Abstract
Background: Diabetic foot ulcer (DFU) is a major complication of diabetes mellitus. Although previous studies have established that inflammation, ischemia and neuropathy contribute to the development of DFU, it is still an unmet medical need due to lack knowledge of cellular and molecular mechanisms associated with DFU. In the present study, we tested our hypothesis that subcutaneous application of human placental mesenchymal stem cells (PMSCs) can accelerate diabetic dermal wound healing by modulating immunoresponse. Methods and Results: By using an in vivo excisional wound healing model in Goto-Kakizaki (GK) rats, we found that injection of PMSCs accelerates wound closure. Further studies revealed that application of PMSCs can regulate inflammation associated with wound healing by controlling secretion of pro- and anti-inflammatory factors, the beneficial effects can be partially blocked by application of antibodies against interleukin-10 (IL-10). Furthermore, in vitro experiments suggested that co-culture of PMSCs with human dermal fibroblasts can significantly inhibit activation of NF-ĸB induced by lipopolysaccharides (LPS), indicating the molecular mechanism of PMSCs mediated immunomodulation. Conclusion: Taken together, our study suggested that the immunomodulation of PMSCs play an important role on diabetic dermal wound healing process, thus PMSCs might represent an attractive choice for treatment of diabetes dermal wound and DFU.
Keywords: Diabetic foot ulcer, placenta-derived mesenchymal stem cell, wound closure
Introduction
Diabetes mellitus is one of the most common metabolic disorders that greatly affect the life quality of the whole human society. It was estimated that the prevalence of type 2 diabetes was 285 million (6.4% of the world population) on 2010, which will keep increasing to 439 million (7.7%) by year of 2030 [1]. In addition to major symptoms, diabetes patients are usually suffering multiple complications, such as diabetic foot ulcer, ketoacidosis, nephropathy, high blood pressure, stroke and so on (www.diabetes.org). Among these complications, diabetic foot ulcer (DFU) is one of the most frequent clinical indications in diabetic patients. The estimates indicate that about 2-3% diabetic patients will develop a foot ulcer each year, and about 15% of them will develop a foot ulcer during their lifetime, which causes a tremendous medical burden worldwide [2,3]. A number of risk factors have been linked to development of DFU, including limb ischemia, neuropathy and impaired dermal wound healing [2,3]. Given the fact that multiple cellular processes are involved in dermal wound healing associated with diabetes, such as inflammatory response, infection, angiogenesis and vasculogenesis, development an effective means for treatment of diabetes associated chronic wound healing remains an unmet medical need.
Stem cell based therapy has emerged as an effective treatment in regenerative medicine for multiple diseases, such as cardiac diseases [4], diabetes [5], and central neuronal degenerations [6]. In addition, increasing clinical and preclinical evidences have suggested that the beneficial effects of cell-based therapy extend beyond the initial concept that implanted stem cells can differentiate and regenerate injured local tissues. There is a more complex process of paracrine factors secreted by the stem cells might also play critical roles in tissue repair [7-11]. In particular, immunomodulation effects of implanted stem cells are considered to be a potential mechanism underlying the beneficial effects of stem-cell based therapy [12-14].
Here, we sought to test a hypothesis that implantation of mesenchymal stem cells derived from human placentas (PMSCs) can ameliorate dermal wound healing by immunomodulation. We found that co-culture of PMSCs with dermal fibroblasts can inhibit in vitro inflammatory response induced by LPS. Further in vivo experiments demonstrated that implantation of PMSCs can accelerate closure of the excisional wounds in diabetic Goto-Kakizaki (GK) rats and the beneficial effects of PMSCs were at least partially through modulating inflammatory response following dermal injury. Thus, our study suggested a potential immunomodulation role of implanted PMSCs in diabetic wound healing.
Materials and methods
Patient selection, tissue processing and mesenchymal stem cell isolation
PMSCs isolation protocol was established from other studies [15]. Briefly, fresh placentas were collected from normal, full-term (38-40 weeks gestation), healthy donors in compliance and approval of the Independent Ethics Committee of the Tongji Hospital affiliated with Tongji University. Written informed consent was signed prior to the study. All tissues were examined by a certified pathologist to verify that they were free of human immunodeficiency virus, toxoplasmosis, cytomegalovirus and rubella virus infections. To maintain recovery of PMSCs, all tissues were kept on ice and processed within three hours after the pathologist evaluation.
The harvested tissues were extensively washed with ice-cold phosphate-buffered saline (PBS) and then manually minced. The tissues were digested in 0.1% collagenase IV (Sigma-Aldrich, St. Louis, MO) at 37°C for 1 h followed by a cell strainer filtration (Falcon 3078, BD Biosciences, San Jose, CA) to eliminate undigested debris. After that, the cells were span down at 2000 rpm for 10 minutes, and red blood cells were lysed by red blood cell lysis buffer for 5 minutes at 37°C. The remaining cells were collected by centrifugation at 300 × g for 5 minutes and cultured in growth medium (DMEM medium containing 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 1% nonessential amino acids). The cells were cultured at 37°C under a 5% CO2 atmosphere for 4 days before the culture medium was first replaced.
Characterization of isolated PMSCs
PMSCs from passage 3 were used for phenotypic marker identification by flow cytometry analysis. The cells were detached with trypsin (0.25% with 0.1% ethylenediaminetetraacetic acid (EDTA) in Hanks’ balanced salt solution (HBSS)) and suspended in ice-cold staining buffer. Approximately 5 × 105 cells were incubated with fluorescence conjugated antibodies for 30 min. The antibodies used were CD29 (APC-conjugated), CD13 (FITC-conjugated), CD73 (PE-conjugated), CD105 (PE-conjugated), CD49b (APC-conjugated), HLA-DR (FITC-conjugated), CD45 (PE-conjugated), and CD34 (FITC-conjugated). All of the antibodies were obtained from Becton Dickinson and Company (BD Pharmingen, San Diego, CA). At least 15,000 events per staining were analyzed by flow cytometry (FACScan, BD Biosciences), and the data analysis was performed with BD FACSDiva software (version 5.0, BD Biosciences).
The pluripotency of the isolated PMSCs was tested by culturing PMSCs in adipogenic or osteogenic culture medium followed by oil red O staining for adipocytes and Alizarin red staining for osteocytes.
Non-contact co-culture of PMSCs and human dermal fibroblasts
Primary human dermal fibroblasts were co-cultured with PMSCs in separate six-well plates in a transwell cell culture system (Corning, Tewksbury, MA) [16]. Pores with a size of 0.4 μm allow exchange of PMSC-produced soluble factors from upper chamber (PMSCs) to lower chamber (fibroblasts). For all studies, the cells were seeded at 5 × 105 cells/chamber and maintained in DMEM for 8 h. Fibroblast cells were serum starved for 24 h prior to any experiment. After serum starvation, fibroblast cells were stimulated with LPS 1 µg/ml in the presence or absence of 5 × 105 PMSCs in the upper chamber. Fibroblasts were harvested at each specified time point, total and cytoplasmic were isolated and analyzed as mentioned.
Animal studies
Animal husbandry and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the Tongji Hospital affiliated with Tongji University. Diabetic Goto-Kakizaki (GK) rats (aged 8-12 weeks) were placed in individual cages and subjected to wounding. The dorsum hair was removed by a clipper. A full-thickness circular (8 mm diameter) excisional wound was created on the dorsum of each rat with a dermal biopsy punch (Miltex, Plainsboro, NJ). Six hours after surgery, 106 PMSCs (a total of 100 µl) and same volume of PBS (control) were subcutaneously injected around the wound area. For recombinant IL-10 (rIL-10) and IL-10 antibody (Life Technologies, Grand Island, NY; purity ~95%) treatment experiments, rIL-10 (100 ng) or IL-10 antibody (200 ng) was subcutaneously injected around wound area after excisional wound surgery. The treatments were continued once every other day throughout the whole experiments.
Analysis of wound closure
Digital photographs of the excisional wounds were taken at day 0, 5, 10 and 15. The wound area was measured by tracing the wound margin and calculated using ImageJ software (NIH). The percentage wound closure was calculated as following formula: Wound closure percentage = (area of original wound-area of actual wound)/area of original wound × 100. Rats were heavily anesthetized using ketamine and dorsal skin was removed using aseptic technique at day 15 after the wound surgery. Each wound was cut out and placed into buffered formalin solution for histopathological examination. Tissue sections were stained with either H&E or Masson’s Trichrome and examined by qualified pathologists blinded by treatments for evaluation of pathological outcomes of different treatments.
Western blot analysis
At the end of co-culture experiments, fibroblasts were collected and lysed by RIPA buffer (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) supplemented with protease and phosphatase inhibitor cocktails (Roche, Applied Science). For each sample, 25 μg of total protein was loaded and separated on 8% SDS polyacrylamide gels. Then proteins were transferred to polyvinylidene difluoride membrane and blotted with primary antibody and horseradish peroxidase conjugated secondary antibody. Peroxidase activity was developed with ECL kits (Pierce). Anti-NF-κB and anti-GAPDH antibodies were used in the experiments.
Statistical analysis
All results are presented as the mean ± SEM. Statistical comparisons between two groups were performed using the Student t-test. Probability (P) values < 0.05 were considered statistically significant. All in vitro experiments were repeated at least in triplicate and data used for analysis.
Results
Characterization of PMSCs
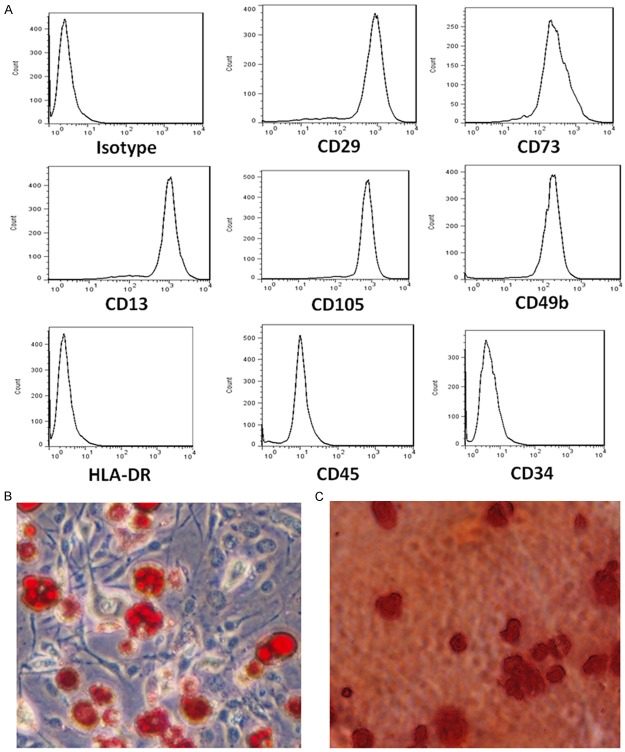
To characterize the cell surface markers of typical PMSCs, flow cytometry was performed to analyze PMSCs. The cells were stained with FITC-, APC-, or PE-conjugated surface marker antibodies and examined by flow cytometry. Cells were dissociated and stained with CD29 (mononuclear cell marker), CD73 (endothelial cell and stem-cell marker), CD13 (mesenchymal stem-cell marker), CD105 (mesenchymal stem-cell marker), CD49b (mesenchymal stem-cell marker), HLA-DR (an MHC class II cell-surface receptor encoded by the human leukocyte antigen; DR is also a marker for immune stimulation), CD45, and CD34. Stained cells and an unstained control cells were subjected to FACS analysis. Most of the PMSCs strongly expressed CD29, CD13, CD73, and CD105, CD49b and were negative for HLA-DR, CD45, and CD34 (Figure 1A), indicating a successful purification of PMSCs. Further analysis demonstrated that the immunophenotype of PMSCs remained unchanged for more than eight cell passages. After culturing with differentiation medium, the isolated PMSCs can also differentiate into adipocytes (Figure 1B) and osteocytes (Figure 1C) as demonstrated by Oil Red O and Alizarin red staining, indicating the functions of PMSCs were preserved after isolation.
Figure 1.
Characterization of isolated PMSCs. (A) Representative images of flow cytometry show the expression of surface markers of the PMSCs isolated from human placenta. Microscope images show that isolated PMSCs can differentiate into adipocytes (B) and osteocytes (C). Scale bar: 50 µm.
PMSCs transplantation promotes wound closure
In order to test whether PMSCs treatment can facilitate dermal wound healing process, we utilized an excisional wound healing animal model in diabetic GK rats. As shown in Figure 2A, full-thickness excisional wounds with a mean diameter of 8 mm were created in GK rats. Six hours later, PMSCs were transplanted around the wound. While the initial wounds are similar between experimental groups, PMSCs treatment (61.01 ± 9.25%) resulted in a significantly smaller wound than that in control animals (93.42 ± 16.98%, P < 0.01 as compared with PMSCs treatment) within 5 days of treatment. Furthermore, the wounds in PMSCs treated group healed around day 15, while the wounds in control group were still very pronounced (11.52 ± 2.34% in PMSCs treated group vs. 40.48 ± 7.56% in control group, P < 0.01).
Figure 2.
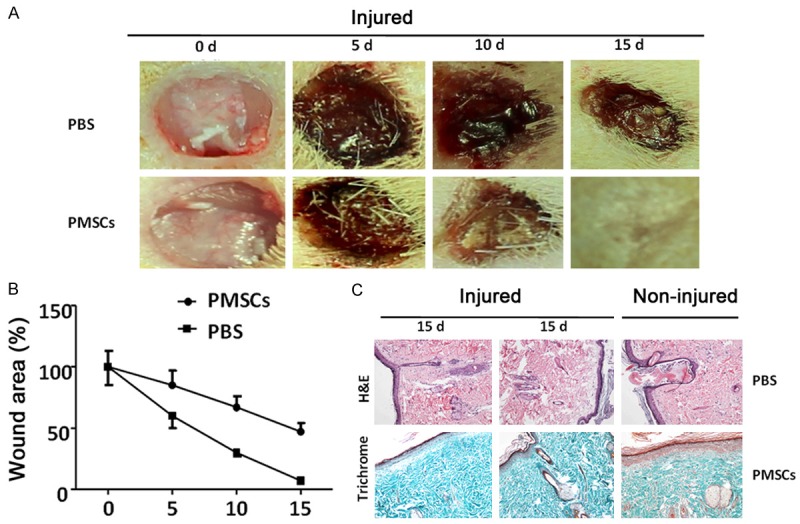
PMSCs treatment enhances diabetic wound healing. A. Representative images show subcutaneous application treatment of PMSCs accelerates diabetic wound closure. B. All wounds were measured and quantified by ImageJ software at day 0, 5, 10 and 15 post-operation. The wound contraction rate is plotted as the percentage reduction of original wound area over time (each group, n = 5; **: P < 0.01). C. H&E staining (upper panels) and Masson trichrome staining (lower panels) show that epidermal layer was obviously thicker in PMSCs-treated group than that of PBS-treated control, more similar to the normal. The PMSCs-treated group exhibited a thick layer of granulation tissue with a large number of microvessels as well. Scale bar: 100 µm.
In accordance with observation of wound closure rates, histochemical analysis revealed increased cellular infiltration and collagen deposition and thick granulation tissue in PMSCs-treated wounds (Figure 2C). The thickness of the newly formed epidermal layer in the PMSCs treated group was much greater than that of the control group on day 15 after transplantation. Furthermore, the alignment of fibers in the healing skin tissue appeared more regular in the PMSCs treated group. Finally, folliculuspili and some other appendices emerged as regenerating skin tissue in PMSCs animals but not in controls.
PMSCs regulated the local inflammatory response during skin wound healing
To explore the molecular mechanism of PMSCs mediated wound healing, we next investigated the in vivo effects of PMSCs on production of local inflammatory cytokines in skin wounds. ELISA analysis showed that PMSCs treatment significantly decreased the local levels of pro-inflammatory cytokines, TNF-α, IL-6 and IL-1, increased the anti-inflammatory cytokine IL-10 (Figure 3A-D). These findings suggested that PMSCs treatment promotes skin wound healing, at least in part, by suppressing pro-inflammatory cytokine secretion as well as by increasing the production of IL-10 at the local wound sites.
Figure 3.
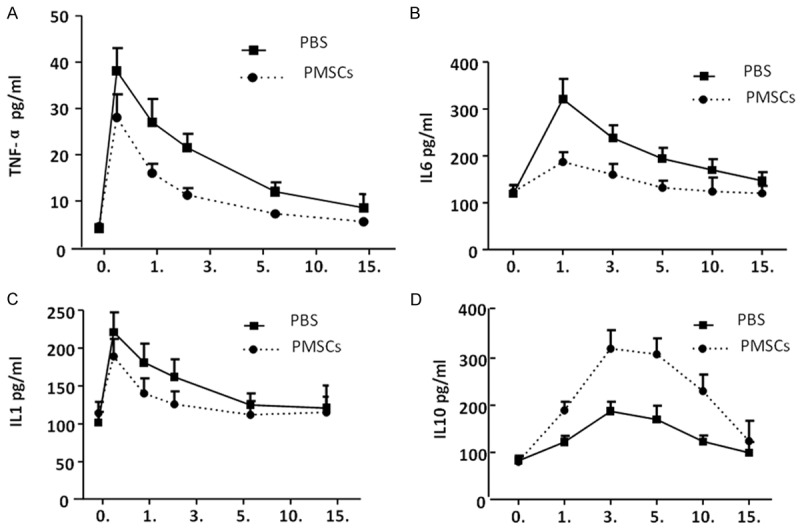
PMSCs treatment regulates secretion of inflammatory factors. Inflammatory factors in blood circulation were determined at indicated time points. PMSCs treatment can effectively inhibit secretion of pro-inflammatory factors, TNF-α (A), IL-1 (B) and IL-6 (C) and promote secretion of anti-inflammatory factor, IL-10 (D). The data were presented as mean ± SEM; *P < 0.05.
PMSCs suppress LPS mediated NF-κB activation in fibroblasts
In order to further dissect molecular mechanisms underlying PMSCs mediated immunomodulation, we used a PMSCs/fibroblasts in vitro co-culture system. As NF-κB plays a central role in regulating the transcription of most of inflammatory factors (such as IL-1β, IL-6 and TNF-α), we tested whether PMSCs have any impact on activation of the NF-κB signaling in fibroblasts. NF-κB-p65 phosphorylation at serine 536 is essential for its activation and transcriptional activity. Therefore, we investigated whether the presence of PMSCs regulates the phosphorylation of p65. To activate NF-κB, LPS was added to culture media. Western blot analysis revealed that LPS treatment alone resulted in rapid and sustained p65 phosphorylation at serine 536 at 30 and 60 min time points. Interestingly, the presence of PMSCs strongly inhibited LPS-induced NF-κB activation in dermal fibroblasts at the 30 and 60 min time points (Figure 4). There was no significant change in total p65 levels after addition of PMSCs cells, suggesting that PMSCs cells might regulate inflammatory response by targeting NF-κB signaling in dermal wound healing.
Figure 4.
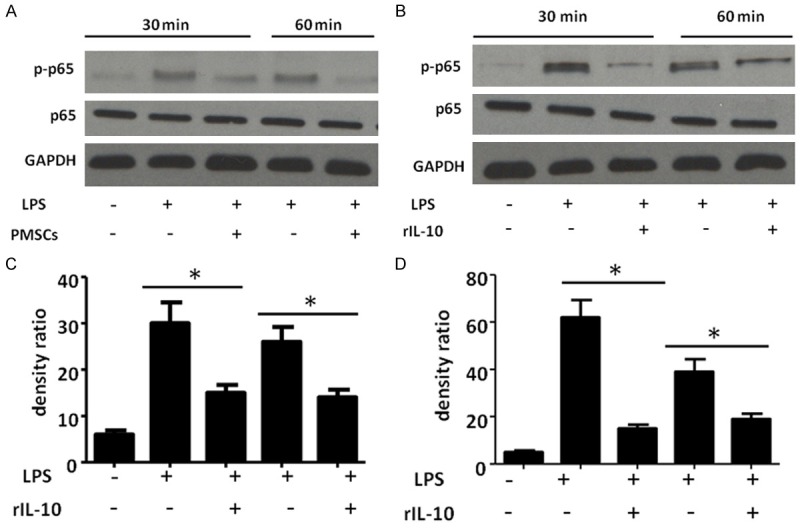
PMSCs inhibit activation of NF-ĸB signaling in fibroblasts. A, B. Western blot analysis shows that LPS induced activation of NF-ĸB signal in fibroblasts is inhibited by co-culture of PMSCs at different time points (30 mins and 60 mins). C, D. Quantification of western blot analysis by ImageJ software. Data are presented as mean ± SEM; n = 6 per group; **P < 0.01.
Modulation of IL-10 signal affects PMSCs mediated diabetic wound healing
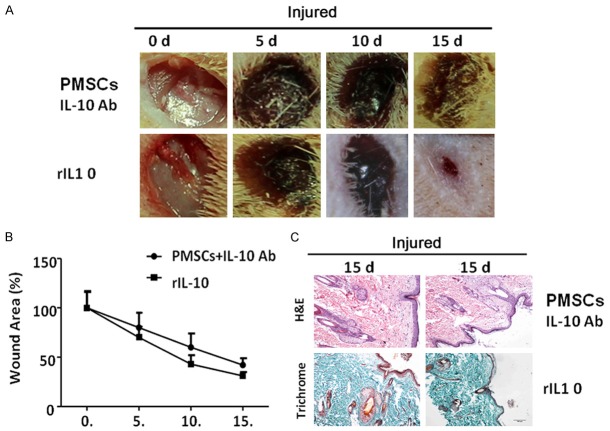
Since we found that PMSCs treatment increased the production of IL-10 at the local wound sites, we hypothesized that PMSCs treatment might modulate inflammatory response by secreting IL-10. In order to test this hypothesis, we co-treated wounds with combination of PMSCs and IL-10 antibody to neutralize production of IL-10 at local wounds. We found that co-treatment significantly delayed wound healing as compared to PMSCs treatment alone (compare Figure 5A, upper panels to Figure 2A lower panels). To further confirm the action of IL-10 in wound healing, recombinant IL-10 was applied to the wounds (subcutaneous injection, 100 ng per wound, injection was started on day 2 and performed once every other day till the end of experiments), we found that rIL-10 treatment enhanced wound healing (Figure 5A, lower panels). These observations were also confirmed by histochemical analysis (Figure 5C). Thus, our study indicated upregulation of IL-10 might contribute to beneficial effects of PMSCs mediated diabetic wound healing.
Figure 5.
Modulation of local IL-10 concentration affects PMSCs mediated diabetic wound healing. A. IL-10 antibody is co-injected with PMSCs after wound surgery (upper panels), the neutralization of IL-10 significantly inhibits PMSCs mediated wound healing. To test the effect of IL-10 signal in wound healing process, recombinant IL-10 (rIL-10, 100 ng) was injected to the wounds, we found injection of rIL-10 enhanced wound healing. B. All wounds were measured and quantified by ImageJ software at day 0, 5, 10 and 15 post-operation. The wound contraction rate is plotted as the percentage reduction of original wound area over time (each group, n = 5; **: P < 0.01). C. H&E staining (upper panels) and Masson trichrome staining (lower panels) show that rIL-10 treated wound exhibits better pathological morphologies than those treated by combination of PMSCs and IL-10 antibody treatment. Scale bar: 100 µm.
Discussion
In the present study, our data suggested that PMSCs treatment can promote dermal wound healing in a diabetic GK rat model. The molecular action of PMSCs mediated wound healing might be through immunomodulation by inhibiting NF-κB mediated pro-inflammatory response and promoting secreting of anti-inflammatory factor IL-10.
There are a number of types of stem cells being used in regenerative medicine, of which mesenchymal stem cells (MSCs) remain to be one of more desirable sources for stem cell-based therapy. It has been shown that MSCs have low tumorigenicity [17,18], immunogenicity [19], and are relatively easy to obtain with minimal ethical issues [20-22] MSCs can be isolated from various tissues [15]. Given the low percentage of MSCs present in the bone marrow (0.001-0.01%), the scientists have worked on isolating MSCs from other sources. One of them is PMSCs which has been used in the present study. Although other group have shown PMSCs can accelerate dermal wound healing through promoting local tissue angiogenesis [15,23], the potential roles of PMSCs on immunomodulation during chronic diabetic wound healing remain largely unknown.
Interestingly, some other study have shown that MSCs treatment can modulate immune reactions in other disease model, such as peripheral artery diseases [24], solid organ transplantations [25], and Crohn’s disease [26] (reviewed in [27]). However, there are few studies focused on the immunomodulation of PMSCs in treatment of diabetic wound. In our study, we didn’t observe obvious immunoresponse after implantation of PMSCs. In addition, we found that application of PMSCs could attenuate inflammatory response following excisional wound healing through NF-ĸB mediated pathway.
Of course, our study cannot address every aspect of PMSCs mediated immunomodulation. For example, in addition to potential role of IL-10 secretion, we also observed that the infiltration of macrophage was less in PMSCs treatment group. Thus, the cellular actions or potential interactions between implanted PMSCs and macrophage might be a direction for future studies. In addition, it has been shown that initial inflammatory response mediated by TGF-β or other factors are beneficial for dermal wound healing. Thus our next question is: do implanted PMSCs play differential roles on regulating initial protective inflammation and following detrimental inflammation? If yes, what are the underlying cellular and molecular mechanisms? Since our data in Figure 5 showed IL-10 antibody could only partially inhibit effects of PMSCs, what are other factors that are involved in PMSCs mediated diabetic wound healing? Finally, PMSCs have been shown to promote angiogenesis of local tissue, which could also contribute to diabetic wound healing. Our future studies will also focus on studying the role of PMSCs on angiogenesis following diabetic wounding with a hope to determine which effects play more important roles. Finally, we will also try to identify the paracrine factors that are secreted by PMSCs. Identification of these factors will allow us to understand the cellular and molecular mechanisms of PMSCs mediated immunomodulation. The answers to these questions will help us gain more insights for the roles of PMSCs in treatment of wound healing and other diseases.
Taken together, our data suggested PMSCs can promote wound healing by immunomodulation through NF-ĸB and IL-10 pathways. Therefore, targeting functional interactions between implanted PMSCs and local immune system might be a potential approach for treatment of different diseases.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81300712 and 81570738), by Shanghai Science and Technology Foundation (No. 13ZR1438000, by Research Fund for the Doctoral Program of Higher Education of China (No. 20130072120014) and by National Natural Science Foundation of China (No. 81401155) and by Shanghai Municipal Commission of Health and Family Planning (2015ZB0502).
Disclosure of conflict of interest
None.
References
- 1.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 2.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricco JB, Thanh Phong L, Schneider F, Illuminati G, Belmonte R, Valagier A, Regnault De La Mothe G. The diabetic foot: a review. J Cardiovasc Surg (Torino) 2013;54:755–762. [PubMed] [Google Scholar]
- 4.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller MJ, Viener HL, Wasserfall C, Brusko T, Atkinson MA, Schatz DA. Autologous umbilical cord blood infusion for type 1 diabetes. Exp Hematol. 2008;36:710–715. doi: 10.1016/j.exphem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S, Kurtzberg J. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010;50:1980–1987. doi: 10.1111/j.1537-2995.2010.02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murry CE, Palpant NJ, Maclellan WR. Cardiopoietry in Motion: Primed Mesenchymal Stem Cells for Ischemic Cardiomyopathy. J Am Coll Cardiol. 2013;61:2339–40. doi: 10.1016/j.jacc.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suncion VY, Schulman IH, Hare JM. Concise review: the role of clinical trials in deciphering mechanisms of action of cardiac cell-based therapy. Stem Cells Transl Med. 2012;1:29–35. doi: 10.5966/sctm.2011-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Niu J, Li X, Li Y, Wang X, Lin J, Zhang F. Hypoxia induces autophagy of bone marrow-derived mesenchymal stem cells via activation of ERK1/2. Cell Physiol Biochem. 2014;33:1467–1474. doi: 10.1159/000358711. [DOI] [PubMed] [Google Scholar]
- 12.Dokic J, Tomic S, Cerovic S, Todorovic V, Rudolf R, Colic M. Characterization and immunosuppressive properties of mesenchymal stem cells from periapical lesions. J Clin Periodontol. 2012;39:807–816. doi: 10.1111/j.1600-051X.2012.01917.x. [DOI] [PubMed] [Google Scholar]
- 13.De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 14.Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375–392. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 15.Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun. 2013;438:410–419. doi: 10.1016/j.bbrc.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roemeling-van Rhijn M, de Klein A, Douben H, Pan Q, van der Laan LJ, Ijzermans JN, Betjes MG, Baan CC, Weimar W, Hoogduijn MJ. Culture expansion induces non-tumorigenic aneuploidy in adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 2013;15:1352–1361. doi: 10.1016/j.jcyt.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Jones M, Varella-Garcia M, Skokan M, Bryce S, Schowinsky J, Peters R, Vang B, Brecheisen M, Startz T, Frank N, Nankervis B. Genetic stability of bone marrow-derived human mesenchymal stromal cells in the Quantum System. Cytotherapy. 2013;15:1323–1339. doi: 10.1016/j.jcyt.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91:40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Castro J, Trigueros C, Madrenas J, Perez-Simon JA, Rodriguez R, Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 2008;12:2552–2565. doi: 10.1111/j.1582-4934.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 22.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 23.Stoff A, Rivera AA, Sanjib Banerjee N, Moore ST, Michael Numnum T, Espinosa-de-Los-Monteros A, Richter DF, Siegal GP, Chow LT, Feldman D, Vasconez LO, Michael Mathis J, Stoff-Khalili MA, Curiel DT. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp Dermatol. 2009;18:362–369. doi: 10.1111/j.1600-0625.2008.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Adesanya TM, Zhang L, Xie N, Chen Z, Fu M, Zhang J, Tan T, Kilic A, Li Z, Zhu H, Xie X. Delivery of placenta-derived mesenchymal stem cells ameliorates ischemia induced limb injury by immunomodulation. Cell Physiol Biochem. 2014;34:1998–2006. doi: 10.1159/000366395. [DOI] [PubMed] [Google Scholar]
- 25.Cortinovis M, Casiraghi F, Remuzzi G, Perico N. Mesenchymal stromal cells to control donor-specific memory T cells in solid organ transplantation. Curr Opin Organ Transplant. 2015;20:79–85. doi: 10.1097/MOT.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 26.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 27.Nery AA, Nascimento IC, Glaser T, Bassaneze V, Krieger JE, Ulrich H. Human mesenchymal stem cells: from immunophenotyping by flow cytometry to clinical applications. Cytometry A. 2013;83:48–61. doi: 10.1002/cyto.a.22205. [DOI] [PubMed] [Google Scholar]