Abstract
Increasing evidences demonstrated that microRNAs (miRNAs) play critical roles in the human tumor development and progression. In our study, we found that miR-330-3p expression was downregulated in gastric cancer cell lines and tissues. Ectopic expression of miR-330-3p suppressed the gastric cancer cell proliferation, colony formation and migration. Overexpression of miR-330-3p promoted E-cadherin expression and inhibited the expression of N-cadherin, vimentin and snail. We identified Musashi-1 (MSI1) as a direct target gene of miR-330-3p in gastric cancer cell. In addition, MSI1 was upregulated in gastric cancer cell lines and tissues and the MSI1 expression was inversely correlated with miR-330-3p expression in gastric cancer tissues. MiR-330-3p expression was increased in gastric cancer cells after treated with histone deacetylase inhibitor trichostatin A (TSA) and DNA methylation inhibitor 5-aza-CdR (AZA). These indicated that downregulated expression of miR-330-3p was partly mediated by gene promoter region hypermethylation. These results suggested that miR-330-3p acted as a tumor suppressor gene in GC.
Keywords: Gastric cancer, microRNAs, miRNAs, miR-330-3p, Musashi-1 (MSI1)
Introduction
Gastric cancer (GC) is the second most common cause of cancer related death and the fourth most common cancer worldwide [1-4]. It is estimated that about one million new GC cases are diagnosed per year [5-7]. Although advances in chemotherapy and surgery have been achieved, the prognosis of GC is still poor with the 5-year overall survival rate below 30% [8-10]. The main reason is that most GC patients are diagnosed at advanced stage [11-13]. Therefore, identification of new biomarkers and therapeutic targets are pivotal for improving GC prognosis.
MicroRNAs (miRNAs) are small, endogenous and noncoding RNAs that regulate gene expression through decreasing the stability and therefore repressing the translation of mRNAs [14-17]. Deregulation of miRNAs is found in many tumors such as gallbladder cancer, osteosarcoma, cutaneous squamous cell carcinoma, bladder cancer, ovarian carcinoma and breast cancer [18-22]. Increasing evidences have demonstrated that miRNAs play an important role in many biological processes such as cell development, proliferation, apoptosis, migration, invasion and differentiation [23-30]. Therefore, it is crucial to study the roles of miRNAs in the development of GC [31,32].
In this study, we showed that miR-330-3p expression was downregulated in gastric cancer cell lines and tissues. Ectopic expression of miR-330-3p suppressed the HGC-27 cell proliferation, colony formation and migration. Musashi-1 (MSI1) was identified as a direct target gene of miR-330-3p in the GC cell.
Materials and methods
Tissue and cell lines cultured and cell transfection
Human GC tissues and adjacent normal tissues were surgically collected from GC patients in our hospital. This study was proved by the Ethics Review Board of the Affiliated Yan An Hospital of Kunming Medical University and written informed consent was collected from each patient. GC cell lines (MGC-803, AGS, SGC-7901, HGC-27) and one normal gastric mucosa cell line (GES) were collected from the Chinese Academy of Science and cultured in the RPMI-1640 medium (Life Technologies, USA) contained with penicillin and streptomycin. miR-330-3p mimic and scramble oligonucleotide were collected from GenePharma (Shanghai, China). Cell transfection was performed using the Lipofectamine 2000 Reagent (Invitrogen, USA) following to manufacturer’s instruction.
Real-time PCR
Total RNA for miRNA and mRNA analyses was isolated from tissues or cells using TRIzol reagent (Invitrogen; USA) according to the manufacturer’s information. The expression of miR-330-3p and MSI1 was determined with SYBR Green PCR master mix (Applied Biosystems, USA) using real-time PCR. The primers were used as following: MSI1 forward 5’-GCTCGACTCCAAAACAATTGACC-3’ and reverse 5’-GGCTGAGCTTTCTTACATTCCAC-3, and for GAPDH were forward 5’-TGTTCGACAGTCAGCCGC-3’ and reverse 5’-GGTGTCTGAGCGATGTGGC-3’. GAPDH and U6 small nuclear RNA was performed as endogenous control for mRNA and miR-330-3p respectively.
Cell growth and colony-forming assay
Cell growth was determined using MTT assays according to the instructions. Cells were seeded in the 96-well plates and MTT reagent was put to the medium, and continue to culture for more than 4 hours. The absorbance was measure on the microtiter plate reader (Molecular Devices, CA) at 490 nm. For colony formation analysis, cells were cultured in the 6-well plate for two weeks. Cell colony was fixed with paraformaldehyde (4%) and stained with crystal violet (1%) (Sigma-Aldrich, USA). Stained colony was counted under the microscope.
Cell migration assay
Wound-healing assay was performed to detect the cell migration. Cells were cultured in the six-well plates. The cell wound was scratched using the sterile plastic tip when cell was reached confluence. After wased with medium twice, cells were cultured for 48 hours and images were taken under microscope to measure the wound.
Luciferase assays
For luciferase assays, HGC-27 cell was cultured in the 24-well plate and transfected with reporter vector and miR-330-3p or scramble using Lipofectamine 2000 according to description. After 48 hours, the activitie of Renilla and firefly luciferase in the cell lysate was measured using a dual-luciferase system (Promega). The ratio of Renilla/firefly luciferase activitie was calculated for normalization.
Western blotting
Total proteins were isolated from cell or tissue and protein concentrations were determined with the BCA protein assay kit (Pierce, USA). Proteins were separated using 12% SDS-PAGE gel and transferred to PVDF membranes (polyvinylidene difluoride; Bio-Rad). The membrane was blocked 5% non-fat milk and incubated with the primary antibody (E-cadherin, N-cadherin, vimentin and snail, MSI1 and GAPDH). The blot was incubated with horseradish peroxidase (HRP) for 1 hour and then was detected using ECL (enhanced chemiluminescence) system (Pierce Biotechnology, IL).
Sodium bisulfite modification and methylation-specific PCR (MS-PCR)
Total DNA from cell or tissues was isolated using Wizard DNA Purification Kit (QIAGEN, Valencia, CA) according to instruction. 10 ng DNA was used to PCR amplification and miR-330-3p CpG islands primers for unmethylated (forward: 5’-TGATTCGTTTTATTATCGGTC-3’, reverse: 5’-AACACTAACGACATCGACG-3’) and methylated (forward: 5’-TATGATTTGTTTTATTATTGGTT-30, reverse: 50-AACACTAACAACATCAA CA ACC-3’).
Statistical analysis
Data were shown as the mean ± SD (standard deviation). Statistical analysis was measured using SPSS 17. Student’s t test was performed to measure differences and P<0.05 was considered significant.
Result
miR-330-3p was downregulated in gastric cancer cell lines and tissues
The expression of miR-330-3p was determined in both GC tissues and cell lines. Of the 40 samples, miR-330-3p expression was downregulated in the 29 patients (29/40, 72.5%) compared to adjacent tissues (Figure 1A). The expression of miR-330-3p was downregulated in the GC tissues compared with adjacent no-tumor tissues (Figure 1B). We also demonstrated that miR-330-3p expression was downregulated in the GC cell lines (MGC-803, MNK-45, SGC-7901, HGC-27) compared with the normal gastric mucosa cell line (GES) (Figure 1C).
Figure 1.
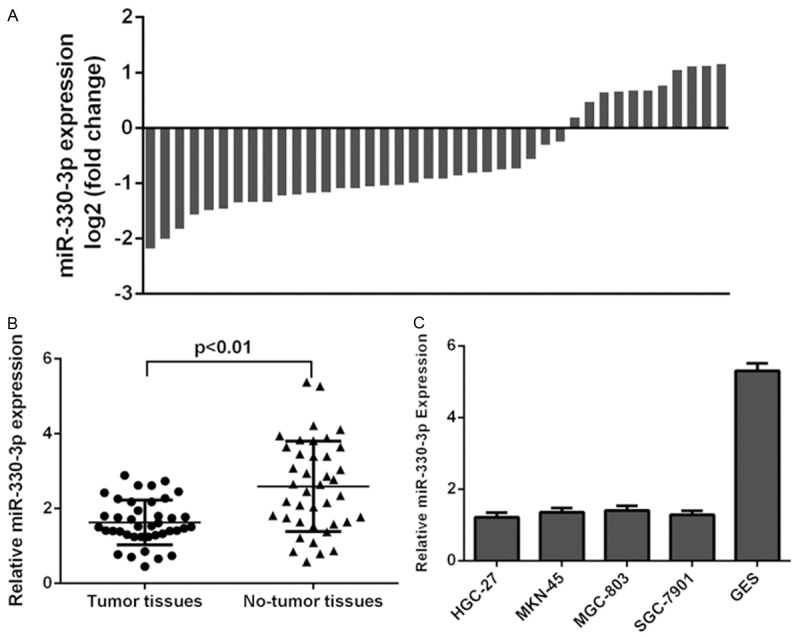
miR-330-3p was downregulated in gastric cancer cell lines and tissues. A: Of the 40 samples, miR-330-3p expression was downregulated in the 29 patients (29/40, 72.5%) compared to adjacent tissues. B: The expression of miR-330-3p was downregulated in the GC tissues compared with adjacent no-tumor tissues. C: miR-330-3p in the GC cell lines (MGC-803, MNK-45, SGC-7901 and HGC-27) and normal gastric mucosa cell line (GES) was measured by using qRT-PCR.
miR-330-3p expression was epigenetically regulated in gastric cancer
HGC-27 and MGC-803 cells were treated with the histone deacetylase inhibitor trichostatin A (TSA) and DNA methylation inhibitor 5-aza-CdR (AZA). miR-330-3p expression was increased in both HGC-27 and MGC-803 cells after treated with TSA or AZA (Figure 2A and 2B). We also measured the methylation status of 4 pairs of GC tissues and adjacent no-tumor tissues by MS-PCR. The methylation level of GC tissues was higher than that of adjacent no-tumor tissues (Figure 2C).
Figure 2.
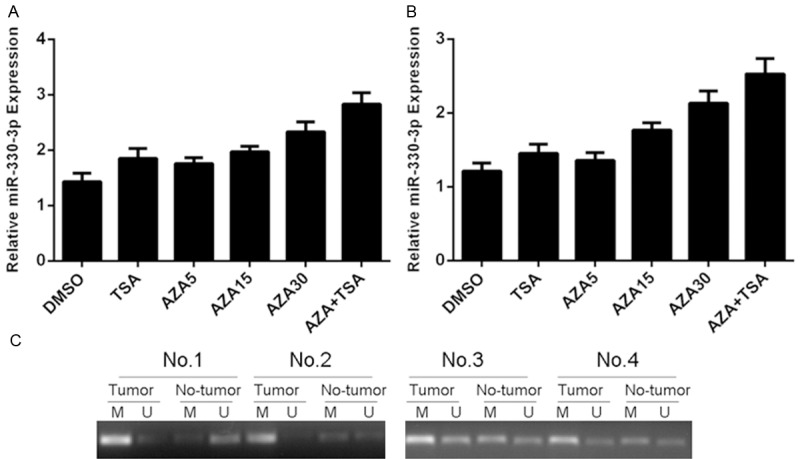
miR-330-3p expression was epigenetically regulated in gastric cancer. A: The expression of miR-330-3p in the HGC-27 cells after treated with TSA or AZA was detected by using qRT-PCR. B: The expression of miR-330-3p in the MGC-803 cells after treated with TSA or AZA was detected by using qRT-PCR. C: The methylation status of 4 pairs of GC tissues and adjacent no-tumor tissues was measured by MS-PCR.
miR-330-3p inhibited gastric cancer cell proliferation, colony formation and migration
The expression of miR-330-3p was increased after treated with miR-330-3p mimic (Figure 3A). Ectopic expression of miR-330-3p suppressed the HGC-27 cell proliferation using MMT assay (Figure 3B). Overexpression of miR-330-3p inhibited the HGC-27 cell colony formation (Figure 3C). Moreover, miR-330-3p overexpression suppressed the HGC-27 cell migration (Figure 3D).
Figure 3.
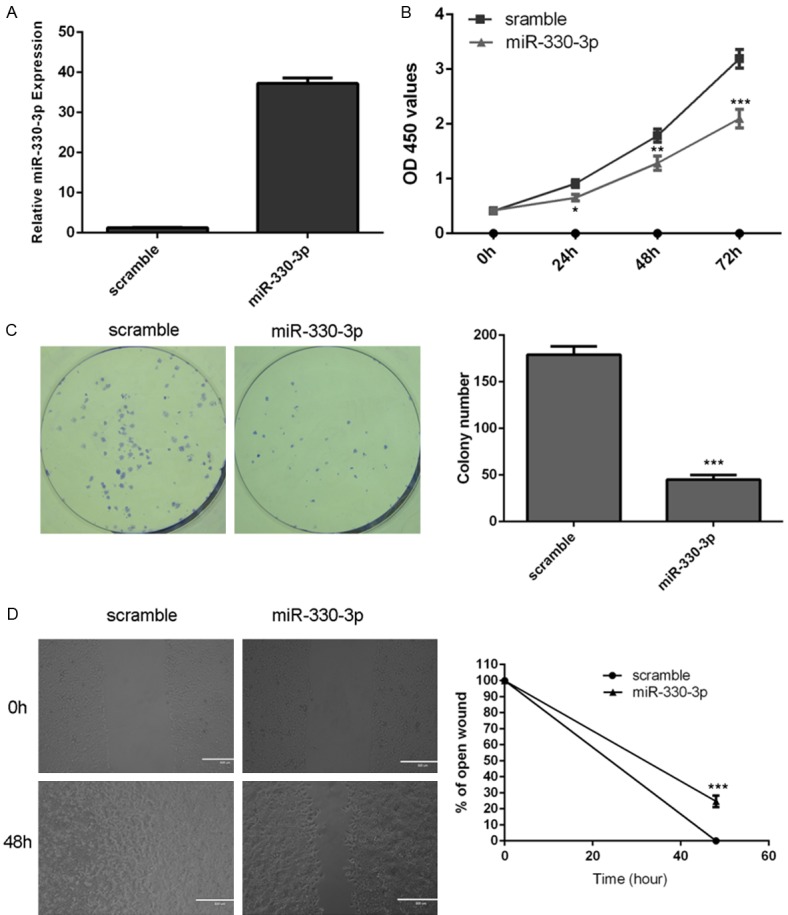
miR-330-3p inhibited gastric cancer cell proliferation, colony formation and migration. A: The expression of miR-330-3p was increased after treated with miR-330-3p mimic. B: Ectopic expression of miR-330-3p suppressed the HGC-27 cell proliferation using MMT assay. C: Overexpression of miR-330-3p inhibited the HGC-27 cell colony formation. D: miR-330-3p overexpression suppressed the HGC-27 cell migration. *P<0.05, **P<0.01 and ***P<0.001.
miR-330-3p repressed gastric cancer cell epithelial mesenchymal transition (EMT)
Overexpression of miR-330-3p promoted the protein expression of E-cadherin and repressed the protein expression of N-cadherin, vimentin and snail using western blot (Figure 4A). In line with this, ectopic expression of miR-330-3p enhanced the mRNA expression of E-cadherin and inhibited the mRNA expression of N-cadherin, vimentin and snail using qRT-PCR (Figure 4B).
Figure 4.
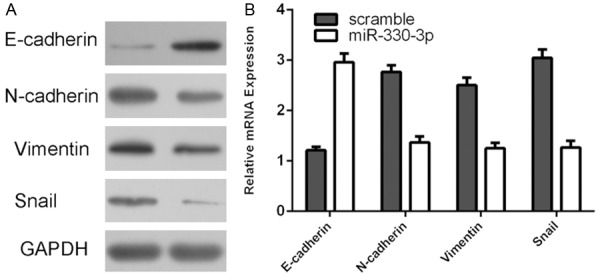
miR-330-3p repressed gastric cancer cell epithelial mesenchymal transition (EMT). A: The protein expression of E-cadherin, N-cadherin, vimentin and snail was measured by western blot. B: The mRNA expression of E-cadherin, N-cadherin, vimentin and snail was measured by qRT-PCR.
miR-330-3p targeted MSI1 in gastric cancer cell
TargetScan online software was used to predict miR-330-3p genes and demonstrated that MSI1 gene 3’ UTR had 8 sequential bases with 5’ of human miR-330-3p (Figure 5A). Overexpression of miR-330-3p decreased the luciferase activity of wild type MSI1 3’ UTR construct, whereas the luciferase activity was not decreased in the mutated type in both HGC-27 (Figure 5B) and MGC-803 (Figure 5C). Ectopic expression of miR-330-3p suppressed the protein expression of MSI1 in both HGC-27 (Figure 5D) and MGC-803 (Figure 5E).
Figure 5.
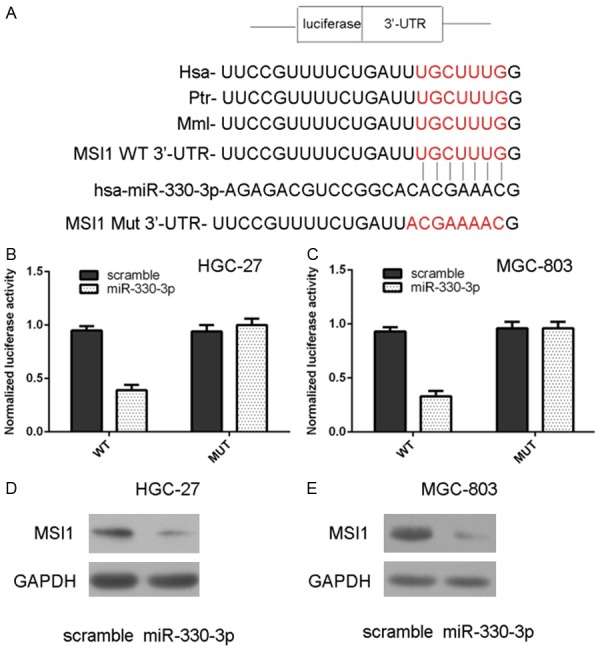
miR-330-3p targeted MSI1 in gastric cancer cell. A: There are 8 sequential bases between MSI1 gene 3’ UTR and 5’ of human miR-330-3p. B, C: Overexpression of miR-330-3p decreased the luciferase activity of wild type MSI1 3’ UTR construct, whereas the luciferase activity was not decreased in the mutated type in both HGC-27 and MGC-803. D: Ectopic expression of miR-330-3p suppressed the protein expression of MSI1 in the HGC-27. E: The protein expression of MSI1 in the MGC-803 cell was determined by using western blot.
MSI1 expression was inversely correlated with miR-330-3p expression in gastric cancer
The expression of MSI1 was determined in GC tissues and cell lines. Of the 40 samples, MSI1 expression was upregulated in the 31 patients (31/40, 77.5%) compared to adjacent tissues (Figure 6A). The expression of MSI1 was upregulated in the GC tissues compared with adjacent no-tumor tissues (Figure 6B). We also demonstrated that MSI1 expression was upregulated in the GC cell lines (MGC-803, MNK-45, SGC-7901, HGC-27) compared with the normal gastric mucosa cell line (GES) (Figure 6C). Moreover, the expression levels of MSI1 were inversely correlated with the expression of miR-330-3p in the gastric cancer tissues (Figure 6D).
Figure 6.
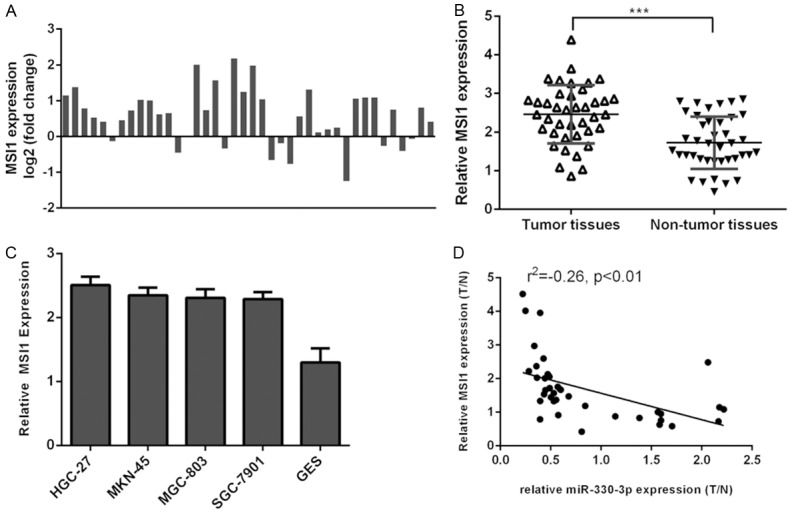
MSI1 expression was inversely correlated with miR-330-3p expression in gastric cancer. A: Of the 40 samples, MSI1 expression was upregulated in the 31 patients (31/40, 77.5%) compared to adjacent tissues. B: The expression of MSI1 was upregulated in the GC tissues compared with adjacent no-tumor tissues. C: The MSI1 expression in the GC cell lines (MGC-803, MNK-45, SGC-7901 and HGC-27) and the normal gastric mucosa cell line (GES) was determined by qRT-PCR. D: The expression levels of MSI1 were inversely correlated with the expression of miR-330-3p in the gastric cancer tissues. ***P<0.001.
Discussion
In this study, we showed that miR-330-3p expression was downregulated in gastric cancer cell lines and tissues. Ectopic expression of miR-330-3p suppressed the HGC-27 cell proliferation, colony formation and migration. Overexpression of miR-330-3p promoted the expression of E-cadherin and inhibited the expression of N-cadherin, vimentin and snail. These data suggested that miR-330-3p repressed gastric cancer cell epithelial mesenchymal transition (EMT). We identified MSI1 as a direct target gene of miR-330-3p in gastric cancer cell. In addition, MSI1 was upregulated in gastric cancer cell lines and tissues and the MSI1 expression was inversely correlated with miR-330-3p expression in gastric cancer tissues. Moreover, miR-330-3p expression was increased in GC cells after treated with histone deacetylase inhibitor trichostatin A (TSA) and DNA methylation inhibitor 5-aza-CdR (AZA). These indicated that downregulated expression of miR-330-3p was partly mediated by gene promoter region hypermethylation. To conclude, these results suggested that miR-330-3p acted as a tumor suppressor gene in GC.
Previous studies showed that miR-330-3p played an important role in the development of multiple tumors [33-38]. For example, Arora et al [38] found that the expression of miR-330-3p classified Brain metastasis (BM) + vs. BM- patients in nonsmall cell lung cancer patients. Lionetti et al [37] showed that deregulated expression of miR-330-3p in the serum existed in primary plasma cell leukemia and was correlated with clinical outcome. Liu et al [33] demonstrated that miR-330-3p expression was upregulated in NSCLC tissues and brain metastasis tissues. Overexpression of miR-330-3p promoted the NSCLC cell cycle distribution and proliferation through targeting EGR2. Meng et al [34] found that miR-330-3p expression was upregulated in esophageal squamous cell carcinoma (ESCC) tissues. miR-330-3p overexpression increased ESCC cell migration, invasion and proliferation through inhibiting PDCD4 expression. However, the role of miR-330-3p in the GC is still unknown. In our study, miR-330-3p expression was downregulated in GC cell lines and clinical GC tissues. Ectopic expression of miR-330-3p suppressed the GC cell proliferation, colony formation and migration.
MSI1 was identified as a candidate direct target of miR-330-3p by using the TargetScan. MSI1 original identified as one of neural stem cell (NSC) marker which is expressed in the nervous system [39,40]. MSI1 is considerated to be one marker of NSCs and neural progenitor cells in mammalian [41]. Moreover, higher expression of MSI1 was correlated with high grade glioma. Additionally, inhibition of MSI1 increased the radiation-induced colon cancer cells apoptosis and cancer regression. MSI1 is an RNA-binding protein (RBP) that plays an important role in the gene expression by binding to a sequence in the 3’ UTR of target mRNAs leading to the translational inhibition [42-44]. Previous studies showed that MSI1 was overexpressed in the cervical cancer tissues and ectopic expression of MSI1 promoted the ectopic cell tumor formation, cell proliferation and increased the cervical cancer cells into the S phase [45]. MSI1 was expressed in a lot of tumors, including retinoblastoma, glioblastoma, and endometrial, colorectal, esophageal and bladder carcinoma [43,46-51]. Morever, Wang et al [52] demonstrated that MSI1 expression was upregulated in GC tissues compare to normal gastric tissues. Kuang et al [53] also showed that MSI1 expression was upregulated in the intestinal metaplasia compared to the normal mucosa. It is reasonable to know that miR-330-3p target genes to inhibit cell proliferation and invasion. Among these putative target genes for miR-330-3p is an inhibitor of MSI1, which was predicted by the TargetScan. Our study proved that ectopic expression of miR-330-3p suppressed the protein expression of MSI1 in both HGC-27 and MGC-803 cell. Data from the Luciferase assays also suggest that MSI1 is one of the direct downstream target genes of miR-330-3p in the GC cell. In line with previous studies, we also found that the expression of MSI1 was upregulated in the GC tissues compared with adjacent no-tumor tissues. In addition, we found that the expression levels of MSI1 were inversely correlated with the expression of miR-330-3p in the gastric cancer tissues.
In conclusion, we demonstrated that miR-330-3p was downregulated in the human GC tissues. Ectopic expression of miR-330-3p suppressed the GC cell proliferation, colony formation and migration. MSI1 was identified as a direct target gene of miR-330-3p. These results suggested that miR-330-3p acted as a tumor suppressor gene in GC.
Disclosure of conflict of interest
None.
References
- 1.Liang J, Liu X, Xue H, Qiu B, Wei B, Sun K. MicroRNA-103a inhibits gastric cancer cell proliferation, migration and invasion by targeting c-Myb. Cell Prolif. 2015;48:78–85. doi: 10.1111/cpr.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai J, Ma M, Cai M, Xu F, Chen J, Wang G, Shuai X, Tao K. Inhibition enhancer of zeste homologue 2 promotes senescence and apoptosis induced by doxorubicin in p53 mutant gastric cancer cells. Cell Prolif. 2014;47:211–218. doi: 10.1111/cpr.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang M, Ren MP, Zhao L, Li CP, Deng MM. miR-485-5p acts as a negative regulator in gastric cancer progression by targeting flotillin-1. Am J Transl Res. 2015;7:2212–2222. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhu W, Zhang J, Huo S, Zhou L, Gu Z, Zhang M. MicroRNA-650 targets ING4 to promote gastric cancer tumorigenicity. Biochem Biophys Res Commun. 2010;395:275–280. doi: 10.1016/j.bbrc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–2158. [PMC free article] [PubMed] [Google Scholar]
- 6.Lam EK, Wang X, Shin VY, Zhang S, Morrison H, Sun J, Ng EK, Yu J, Jin H. A microRNA contribution to aberrant Ras activation in gastric cancer. Am J Transl Res. 2011;3:209–218. [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong G, Yang L, Chen Y, Fan Z. Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution. Am J Transl Res. 2015;7:2262–2269. [PMC free article] [PubMed] [Google Scholar]
- 8.Xia P, Song CL, Liu JF, Wang D, Xu XY. Prognostic value of circulating CD133(+) cells in patients with gastric cancer. Cell Prolif. 2015;48:311–317. doi: 10.1111/cpr.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Zhang S, Zhang J, Lam E, Liu X, Sun J, Feng L, Lu H, Yu J, Jin H. Annexin A6 is down-regulated through promoter methylation in gastric cancer. Am J Transl Res. 2013;5:555–562. [PMC free article] [PubMed] [Google Scholar]
- 10.Gigek CO, Chen ES, Calcagno DQ, Wisnieski F, Burbano RR, Smith MA. Epigenetic mechanisms in gastric cancer. Epigenomics. 2012;4:279–294. doi: 10.2217/epi.12.22. [DOI] [PubMed] [Google Scholar]
- 11.Yin M, Hu Z, Tan D, Ajani JA, Wei Q. Molecular epidemiology of genetic susceptibility to gastric cancer: focus on single nucleotide polymorphisms in gastric carcinogenesis. Am J Transl Res. 2009;1:44–54. [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H, Okamoto K, Otsuji E. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174–1179. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma YY, Tao HQ. Microribonucleic acids and gastric cancer. Cancer Sci. 2012;103:620–625. doi: 10.1111/j.1349-7006.2011.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278–283. doi: 10.1111/cpr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Yu X, Shen J, Liu Y, Chan MT, Wu WK. MicroRNA dysregulation in rhabdomyosarcoma: a new player enters the game. Cell Prolif. 2015;48:511–516. doi: 10.1111/cpr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen J, Niu W, Zhou M, Zhang H, Ma J, Wang L. MicroRNA-410 suppresses migration and invasion by targeting MDM2 in gastric cancer. PLoS One. 2014;9:e104510. doi: 10.1371/journal.pone.0104510. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Zhu M, Zhang N, He S. Similarly up-regulated microRNA-106a in matched formalin-fixed paraffin-embedded and fresh frozen samples and the dynamic changes during gastric carcinogenesis and development. Pathol Res Pract. 2014;210:909–15. doi: 10.1016/j.prp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914–13924. doi: 10.18632/oncotarget.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Li Z. The role of miRNAs in cutaneous squamous cell carcinoma. J Cell Mol Med. 2016;20:3–9. doi: 10.1111/jcmm.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majid S, Dar AA, Saini S, Deng G, Chang I, Greene K, Tanaka Y, Dahiya R, Yamamura S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS One. 2013;8:e67686. doi: 10.1371/journal.pone.0067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim M, Chen X, Chin LJ, Paranjape T, Speed WC, Kidd KK, Zhao H, Weidhaas JB, Slack FJ. Extensive sequence variation in the 3’ untranslated region of the KRAS gene in lung and ovarian cancer cases. Cell Cycle. 2014;13:1030–1040. doi: 10.4161/cc.27941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie T, Zhang J, Peng C, Lin Y, Chen J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget. 2014;5:7013–26. doi: 10.18632/oncotarget.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing’s sarcoma. Cell Prolif. 2015;48:1–6. doi: 10.1111/cpr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Li Z, Chan MT, Wu WK. microRNA deregulation in keloids: an opportunity for clinical intervention? Cell Prolif. 2015;48:626–630. doi: 10.1111/cpr.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Li Z, Liu J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015;48:271–277. doi: 10.1111/cpr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X, Li Z, Yu J, Chan MT, Wu WK. MicroRNAs predict and modulate responses to chemotherapy in colorectal cancer. Cell Prolif. 2015;48:503–510. doi: 10.1111/cpr.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X, Li Z, Chen G, Wu WK. MicroRNA-10b Induces Vascular Muscle Cell Proliferation Through Akt Pathway by Targeting TIP30. Curr Vasc Pharmacol. 2015;13:679–686. doi: 10.2174/1570161113666150123112751. [DOI] [PubMed] [Google Scholar]
- 28.Lei R, Tang J, Zhuang X, Deng R, Li G, Yu J, Liang Y, Xiao J, Wang HY, Yang Q, Hu G. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene. 2014;33:1287–1296. doi: 10.1038/onc.2013.65. [DOI] [PubMed] [Google Scholar]
- 29.Wan X, Ding X, Chen S, Song H, Jiang H, Fang Y, Li P, Guo J. The functional sites of miRNAs and lncRNAs in gastric carcinogenesis. Tumour Biol. 2015;36:521–532. doi: 10.1007/s13277-015-3136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Jiang C, Li D, Wang R, Wang W. MiRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in gastric cancer. Tumour Biol. 2014;35:9801–6. doi: 10.1007/s13277-014-2273-6. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 32.Duan JH, Fang L. MicroRNA-92 promotes gastric cancer cell proliferation and invasion through targeting FXR. Tumour Biol. 2014;35:11013–9. doi: 10.1007/s13277-014-2342-x. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Shi H, Liu B, Li J, Liu Y, Yu B. miR-330-3p controls cell proliferation by targeting early growth response 2 in non-small-cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2015;47:431–440. doi: 10.1093/abbs/gmv032. [DOI] [PubMed] [Google Scholar]
- 34.Meng H, Wang K, Chen X, Guan X, Hu L, Xiong G, Li J, Bai Y. MicroRNA-330-3p functions as an oncogene in human esophageal cancer by targeting programmed cell death 4. Am J Cancer Res. 2015;5:1062–1075. [PMC free article] [PubMed] [Google Scholar]
- 35.Petty RD, McCarthy NE, Le Dieu R, Kerr JR. MicroRNAs hsa-miR-99b, hsa-miR-330, hsa-miR-126 and hsa-miR-30c: Potential Diagnostic Biomarkers in Natural Killer (NK) Cells of Patients with Chronic Fatigue Syndrome (CFS)/ Myalgic Encephalomyelitis (ME) PLoS One. 2016;11:e0150904. doi: 10.1371/journal.pone.0150904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medina-Villaamil V, Martinez-Breijo S, Portela-Pereira P, Quindos-Varela M, Santamarina-Cainzos I, Anton-Aparicio LM, Gomez-Veiga F. Circulating MicroRNAs in blood of patients with prostate cancer. Actas Urol Esp. 2014;38:633–639. doi: 10.1016/j.acuro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Lionetti M, Musto P, Di Martino MT, Fabris S, Agnelli L, Todoerti K, Tuana G, Mosca L, Gallo Cantafio ME, Grieco V, Bianchino G, D’Auria F, Statuto T, Mazzoccoli C, De Luca L, Petrucci MT, Offidani M, Di Raimondo F, Falcone A, Caravita T, Omede P, Morabito F, Tassone P, Boccadoro M, Palumbo A, Neri A. Biological and clinical relevance of miRNA expression signatures in primary plasma cell leukemia. Clin Cancer Res. 2013;19:3130–3142. doi: 10.1158/1078-0432.CCR-12-2043. [DOI] [PubMed] [Google Scholar]
- 38.Arora S, Ranade AR, Tran NL, Nasser S, Sridhar S, Korn RL, Ross JT, Dhruv H, Foss KM, Sibenaller Z, Ryken T, Gotway MB, Kim S, Weiss GJ. MicroRNA-328 is associated with (non-small) cell lung cancer (NSCLC) brain metastasis and mediates NSCLC migration. Int J Cancer. 2011;129:2621–2631. doi: 10.1002/ijc.25939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Good P, Yoda A, Sakakibara S, Yamamoto A, Imai T, Sawa H, Ikeuchi T, Tsuji S, Satoh H, Okano H. The human Musashi homolog 1 (MSI1) gene encoding the homologue of Musashi/Nrp-1, a neural RNA-binding protein putatively expressed in CNS stem cells and neural progenitor cells. Genomics. 1998;52:382–384. doi: 10.1006/geno.1998.5456. [DOI] [PubMed] [Google Scholar]
- 40.Ratti A, Fallini C, Cova L, Fantozzi R, Calzarossa C, Zennaro E, Pascale A, Quattrone A, Silani V. A role for the ELAV RNA-binding proteins in neural stem cells: stabilization of Msi1 mRNA. J Cell Sci. 2006;119:1442–1452. doi: 10.1242/jcs.02852. [DOI] [PubMed] [Google Scholar]
- 41.Schulenburg A, Cech P, Herbacek I, Marian B, Wrba F, Valent P, Ulrich-Pur H. CD44-positive colorectal adenoma cells express the potential stem cell markers musashi antigen (msi1) and ephrin B2 receptor (EphB2) J Pathol. 2007;213:152–160. doi: 10.1002/path.2220. [DOI] [PubMed] [Google Scholar]
- 42.Shi C, Zhang Z. miR-761 inhibits tumor progression by targeting MSI1 in ovarian carcinoma. Tumour Biol. 2016;37:5437–5443. doi: 10.1007/s13277-015-4377-z. [DOI] [PubMed] [Google Scholar]
- 43.Moghbeli M, Sadrizadeh A, Forghanifard MM, Mozaffari HM, Golmakani E, Abbaszadegan MR. Role of Msi1 and PYGO2 in esophageal squamous cell carcinoma depth of invasion. J Cell Commun Signal. 2016;10:49–53. doi: 10.1007/s12079-015-0314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong IS, Kang KS. The effects of Hedgehog on the RNA-binding protein Msi1 in the proliferation and apoptosis of mesenchymal stem cells. PLoS One. 2013;8:e56496. doi: 10.1371/journal.pone.0056496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Yang WT, Zheng PS. Msi1 promotes tumor growth and cell proliferation by targeting cell cycle checkpoint proteins p21, p27 and p53 in cervical carcinomas. Oncotarget. 2014;5:10870–10885. doi: 10.18632/oncotarget.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jullien PE, Mosquna A, Ingouff M, Sakata T, Ohad N, Berger F. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol. 2008;6:e194. doi: 10.1371/journal.pbio.0060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moghbeli M, Rad A, Farshchian M, Taghehchian N, Gholamin M, Abbaszadegan MR. Correlation Between Meis1 and Msi1 in Esophageal Squamous Cell Carcinoma. J Gastrointest Cancer. 2016;47:273–7. doi: 10.1007/s12029-016-9824-6. [DOI] [PubMed] [Google Scholar]
- 48.Gao C, Han C, Yu Q, Guan Y, Li N, Zhou J, Tian Y, Zhang Y. Downregulation of Msi1 suppresses the growth of human colon cancer by targeting p21cip1. Int J Oncol. 2015;46:732–740. doi: 10.3892/ijo.2014.2749. [DOI] [PubMed] [Google Scholar]
- 49.Nikpour P, Baygi ME, Steinhoff C, Hader C, Luca AC, Mowla SJ, Schulz WA. The RNA binding protein Musashi1 regulates apoptosis, gene expression and stress granule formation in urothelial carcinoma cells. J Cell Mol Med. 2011;15:1210–1224. doi: 10.1111/j.1582-4934.2010.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma L, Xu YL, Ding WJ, Shao HF, Teng YC. Prognostic value of Musashi-1 in endometrioid adenocarcinoma. Int J Clin Exp Pathol. 2015;8:4564–4572. [PMC free article] [PubMed] [Google Scholar]
- 51.Chen HY, Lin LT, Wang ML, Lee SH, Tsai ML, Tsai CC, Liu WH, Chen TC, Yang YP, Lee YY, Chang YL, Huang PI, Chen YW, Lo WL, Chiou SH, Chen MT. Musashi-1 regulates AKT-derived IL-6 autocrinal/paracrinal malignancy and chemoresistance in glioblastoma. Oncotarget. 2016;7:42485–42501. doi: 10.18632/oncotarget.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Ong CW, Shi J, Srivastava S, Yan B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B, Salto-Tellez M. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer. 2011;105:658–665. doi: 10.1038/bjc.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuang RG, Kuang Y, Luo QF, Zhou CJ, Ji R, Wang JW. Expression and significance of Musashi-1 in gastric cancer and precancerous lesions. World J Gastroenterol. 2013;19:6637–6644. doi: 10.3748/wjg.v19.i39.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]


