Abstract
mTOR inhibitors have potent antiangiogenic and anti-lymphangiogenic effects in addition to their growth inhibitory effects in head and neck squamous cell carcinoma (HNSCC). Lymphatogenous spread is much more predominant in HNSCC than hematogenous spread and significantly decreases survival. In this study we evaluated the effects of rapamycin on targeting tumor-stroma crosstalk in HNSCC. HNSCC tumor cells (FaDu) and human lymphatic endothelial cells (HMEC-1A) were co-cultured in various combinations using transwell cell culture inserts to study tumor-stroma crosstalk and the effects of mTOR inhibitor rapamycin. Levels of growth factors and cytokines in cell culture media were measured using Milliplex bead immunoassay (EMD Millipore) and ELISA assay (R&D Systems). We found that conditioned media collected from tumor cells or co-culture with tumor cells significantly increased the invasiveness of lymphatic and blood vascular endothelial cells (P<0.05), while there was no effect of conditioned media collected from endothelial cell cultures or co-culture with endothelial cells on tumor cell invasiveness. There was a significant effect of rapamycin on both baseline and tumor cell stimulated invasiveness of endothelial cells (P<0.05). Importantly the level of IL-6 secreted in media increased significantly in tumor-endothelial cell co-culture compared to monocultures. Rapamycin significantly suppressed secretion of IL-6 by tumor cells (P<0.05). Thus, HNSCC cells produce chemotactic stimuli that promote endothelial cell invasion toward tumor cells that can stimulate lymphangiogenesis. Rapamycin effectively reverted the stimulatory effect of IL-6 secreted by tumor cells on endothelial cell invasiveness.
Keywords: Head and neck squamous cell carcinoma, lymphangiogenesis, crosstalk, mTOR inhibitors, Interleukin-6
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide [1]. Over 45,000 Americans develop HNSCC and over 11,000 die from it annually [2]. HNSCC is characterized by high locoregional recurrence rates reaching approximately 50% in advanced-stage disease. The 5-year survival rate of HNSCC patients is 45% and has not changed in decades. The current standard of care for patients with locally advanced disease is platinum-based chemoradiotherapy. However cisplatin-based chemoradiotherapy results in significantly worse toxicity compared to patients receiving radiotherapy alone (77% versus 34%) [3]. Alternative treatment strategies such as molecular targeted therapies are clearly needed. The only FDA-approved molecular targeted agent for HNSCC is cetuximab (Erbitux). Unfortunately, in several studies chemoradiotherapy with cetuximab demonstrated significantly worse outcomes in locally advanced HNSCC compared to cisplatin-based chemoradiotherapy [4]. Emerging data from several studies indicate that cetuximab is not a promising alternative to cisplatin and suggests a need for other molecular targeted agents. mTOR inhibitors are promising alternatives. mTOR inhibitors have been shown to be well tolerated with mild, manageable and reversible toxicities compared to cisplatin. They have proven to be safe and efficacious even in long term use in renal transplant and coronary stent patients. Importantly, metastasis to regional lymph nodes occurs in 30-40% of HNSCC patients and is associated with poor prognosis and reduced survival rates [5,6]. mTOR inhibitors exhibit potent antitumor, antiangiogenic and anti-lymphatic properties in HNSCC preclinical models [7,9]. The mechanisms include a marked decrease in VEGF production, inhibition of vascular endothelial cells responses to VEGF stimulation, suppression of VEGFR-3 expression and rapamycin-induced release of soluble VEGFR-2 that acts as a VEGF C and D trap [10]. In the current study we evaluated the effects of rapamycin, an mTOR inhibitor, on the tumor-stroma crosstalk in HNSCC.
Materials and methods
Cell lines
HMEC-1A, a human lymphatic endothelial cell line [11] was maintained in MCDB 131 medium (Sigma-Aldrich), supplemented with 20 mM HEPES, 1 ug/ml hydrocortisone, 10 ng/ml EGF and 10% fetal bovine serum (FBS). HNSCC cell line FaDu established from a hypopharyngeal SCC was procured from ATCC. FaDu cultures were maintained in MEM media supplemented with 10% FBS and non-essential amino acids.
Cell line transfections
Amaxa Nucleofector Kits (Amaxa Inc., Gaithersburg, MD) was used for transfection of pcDNA3 plasmid encoding AU1-tagged rapamycin-resistant mutant mTOR (S2035I) or empty vector into FaDu and HMEC-1A cells according to the manufacturer’s instructions. Stably transfected clones were selected and expanded in growth medium containing G418 (500 μg/ml).
Co-culture proliferation and invasion assays
To evaluate the interaction between normal endothelial cells and test the effects of rapamycin in HNSCC cells we employed co-culture sytems. HMEC-1A and FaDu cells were cultured using transwell cell culture inserts. One of the cell types was seeded in the lower compartment (wells in a 24-well plate), while another cell type was seeded in the top compartment (transwell inserts).
The effects of co-culturing the two cell types and rapamycin (purchased from LC Laboratories, Woburn, MA) effects on proliferation of tumor and endothelial cells were assessed using CellTiter 96 AQueous cell proliferation assay (Promega Corp., Madison, WI) according to the manufacturer’s instructions. Transwell inserts with 0.4-μm pore size were used (BD biosciences, Bedford, MA).
Co-culture cell invasion assays were performed using 24-well plates and transwell inserts with an 8-μm pore size (BD biosciences, Bedford, MA). The transwells were collagenized using 100 μL of 15 μg/mL collagen solution (Sigma-Aldrich, from human fibroblasts) and incubation performed at 37°C for 4 hours. Endothelial cells (50,000 per well) or tumor cells (20,000 per well) were seeded into 24-well plates in 600 μL of MCDB 131 media (Sigma-Aldrich, containing 0% FBS (medium with no serum is used to limit cell proliferation and observe the true effects due to cell invasion). Some control wells had cell-free 0% FBS MCDB 131 medium. The cells seeded in the plates were allowed to adhere at 37°C for 4 hours. Collagenized transwells were washed and seeded with the same number of endothelial or tumor cells as above. Seeding of these cells were performed in the following combinations: endothelial cells over control media, endothelial cells over tumor cells, tumor cells over control media, and tumor cells over endothelial cells. Co-cultures were then incubated at 37°C for 22-24 hours. After invasion had occurred, noninvasive cells were removed by swabbing. Cells were then fixed with methanol and stained with 0.2% Crystal Violet. The total number of migrated cells were counted using a light microscopy at × 200 magnification.
Western blot analysis
Soluble proteins were extracted as previously described [12] and expression of tumor and lymphatic biomarkers were evaluated by western blot using the following primary antibodies: phospho-S6 ribosomal protein (serine 235/236, 1:100), actin (1:3,000; all antibodies above-Cell Signaling, Beverly, MA) and AU1 (1:2,000 dilution; Bethyl Laboratories, Montgomery, TX). The expression levels of each marker were quantified after normalizing to actin immunoblotting.
Cytokine analysis
The effects of rapamycin treatment on secretion of cytokines into culture medium were determined using ELISA assays (R&D Systems, Minneapolis, MN) or MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead multiplex assay (EMD Millipore, Billerica, MA) according to manufacturer’s instructions.
Statistical analysis
Two-tailed, unpaired Student’s t test was used to analyze the differences between experimental groups. Statistical analysis was performed using GraphPad InStat version 3.06 for Windows (GraphPad Software); P values of less than 0.05 were considered statistically significant.
Results
Characterization of rapamycin-resistant clones
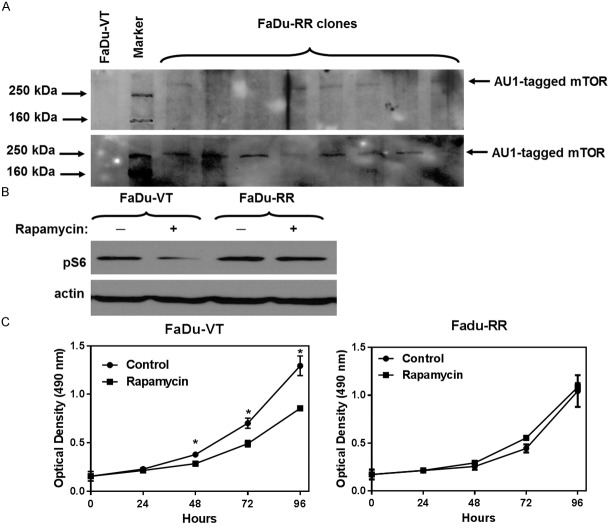
We generated stable FaDu clones overexpressing exogenous RR-mTOR tagged with AU1 (Figure 1A). The clone with the highest expression of AU1-tagged mTOR was evaluated for its response to rapamycin treatment using pS6 WB analysis. We compared the effect of mTOR inhibition on pS6 expression in vector-transfected control clones and rapamycin-resistant (RR) clones. Effect of the drug on S6 phosphorylation was significantly attenuated in RR cells (Figure 1B). Cell proliferation assay showed that the growth of VT-FaDu cell line was suppressed by rapamycin (100 ng/ml), while the RR-FaDu clone was insensitive (Figure 1C).
Figure 1.
Generation and characterization of stable FaDu clones overexpressing rapamycin-resistant mTOR (Ser2035→Ile). A: Detection of AU1-tagged mTOR in transfected FaDu clones. B: m-TOR inhibition downregulates S6 phosphorylation in vector-transfected FaDu clone (FaDu-VT), while there is no change in S6 phosphorylation in rapamycin-resistant FaDu clone (FaDu-RR). C: Cell proliferation assay. Cells were seeded as highlighted in materials and methods. The effect on cell proliferation was determined at different time points using MTS assay. Data represents mean optical density ± SD for n≥4 experiments. *P<0.05, Two sample independent student’s t test. Growth of FaDu-VT cells was suppressed by rapamycin (100 ng/ml), while the FaDu clone was insensitive to Rapamycin treatment.
Invasion and proliferation of FaDu and HMEC-1A cells in co-culture: Tumor cells stimulate lymphatic endothelial cell invasiveness
To evaluate the interaction between normal human microvascular lymphatic endothelial cells HMEC-1A and HNSCC FaDu cells we employed co-culture systems. When studying cell invasiveness we found that HNSCC cells significantly increased the invasiveness of lymphatic endothelial cells (P<0.05) (Figure 2), while there was no effect of lymphatic endothelial cells on tumor cell invasiveness. Co-culture of the lymphatic endothelial cells with tumor cells increased their invasiveness by more than two-fold. Next, we assessed the effects of co-culture of FaDu tumor cells and HMEC-1A endothelial cells on their proliferation by using cell culture inserts with 0.4 µm pore size. This allows for exchange of growth factors/cytokines between the cells in the two compartments, but prevents cell invasion. There was no significant effect of tumor cells on proliferation of endothelial cells in this model (Figure 3). Similarly there was only a minor effect of endothelial cells on proliferation of tumor cells (data not shown).
Figure 2.
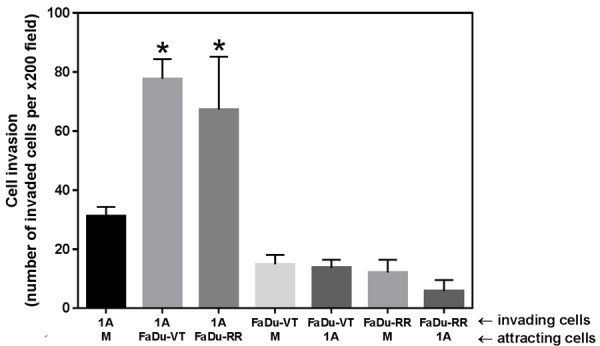
Graph depicting invasion of HMEC-1A (1A), Fadu-VT, or FaDu-RR cells toward cell-free media (M), Fadu-VT, FaDu-RR, or HMEC-1A (1A) cells. Data represents mean ± SD for number of invading cells for n>3 experiments. *P<0.05, Two sample independent student’s t test with * indicates significance relative to invasion of HMEC-1A toward cell-free media.
Figure 3.
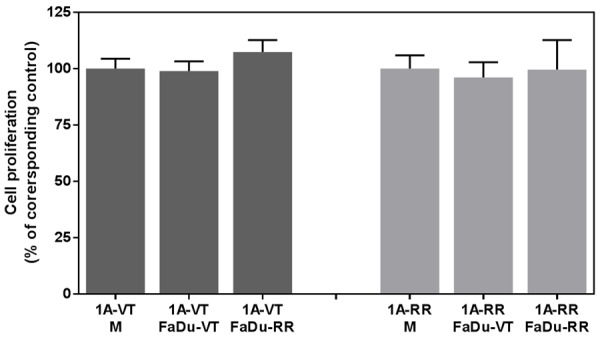
Effect of co-culture with FaDu cancer cells on the proliferation of endothelial cells HMEC-1A (1A). VT-vector-transfected; RR-rapamycin-resistant mTOR transfected cells. No significant effect of tumor cells on proliferation of endothelial cells was observed.
Effects of rapamycin on invasiveness and proliferation of endothelial and tumor cells
Importantly, the effect of rapamycin on cell proliferation varied significantly depending on the combination of endothelial and tumor cells used (Figure 4). Specifically, co-culture of rapamycin-resistant FaDu tumor cells with endothelial cells sensitive to rapamycin had no effect on the growth-inhibitory effect of rapamycin against endothelial cells. Rapamycin caused approximately 30% reduction in proliferation of endothelial cells grown in co-culture with vector-transfected or rapamycin-resistant mTOR-transfected FaDu tumor cells. In other words we did not observe any “protective” effect of rapamycin-resistant tumor cells against growth-inhibitory effect of rapamycin in endothelial cells (Figure 4). However, surprisingly there was an obvious difference in the effect of rapamycin on the growth of vector-transfected FaDu tumor cells co-cultured with either vector-transfected endothelial cells or with rapamycin-resistant mTOR-transfected endothelial cells. Rapamycin-resistant endothelial cells reduced the growth-inhibitory effect of rapamycin in vector-transfected FaDu tumor cells. We further studied the interaction between normal endothelial cells and HNSCC cells and evaluated the effects of rapamycin on this interaction using transwell inserts and conditioned media (media harvested from cultured cells that is typically enriched in cytokines and growth factors secreted by the cells).
Figure 4.
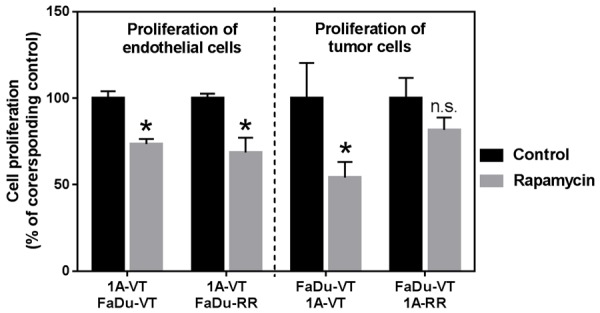
Cell proliferation of HMEC-1A (1A) shown on the left and FaDu cancer cells shown on the right that were co-cultured and treated with mTOR inhibitor rapamycin (0 and 10 ng/ml). VT-vector-transfected; RR-rapamycin-resistant mTOR transfected cells. Data is expressed as percent of control. Data represents mean ± SD for n>3 expeirments. Data was analyzed using Two sample independent student’s t test. *indicates P<0.05 relative to corresponding control, n.s.-not statistically significant.
When studying cell invasiveness we found that conditioned media collected from FaDu tumor cells also significantly increased the invasiveness of HMEC-1A cells (P<0.5) (Figure 5A), while there was no effect of conditioned media collected from endothelial cell cultures on tumor cell invasiveness. Conditioned media collected from tumor cell cultures increased the invasiveness of endothelial cells by almost two-fold.
Figure 5.
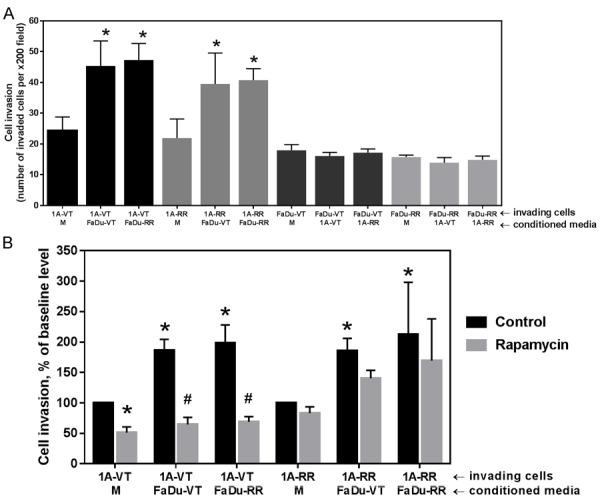
A: Graph depicting invasion of vector-transfected endothelial cells HMEC-1A (1A-VT), rapamycin-resistant endothelial cells (1A-RR), vector-transfected tumor cells (FaDu-VT) or rapamycin-resistant tumor cells (FaDu-RR) cells toward conditioned media of the same cell type (M, bottom row) or conditioned meda from the indicated cell types (bottom row under horizonthal axis). B: Effect of treatment with rapamycin (10 ng/ml) on the invasiveness of endothelial cells. Under horizonthal axis: first row-invading cells; second row-conditioned media of the same cell type (M) or indicated type of conditioned media. Data represents mean ± SD for n>3 expeirments. Data was analyzed using Two sample independent student’s t test. * indicates P<0.05 relative to invasion toward conditioned media of the same cell type (baseline). #indicates P<0.05 relative to corresonding vehicle-treated (DMSO) control.
We then evaluated the effects of rapamycin on the invasiveness of endothelial cells, including endothelial cell invasion stimulated by conditioned media collected from FaDu tumor cell cultures (Figure 5B). Conditioned media was added into the lower compartment (a well in a 24-well plate). Cells were seeded in the top compartment (transwell inserts) and treated with 10 ng/ml of rapamycin. There was a significant effect of rapamycin on both baseline invasiveness of vector-transfected rapamycin-sensitive endothelial cells as well as the invasiveness of endothelial cells stimulated by conditioned media collected from tumor cell cultures. Rapamycin effectively reverted the stimulatory effect of cytokines secreted by tumor cells on endothelial cell invasion (Figure 5B). Endothelial cells transfected with rapamycin-resistant mTOR show only modest sensitivity to rapamycin treatment as expected (Figure 5B).
In order to differentially assess direct anti-vascular and crosstalk-mediated effects of rapamycin (Table 1) we measured a reduction in proliferation of endothelial cells co-cultured with tumor cells (VT or RR). Various combinations of endothelial cells co-cultured with vector-transfected (VT) or rapamycin-resistant mTOR transfected (RR) TC were treated with 10 ng/ml of rapamycin for differential assessment of direct antivascular and crosstalk-mediated effects of rapamycin (Table 1). We can conclude that anti-vascular growth inhibitory pro-perties of rapamycin are primarily a result of drug’s direct effects on endothelial cells, and not cross-talk mediated, effects (Table 1).
Table 1.
Assesment of direct anti-vascular and crosstalk-mediated effects of rapamycin in various co-culture combinations
| # | Co-culture | Inhibition of EC growth is a measure of the following effects of the drug | Percent inhibition of EC growth (to corresponding control) |
|---|---|---|---|
| 1 | 1A-VT+FaDu-VT | Direct and crosstalk-mediated | 40.4±6.3% |
| 2 | 1A-VT+FaDu-RR | Only direct | 37.2±7.1% |
| 3 | 1A-RR+FaDu-VT | Only crosstalk-mediated | 3.5±3.2% |
Cytokine secretion: co-culture stimulates IL-6 secretion, while rapamycin significantly suppresses secretion of IL-6 in sensitive cells
Next, using Multiplex bead immunoassay we evaluated levels of various growth factors and cytokines that endothelial and tumor cells release into cell culture media. While rapamycin had a moderate effect on the levels of several growth factors and cytokines (sVEGFR-1, HGF, FGF-2, TNF-alpha), in the evaluated co-culture models there was a distinct differential effect of the drug on the levels of secreted IL-6. When vector-transfected endothelial cells were co-cultured with any of the FaDu tumor cells (VT, RR), rapamycin caused a significant decrease in the level of IL-6 (Figure 6A). On the contrary, rapamycin treatment lead to an upregulation in the level of IL-6 when rapamycin-resistant endothelial cells were co-cultured with VT or RR FaDu cells. Since IL-6 is known to have mitogenic properties this may explain how co-culture of rapamycin-resistant endothelial cells with rapamycin-sensitive tumors cells can reduce growth-inhibitory effects of rapamycin against head and neck tumor cells.
Figure 6.
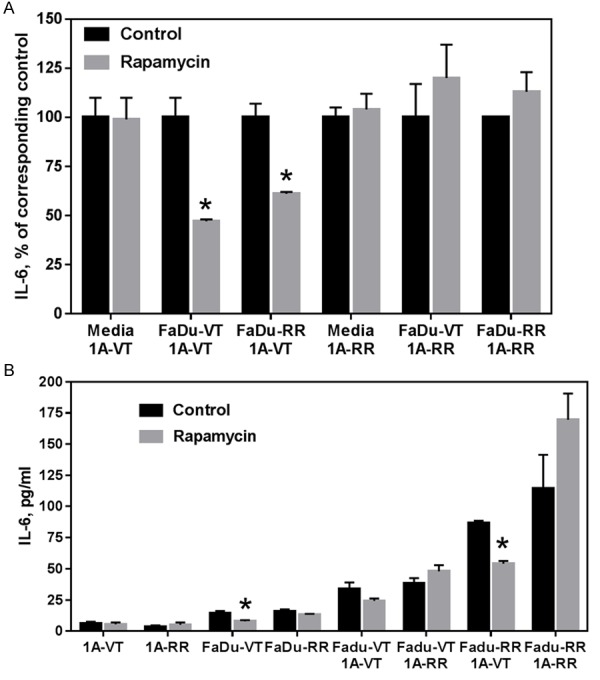
A: The results of MILLIPLEX MAP assay. Levels of IL-6 in cell culture media after treatment with vehicle (DMSO) or rapamycin (10 ng/ml) for 4 days. B: Levels of secreted IL-6 in cell culture media after treatment with vehicle (DMSO) or rapamycin (10 ng/ml) for 4 days by ELISA assay. VT-vector-transfected; RR-rapamycin-resistant mTOR transfected cells; 1A-HMEC-1A cells. Data represents mean + SD for n>3 experiments. Data was analyzed using Two sample independent student’s t test. *indicates P<0.05 relative to corresponding DMSO-treated control.
These observations were confirmed using ELISA assay (Figure 6B). Endothelial cells secrete much lower levels of IL-6 compared to tumor cells. Importantly co-culture of endothelial and tumor cells results in a significant upregulation of IL-6 secretion. It can be noted that rapamycin treatment inhibits IL-6 secretion in rapamycin-sensitive tumor cells. In the case of co-culture of endothelial and tumors cells the effect of rapamycin depends on the status of both cell types. Rapamycin suppressed IL-6 secretion in co-culture combinations with rapamycin-sensitive endothelial cells.
Discussion
In the present study, we sought to investigate crosstalk mechanisms between HNSCC tumor cells and lymphatic endothelial cells and determine whether mTOR inhibition has a significant effect on this crosstalk. Our results suggest that the ‘anti-tumor’ activity of mTOR inhibitors in HNSCC confers a significant anti-vascular. We found that although HNSCC cells have minimal effect on endothelial cell proliferation, there was a significant upregulation in the invasiveness of lymphatic endothelial cells when they were co-cultured with head and neck carcinoma cells. This suggests that tumor cells produce chemotactic stimuli that promote endothelial cell invasion towards tumor cells. This process can play an important role in head and neck cancer lymph node metastasis. Lymphatogenous spread is a significant problem and much more predominant in HNSCC than hematogenous spread. Rapamycin treatment significantly suppressed baseline invasiveness of both endothelial and tumor cells and also suppressed stimulatory effect of tumor cells on invasion of endothelial cells. Interestingly, although tumor cells promote endothelial cell invasion, there appears to be a direct effect of rapamycin on the invasion potential of endothelial cells since co-culture of rapamycin-sensitive endothelial cells with rapamycin-resistant tumor cells does not result in attenuation of drug’s effects on sensitive endothelial cells.
Interleukin-6 (IL-6) is a pleiotropic cytokine that acts as a multifunctional regulator of immune response and hematopoiesis [13]. Furthermore several studies identified IL-6 as an important trigger in the progression of lymphangiogenesis and endothelial tube formation within the tumor [14,17]. One of the unexpected findings in this study is the attenuation of growth-inhibitory effects of mTOR inhibitor rapamycin on head and neck tumor cells when those were co-cultured with rapamycin-resistant endothelial cells emphasizing the role of tumor stroma in the drug’s efficacy. It is not clear whether tumor stroma in different patients can vary significantly in its sensitivity to mTOR inhibition. However our results suggest the ‘anti-tumor’ activity of mTOR inhibitors in HNSCC can have a significant anti-vascular component. Among several cytokines tested only IL-6 showed a significant response to rapamycin treatment and was associated with biological effects of the drug. Since sIL-6 is known to have mitogenic properties [18,20] this could explain how co-culture of rapamycin-resistant endothelial cells with rapamycin-sensitive tumors cells can reduce growth-inhibitory effects of rapamycin against head and neck tumor cells. IL-6 is one of the cytokines that is significantly increased in subjects with laryngeal dysplasia and larynx squamous cell carcinoma patients compared to controls [21]. Upregulated levels of IL-6 are associated with unfavorable prognosis, recurrences and poor response to chemoradiotherapy in HNSCC [22,24]. Upregulation of IL-6 expression was found to be one of the causes of resistance to EGFR tyrosine kinase inhibitor erlotinib in HNSCC [25].
Our findings suggest that sIL-6 can be a potential biomarker of response to mTOR inhibitor therapy. We hypothesize that efficacy of mTOR-targeted therapy can correlate with changes in the level of released sIL-6 after treatment. In our preclinical studies of rapalogues to date we identified potential biomarkers of antiangiogenic and anti-lymphangeogenic effects of mTOR inhibitors, such as VEGF-A, VEGF-C, FGF-2, soluble VEGFR-2, VEGFR-3 [8-10] and now IL-6. These biomarkers are currently being evaluated in Phase II clinical trial of the mTOR inhibitor everolimus.
Acknowledgements
The study was supported by grant # R01CA102363 from the National Cancer Institute to C.O. Nathan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 4.Riaz N, Sherman E, Koutcher L, Shapiro L, Katabi N, Zhang Z, Shi W, Fury M, Wong R, Wolden S, Rao S, Lee N. Concurrent Chemoradiotherapy With Cisplatin Versus Cetuximab for Squamous Cell Carcinoma of the Head and Neck. Am J Clin Oncol. 2016;39:27–31. doi: 10.1097/COC.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiura T, Inoue Y, Matsuki R, Ishii K, Takahashi M, Abe M, Shirasuna K. VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: Implications for use as a prognostic marker. Int J Oncol. 2009;34:673–680. doi: 10.3892/ijo_00000193. [DOI] [PubMed] [Google Scholar]
- 6.Warburton G, Nikitakis NG, Roberson P, Marinos NJ, Wu T, Sauk JJ Jr, Ord RA, Wahl SM. Histopathological and lymphangiogenic parameters in relation to lymph node metastasis in early stage oral squamous cell carcinoma. J Oral Maxillofac Surg. 2007;65:475–484. doi: 10.1016/j.joms.2005.12.074. [DOI] [PubMed] [Google Scholar]
- 7.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, Molinolo AA, Gutkind JS. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 8.Ekshyyan O, Rong Y, Rong X, Pattani KM, Abreo F, Caldito G, Chang JK, Ampil F, Glass J, Nathan CO. Comparison of radiosensitizing effects of the mammalian target of rapamycin inhibitor CCI-779 to cisplatin in experimental models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2009;8:2255–2265. doi: 10.1158/1535-7163.MCT-08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan CO, Amirghahari N, Rong X, Giordano T, Sibley D, Nordberg M, Glass J, Agarwal A, Caldito G. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67:2160–2168. doi: 10.1158/0008-5472.CAN-06-2449. [DOI] [PubMed] [Google Scholar]
- 10.Ekshyyan O, Moore-Medlin TN, Raley MC, Sonavane K, Rong X, Brodt MA, Abreo F, Alexander JS, Nathan CA. Anti-lymphangiogenic properties of mTOR inhibitors in head and neck squamous cell carcinoma experimental models. BMC Cancer. 2013;13:320. doi: 10.1186/1471-2407-13-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells SR, Jennings MH, Rome C, Hadjivassiliou V, Papas KA, Alexander JS. Alpha-, gamma- and delta-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J Nutr Biochem. 2010;21:589–597. doi: 10.1016/j.jnutbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Nathan CO, Amirghahari N, Abreo F, Rong X, Caldito G, Jones ML, Zhou H, Smith M, Kimberly D, Glass J. Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2004;10:5820–5827. doi: 10.1158/1078-0432.CCR-03-0483. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinriki S, Jono H, Ueda M, Ota K, Ota T, Sueyoshi T, Oike Y, Ibusuki M, Hiraki A, Nakayama H, Shinohara M, Ando Y. Interleukin-6 signalling regulates vascular endothelial growth factor-C synthesis and lymphangiogenesis in human oral squamous cell carcinoma. J Pathol. 2011;225:142–150. doi: 10.1002/path.2935. [DOI] [PubMed] [Google Scholar]
- 15.Culig Z. Interleukin-6 as a therapy target in oral squamous carcinoma. Expert Opin Ther Targets. 2013;17:53–59. doi: 10.1517/14728222.2013.733368. [DOI] [PubMed] [Google Scholar]
- 16.Huang YH, Yang HY, Hsu YF, Chiu PT, Ou G, Hsu MJ. Src contributes to IL6-induced vascular endothelial growth factor-C expression in lymphatic endothelial cells. Angiogenesis. 2014;17:407–418. doi: 10.1007/s10456-013-9386-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35:1787–1795. doi: 10.3892/or.2016.4544. [DOI] [PubMed] [Google Scholar]
- 18.Kanazawa T, Nishino H, Hasegawa M, Ohta Y, Iino Y, Ichimura K, Noda Y. Interleukin-6 directly influences proliferation and invasion potential of head and neck cancer cells. Eur Arch Otorhinolaryngol. 2007;264:815–821. doi: 10.1007/s00405-007-0264-6. [DOI] [PubMed] [Google Scholar]
- 19.Bran G, Götte K, Riedel K, Hörmann K, Riedel F. IL-6 antisense-mediated growth inhibition in a head and neck squamous cell carcinoma cell line. In Vivo. 2011;25:579–584. [PubMed] [Google Scholar]
- 20.Scherzad A, Steber M, Gehrke T, Rak K, Froelich K, Schendzielorz P, Hagen R, Kleinsasser N, Hackenberg S. Human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2. Int J Oncol. 2015;47:391–397. doi: 10.3892/ijo.2015.3009. [DOI] [PubMed] [Google Scholar]
- 21.Eyigor M, Eyigor H, Osma U, Yilmaz MD, Erin N, Selcuk OT, Sezer C, Gultekin M, Koksoy S. Analysis of serum cytokine levels in larynx squamous cell carcinoma and dysplasia patients. Iran J Immunol. 2014;11:259–268. [PubMed] [Google Scholar]
- 22.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, Wolf GT, Teknos TN. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 23.Meyer F, Samson E, Douville P, Duchesne T, Liu G, Bairati I. Serum prognostic markers in head and neck cancer. Clin Cancer Res. 2010;16:1008–1015. doi: 10.1158/1078-0432.CCR-09-2014. [DOI] [PubMed] [Google Scholar]
- 24.Jinno T, Kawano S, Maruse Y, Matsubara R, Goto Y, Sakamoto T, Hashiguchi Y, Kaneko N, Tanaka H, Kitamura R, Toyoshima T, Jinno A, Moriyama M, Oobu K, Kiyoshima T, Nakamura S. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable prognosis in oral squamous cell carcinoma. Oncol Rep. 2015;33:2161–2168. doi: 10.3892/or.2015.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanam A, Love-Homan L, Joseph TS, Espinosa-Cotton M, Simons AL. Upregulated interleukin-6 expression contributes to erlotinib resistance in head and neck squamous cell carcinoma. Mol Oncol. 2015;9:1371–1383. doi: 10.1016/j.molonc.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]