell apoptosis was analyzed by flow cytometry. (A) Flow cytometry was used to detect Tca8113 cell apoptosis. (B) The number of apoptotic cells in each group was obtained based on (A). Results are mean ± SD of triplicate measurements from three separate experiments. ***P<0.001.
Discussion
OSCC is the most common type of head and neck squamous cell carcinoma, with approximately 500000 new cases reported every year. With invasive growth, high local invasion ability, tendency to cervical lymphatic metastasis and poor prognosis, OSCC is mainly treated by surgery, chemotherapy and radiotherapy [2]. Some chemotherapeutic agents and radiotherapy kill sensitive cancer cells by causing DNA damage, but the tolerant cell subsets still survive and proliferate, severely affecting the therapeutic effects [14]. In this study, OSCC Tca8113 cells were treated by DOX or irradiated by γ ray. Immunofluorescence assay showed that these treatments induced cellular DNA damage, especially in the DOX group, further verifying that the cells showed different sensitivities to various DNA-damaging agents. Under the stress of DNA damage, HOTAIR expression was up-regulated more significantly in the DOX group than that in the irradiation group. On one hand, DNA-damaging agents have specific targets. On the other hand, cells irradiated by γ ray can resist DNA damage by inducing cell cycle arrest or performing repair within 24 h [15]. Thus, HOTAIR may mediate the resistance of tumor cells to DOX and irradiation. Though the correlation between HOTAIR and resistance to chemoradiotherapy remains elusive, HOTAIR is a potential target for predicting and regulating the sensitivity and tolerance of OSCC to chemotherapy and radiotherapy.
DNA damage stress can induce the changes of lncRNA expressions, involving anti-oncogene p53 and other transcription factors. For example, lincRNA-p21 expression is up-regulated in this process depending on p53 [9]. Similarly, small nucleolar RNA that is derived from growth-arrest-specific 5 lncRNA is expressed in colon cancer cells and tissues with DNA damage response depending on p53, without being related with DICER [16]. We herein found that HOTAIR and p53 mRNA expression levels were up-regulated under DNA damage stress. After p53 interference, the background HOTAIR expression did not change, but its up-regulatory effects were attenuated, indicating that DNA damage-induced HOTAIR expression may be associated with p53. However, the mechanism for p53-induced regulation still needs in-depth studies. Since cell apoptosis was facilitated by p53 expression but suppressed by p53-regulated HOTAIR expression, p53 played complicated roles [17-19].
It has previously been reported that HOTAIR was highly expressed in breast, liver and pancreatic cancers, which participated in tumor progression by inhibiting the expressions of anti-oncogenes through histone methylation, as a novel prognostic factor [20-23]. In gastric cancer, HOTAIR is abnormally highly expressed, being associated with poor prognosis such as liver metastasis and peritoneal dissemination [24]. High HOTAIR expression is related with epigenetic mechanism. As to esophageal squamous cell carcinoma, HOTAIR directly inhibits WIF-1 expression by activating the methylation of histone H3K27 in the promoter region and then activates the Wnt/β-catenin signaling pathway. For colon and breast cancers, HOTAIR participates in epithelial-mesenchymal transition and maintains the “stemness” of cancer stem cells [25,26].
In summary, for the first time, we explored the biological functions of HOTAIR in OSCC Tca8113 cells. Overexpression of HOTAIR promoted the proliferation of these cells and inhibited their apoptosis. After interference of HOTAIR expression, the cells were arrested in the G2/M or M phase. Hence, HOTAIR may play a crucial role in the onset and progression of OSCC. Nevertheless, the regulatory effects of HOTAIR on Tca8113 cells remain unclear. The findings provide a potentially eligible target for the treatment of OSCC.
Disclosure of conflict of interest
None.
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 3.Caley DP, Pink RC, Trujillano D, Carter DR. Long noncoding RNAs, chromatin, and development. ScientificWorldJournal. 2010;10:90–102. doi: 10.1100/tsw.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa FF. Non-coding RNAs: lost in translation? Gene. 2007;386:1–10. doi: 10.1016/j.gene.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talukder KA, Azmi IJ, Ahmed KA, Hossain MS, Kabir Y, Cravioto A, Sack DA, Nur-E-Kamal A. Activation of p53/ATM-dependent DNA damage signaling pathway by shiga toxin in mammalian cells. Microb Pathog. 2012;52:311–317. doi: 10.1016/j.micpath.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Ann Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 8.Lowndes NF, Toh GW. DNA repair: the importance of phosphorylating histone H2AX. Curr Biol. 2005;15:R99–R102. doi: 10.1016/j.cub.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, Wang Y, Kong B, Langerød A, Børresen-Dale AL, Kim SK, van de Vijver M, Sukumar S, Whitfield ML, Kellis M, Xiong Y, Wong DJ, Chang HY. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena A, Carninci P. Long non-coding RNA modifies chromatin: epigenetic silencing by long non-coding RNAs. BioEssays. 2011;33:830–839. doi: 10.1002/bies.201100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan YF, Feng L, Wei LY, Song LJ, Zhang XQ, Liang HX, Li ZQ, Tao FB. [Study on expression profile of long non-coding RNA in hepatoceilular carcinoma] . Chinese Journal of Experimental Surgery. 2011;3:406–407. [Google Scholar]
- 14.Petera J, Sirak I, Beranek M, Vosmik M, Drastikova M, Paulikova S, Soumarova R. Molecular predictive factors of outcome of radiotherapy in cervical cancer. Neoplasma. 2011;58:469–475. doi: 10.4149/neo_2011_06_469. [DOI] [PubMed] [Google Scholar]
- 15.Janicke RU, Engels IH, Dunkern T, Kaina B, Schulze-Osthoff K, Porter AG. Ionizing radiation but not anticancer drugs causes cell cycle arrest and failure to activate the mitochondrial death pathway in MCF-7 breast carcinoma cells. Oncogene. 2001;20:5043–5053. doi: 10.1038/sj.onc.1204659. [DOI] [PubMed] [Google Scholar]
- 16.Krell J, Frampton AE, Mirnezami R, Harding V, De Giorgio A, Roca Alonso L, Cohen P, Ottaviani S, Colombo T, Jacob J, Pellegrino L, Buchanan G, Stebbing J, Castellano L. Growth arrest-specific transcript 5 associated snoRNA levels are related to p53 expression and DNA damage in colorectal cancer. PLoS One. 2014;9:e98561. doi: 10.1371/journal.pone.0098561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin J, Liu GT. [MDM2 and p53] . Cancer. 2001;06:663–666. [Google Scholar]
- 18.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tovar C, Graves B, Packman K, Filipovic Z, Higgins B, Xia M, Tardell C, Garrido R, Lee E, Kolinsky K, To KH, Linn M, Podlaski F, Wovkulich P, Vu B, Vassilev LT. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73:2587–2597. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 22.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Chen S, Yang G, Gu F, Li M, Zhong B, Hu J, Hoffman A, Chen M. Long noncoding RNA HOTAIR as an independent prognostic marker in cancer: a meta-analysis. PLoS One. 2014;9:e105538. doi: 10.1371/journal.pone.0105538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajjari M, Behmanesh M, Sadeghizadeh M, Zeinoddini M. Up-regulation of HOTAIR long non-coding RNA in human gastric adenocarcinoma tissues. Med Oncol. 2013;30:670. doi: 10.1007/s12032-013-0670-0. [DOI] [PubMed] [Google Scholar]
- 25.Pádua Alves C, Fonseca AS, Muys BR, de Barros E Lima Bueno R, Bürger MC, de Souza JE, Valente V, Zago MA, Silva WA Jr. Brief report: The lincRNA Hotair is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells. 2013;31:2827–2832. doi: 10.1002/stem.1547. [DOI] [PubMed] [Google Scholar]
- 26.Inaba H, Kuboniwa M, Sugita H, Lamont RJ, Amano A. Identification of signaling pathways mediating cell cycle arrest and apoptosis induced by Porphyromonas gingivalis in human trophoblasts. Infect Immun. 2012;80:2847–2857. doi: 10.1128/IAI.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]