Abstract
MicroRNA-22-3p (miR-22-3p) is downregulated in hepatocellular carcinoma (HCC), which contributes to the development and progression of HCC. In this study, berberine treatment upregulated miR-22-3p expression in HepG2 cells. Therefore, we investigated whether berberine suppresses the proliferation of HCC cells and explored the underlying mechanism. The HCC HepG2 cell line was treated with a gradient of berberine concentrations (0-300 μM) for 48 h, and 100 μM berberine inhibited cell growth at 24 h. The HepG2 cells were then incubated with 100 μM berberine for 0-48 h, and after treatment for 24 h, berberine markedly suppressed HepG2 cell growth and significantly upregulated miR-22-3p expression. Berberine also downregulated the expression of SP1, CCND1, and BCL2, determined with western blotting. Dual luciferase reporter assays and western blot analyses showed that miR-22-3p directly targeted SP1, thereby suppressing the expression of its downstream targets, CCND1 and BCL2. SP1 knockdown with small interfering RNA also reduced CCND1 and BCL2 expression in HepG2 cells. Therefore, we conclude that berberine treatment suppresses cancer cell growth by regulating miR-22-3p and SP1 and its downstream targets, CCND1 and BCL2, in HCC.
Keywords: Berberine, hepatocellular carcinoma, miR-22-3p, SP1
Introduction
Liver cancer is the fifth most prevalent form of cancer and the third leading cause of cancer-related death worldwide, followed by lung and colon cancers [1]. Hepatocellular carcinoma (HCC) is one of the most common forms of adult liver cancer, representing over 90% of all cases of primary liver cancer [1,2]. Despite extensive efforts to improve the diagnosis and therapeutic strategies for HCC, the average survival of HCC patients has been improved only slightly in the past decade, so the discovery of new treatment modalities is essential [2-4].
Berberine, the major alkaloid component of Huang Lian (Rhizoma coptidis) and other medicinal herbs, is a commonly used medicine in China [5,6]. Berberine is a strong inhibitory drug used in the treatment of inflammation because it has both antimicrobial and anti-inflammatory effects [7,8]. Studies have recently shown that berberine also has antitumor activity, inhibiting the growth, invasion, and metastatic capacities of several cancers [6,9-11]. Recent studies of HCC have focused on the inhibition of cell growth, cell-cycle arrest, the induction of apoptosis, and the prevention of metastasis in this cancer [12-18].
The microRNAs (miRNAs) are a very large gene family encoding small noncoding RNAs of approximately 17-25 nucleotides [19]. These regulate the expression of protein-coding genes at the posttranscriptional level by binding to the 3’-untranslated regions (3’-UTRs) of their target miRNAs [20]. Among the miRNAs, miR-22-3p is a 22-nucleotide noncoding RNA that was originally identified as a tumor suppressor in HeLa cells [21,22]. Its expression has since been detected in a variety of tissues, including the liver, breast, lung, skin, and gastric cancer [23,24]. Several studies have also shown that miR-22-3p is associated with many important biological processes, including neuroprotection, tumorigenesis, and various other tumor progressions [25]. However, the roles of miR-22-3p in the progression of various tumors are inconsistent. In some studies, miR-22-3p was reported to act as an oncogene, promoting malignancy in breast cancer, lung cancer, and multiple myeloma [26-29]. While, several reports have also shown that it may act as a tumor suppressor in gastric cancer and esophageal squamous cell carcinoma [30,31].
In this study, we found that berberine treatment inhibited the proliferation of HepG2 cells in a time- and concentration-dependent manner. Berberine also upregulated the expression of miR-22-3p and downregulated the expression of SP1 and its downstream targets, CCND1 (a cell-cycle-related protein) and BCL2 (an apoptosis-related protein), in HepG2 cells. We demonstrate that an miR-22-3p mimic and inhibitor also regulated SP1, CCDN1, and BCL2 expression, confirming that SP1 is a direct target of miR-22-3p. In conclusion, we show that the overexpression of miR-22-3p suppresses the growth of HepG2 cells by directly targeting SP1. Our results indicate that miR-22-3p functions as a tumor suppressor and is a potential therapeutic target in HCC patients.
Materials and methods
Cell lines and culture conditions
The human HCC cell line HepG2 was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). It was routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) at 37°C in a humidified atmosphere of 5% CO2.
miRNAs and small interfering RNAs (siRNAs)
The miRNA mimic, inhibitor, and their negative controls were purchased from Thermo Scientific Dharmacon (Lafayette, CO, USA), and were transiently transfected with a final concentration of 100 nmol/L using Lipofectamine® 2000 (Invitrogen) according to the manufacturer’s instructions. After incubation for 5 h, the medium was replaced with DMEM containing 10% FBS. To knockdown human SP1 expression, cells were transfected with the appropriate siRNA using Lipofectamine® 2000 (Invitrogen). After 48 h, the levels of protein expression were measured with western blotting.
Quantitative real-time reverse transcription-PCR (qRT-PCR)
Total RNA was extracted from cultured cells with TRIzol® Reagent (Invitrogen) and reverse transcribed to cDNA with the PrimeScript RT-PCR Kit (TaKaRa, Shiga, Japan). Real-time PCR was performed with a SYBR® Premix Ex Taq™ II kit (TaKaRa), according to the manufacturer’s protocol, on an MX3005P QPCR System (Stratagene, La Jolla, CA, USA). All the reactions were performed in triplicate. The -delta delta Ct (-ΔΔCt) method for the relative quantification of gene expression was used to determine the miRNA expression levels. The forward and reverse primers for miR-22-3p were 5’-AAGCTGCCAGTTGAAGAACTGTA-3’ and Universal Primer (Qiagen, Hilden, Germany), respectively, and for u6 were 5’-CTCGCTTCGGCAGCACA-3’ and sn-RNA 5’-AACGCTTCACGAATTTGCGT-3’, respectively. All the primers were synthesized by Shanghai Sangon Biological Engineering Technology and Services Co., Ltd (Shanghai, China).
Western blotting
Western blotting assay was performed as following. Cells were harvested and lysed with M-PER Protein Extraction Reagent (Pierce, Rockford, IL, USA) supplemented with protease inhibitor cocktail. Protein concentrations of the extracts were measured with the bicinchoninic acid assay (Pierce, CA, USA) and equalized with the extraction reagent. Equal amounts of the extracts were loaded and subjected to SDS/PAGE, transferred onto nitrocellulose membranes, and then blotted as previously described. The primary antibodies used were: anti-SP1 (Abnova, Taipei, Taiwan), and anti-survivin (Ser473), anti-BCL2, and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology, Danvers, MA, USA).
Cell viability assay
HepG2 cells (500 cells per well) were plated into six individual wells of a 96-well plate in 100 μL of complete medium and allowed to attach overnight. The cells were then treated with 0-300 μM berberine for 48 h. After treatment, 20 μL of Cell Counting Kit-8 (CCK-8; Beyotime, Nanjing, China) was added to each well and the plates incubated for 2 h at 37°C. The Thermo Multiskan™ Spectrum Reader (Thermo Scientific) was used to measure the absorbance at 570 nm.
Measurement of the cell cycle
After treatment with 100 μM berberine for 24 h, HepG2 cells (1 × 106) were fixed in 70% ethanol at -20°C overnight. After the cells were washed, they were incubated with 0.25 mg/mL RNase A at 37°C for 30 min. Then 5 μL of propidium iodide (PI; KeyGen, Nanjing, China) was added to the cell suspension, which was incubated at room temperature for a further 30 min in the dark. The mixture was analyzed for cell-cycle progression with a FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA, USA).
Measurement of apoptosis
HepG2 cells were plated in six-well plates at a density of 2 × 106 cells/well and treated with 100 μM berberine for 24 h. The cells were later collected, gently vortexed, resuspended in binding buffer at a concentration of 3 × 106/mL, and 100 μL of the cell suspension was added to 5 μL of annexin V-FITC and 10 μL of PI. The samples were mixed for 15 min in the dark at room temperature, and 400 μL of phosphate-buffered saline was added to the solution. A FACScan flow cytometer (BD Biosciences) was used to count the cells (1 × 104) at an excitation wavelength of 490 nm. The CellQuest software was used for data collection and processing.
3’-UTR luciferase reporter assays
A human SP1 3’-UTR luciferase reporter construct was prepared by cloning the human SP1 mRNA 3’-UTR sequence into pMIR-REPORT (Ambion, Austin, TX, USA). The SP1 3’-UTR mutant binding sites were synthesized with PCR. HepG2 cells were cotransfected with 100 ng of the luciferase reporter plasmid and 40 ng of the Renilla-luciferase-encoding pRL-TK plasmid (Promega, Madison, WI, USA). The final concentration of RNA (100 nM) was determined with Jet-siENDO transfection reagent (Polyplus-Transfection, Graffenstaden, France). After incubation for 24 h, the luciferase activity was measured with the Dual-Luciferase Reporter Assay System (Promega, Sunnyvale, CA, USA), and the data were normalized for transfection efficiency by dividing the firefly luciferase activity by the Renilla luciferase activity.
Statistical analysis
All experiments were carried out at least 3 times with triplicate samples. The differences between groups were analyzed by one-way ANOVA when there were more than two groups. In all cases, differences were considered to be statistically significant at P < 0.05. All analyses were performed with SPSS 17.0 (Chicago, IL, USA).
Results
Berberine treatment inhibits cell proliferation and upregulates miRNA-22-3p expression in HepG2 cells
Berberine has shown antitumor effects by inhibiting the growth, invasion, and metastatic capacity of a variety of cancers [10,15]. To examine the effects of berberine on HCC cells, concentrations of 0-200 μM berberine were used to treat HepG2 cells for 48 h. The ability of berberine to inhibit cell proliferation at different time points was tested with a Cell Counting Kit-8 (CCK-8) assay. Berberine inhibited the proliferation of HepG2 cells in a time-dependent manner from 12 to 48 h, and also showed a concentration-dependent effect from 0 to 200 μM (Figure 1A). Real-time PCR was used to measure the expression of miR-22-3p with time in HepG2 cells during treatment with 100 μM berberine for 0-48 h. As shown in Figure 1B, miR-22-3p levels increased after treatment with berberine for 12 h (two-fold increase), 24 h (three-fold increase), and 48 h (five-fold increase).
Figure 1.
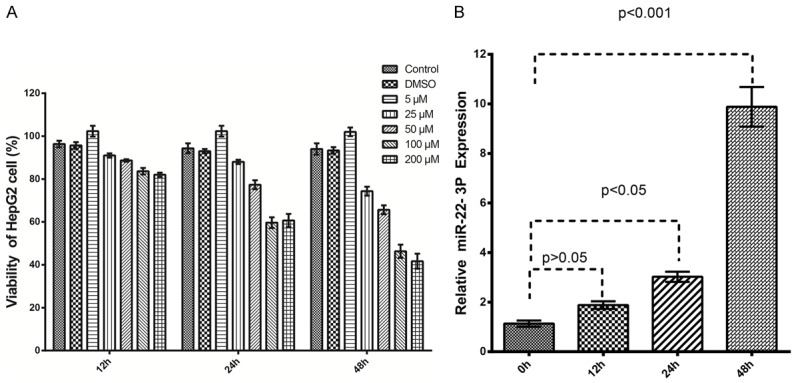
Berberine treatment inhibits cell proliferation and upregulates miRNA-22-3p expression in HepG2 cells. A: HepG2 cells were stimulated with 0-200 μM berberine for 0-48 h. Inhibition of cell proliferation by berberine was measured with an MTT assay. B: HepG2 cells stimulated with 100 μM berberine for up to 48 h compared with the untreated control. miRNA-22-3p expression was measured with qRT-PCR and is expressed as fold increases.
Berberine treatment induces cell-cycle arrest and apoptosis in HCC cells
Because berberine treatment inhibited HepG2 cell proliferation, we investigated whether berberine affects the cell cycle and/or apoptosis. HepG2 cells were treated with 100 μM berberine, and the cell-cycle distribution and levels of apoptotic cells were measured at different time points from 0 to 48 h. Cell-cycle arrest in G2/M phase was observed after treatment for 24 h (Figure 2). As shown in Figure 3, berberine treatment also induced significant apoptosis in the HepG2 cells (P < 0.01). These results indicate that berberine treatment inhibits HepG2 cell growth by inhibiting cell-cycle progression and inducing apoptosis.
Figure 2.
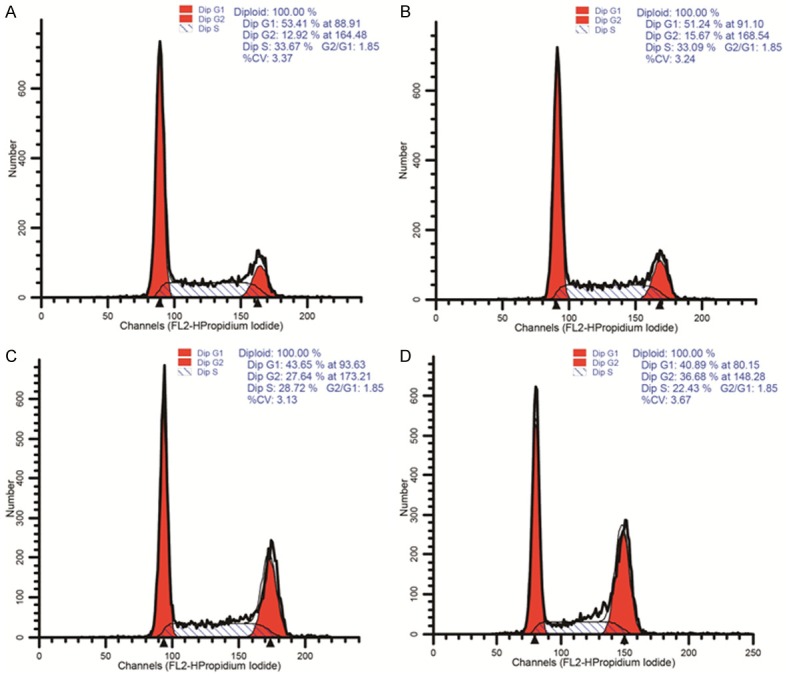
Berberine treatment induces cell-cycle arrest in HCC cells. Stimulated HepG2 cells and untreated control cells were harvested and analyzed with flow cytometry, and the cell-cycle distributions were determined.
Figure 3.
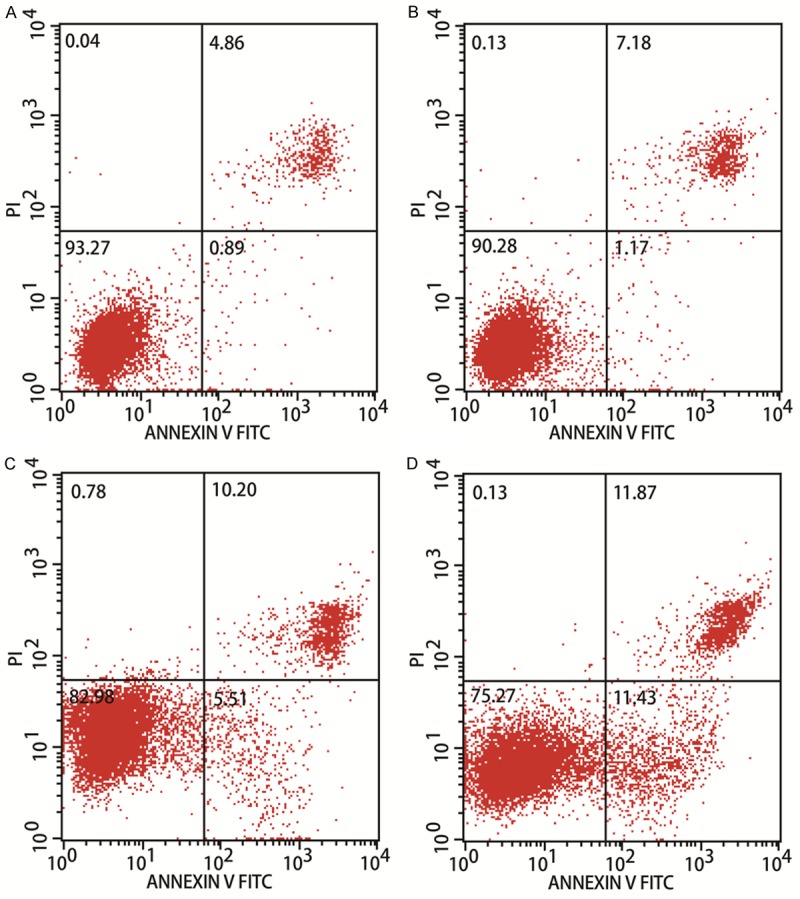
Berberine treatment induces apoptosis in HCC cells. Berberine stimulated HepG2 cells and untreated control cells were harvested, stained with annexin V and propidium iodide, and measured with flow cytometry.
Berberine treatment downregulates the expression of SP1 and its downstream targets, CCND1 and BCL2, in HCC
Various studies have shown that SP1 might be a target gene of miR-22-3p in different cancers [23,28,32,33]. Here, as mentioned above we found that berberine treatment upregulates miR-22-3p expression. To confirm that berberine treatment influences SP1 expression, we used western blot to investigate SP1 expression at different time points from 0 to 48 h after berberine treatment. And we found that after 48 h, SP1 was downregulated almost three-fold. Berberine also downregulated the expression of CCND1 and BCL2 in a time-dependent manner (Figure 4A). These results confirm that berberine treatment downregulates the expression of SP1 and its downstream targets, CCND1 and BCL2.
Figure 4.
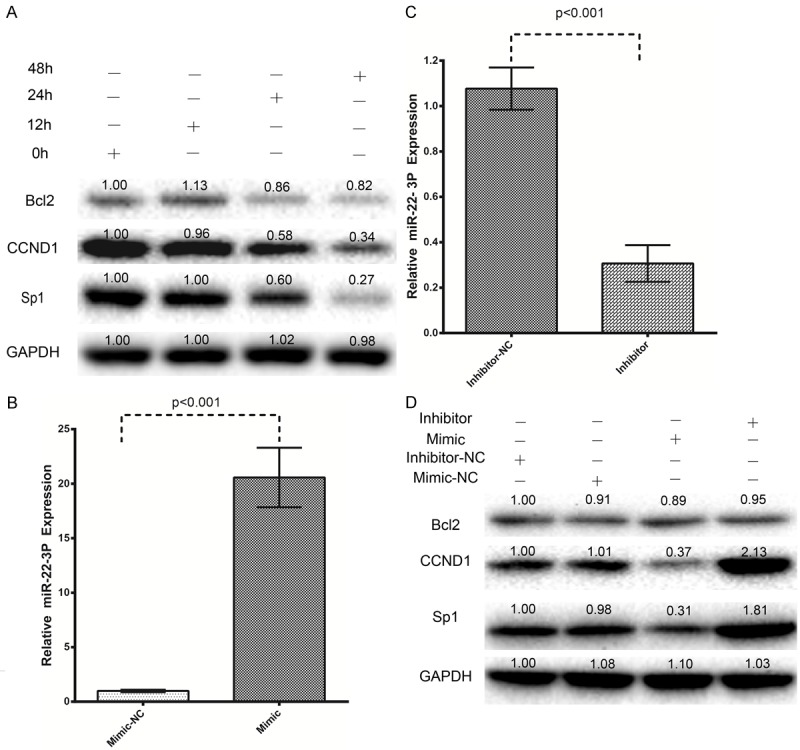
Berberine treatment downregulates SP1 and its downstream targets, CCND1 and BCL2, in hepatocellular carcinoma cells, and an miR-22-3p mimic and inhibitor regulated SP1, CCND1, and BCL2 expression in hepatocellular carcinoma cells. A: HepG2 cells stimulated with 100 μM berberine for up to 48 h were compared with untreated control cells. The expression of SP1, CCND1, and BCL2 was then measured with western blots. B: Transfection of HepG2 cells with an miR-22-3p mimic increased the expression of miR-22-3p. C: Transfection with an miR-22-3p inhibitor downregulated the expression of miR-22-3p, as determined with real-time PCR (qRT-PCR). D: The miR-22-3p mimic and inhibitor regulated SP1, CCND1, and BCL2 expression, determined with western blots.
miR-22-3p mimic and inhibitor regulate SP1 and consequently CCND1 and BCL2 expression in HCC
Based on these results, we speculated that the effect of berberine on SP1 expression might be mediated by miR-22-3p. To confirm the effects of miR-22-3p, we performed gain-of-function and loss-off-function experiments with an miR-22-3p mimic and an miR-22-3p inhibitor. Figure 4B shows that the transfection of HepG2 cells with 50 nM miR-22-3p mimic for 24 h caused a 22-fold increase in miR-22-3p expression. Figure 4C shows that the transfection of HepG2 cells with 50 nM miR-22-3p hairpin inhibitor caused a three-fold reduction in miR-22-3P expression. As shown in Figure 4D, miR-22-3p overexpression caused the downregulation of SP1, CCND1, and BCL2, whereas these proteins were upregulated when miR-22-3p was inhibited (Figure 4D). These data confirm that miR-22-3p plays a regulatory role in SP1 expression, and also influences the expression of its downstream targets, CCND1 and BCL2. SP1 is strongly associated with antitumor effects in HCC and is a putative target of miR-22-3p. Therefore, we examined the relationship between miR-22-3p and SP1.
SP1 is a direct target of miR-22-3p
Because both the miR-22-3p mimic and inhibitor significantly influenced SP1 expression, the 3’-UTR segment of SP1 was cloned into a reporter plasmid downstream from the luciferase gene to detect whether SP1 is a target gene of miR-22-3p. Figure 5A shows that the miR-22-3p mimic caused the substantial downregulation of reporter gene expression, and the luciferase activity was reduced from approximately 1.0 to 0.5. However, the downregulation of SP1 expression did not affect miR-22-3p expression in HepG2 cells (Figure 5B). Taken together, these results demonstrate that SP1 is a direct target of miR-22-3p.
Figure 5.
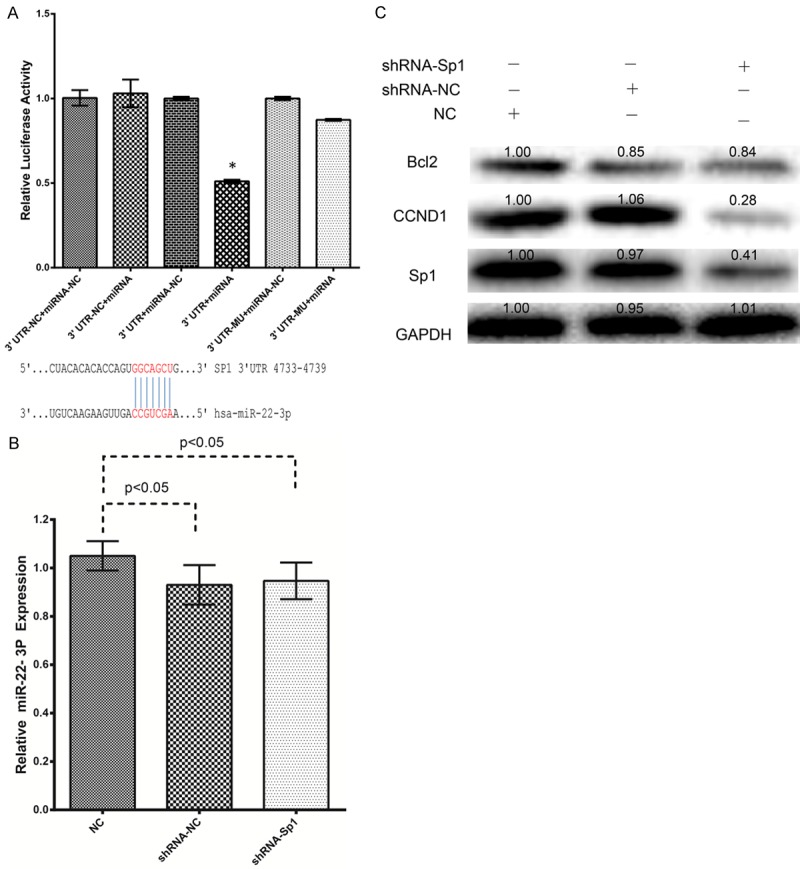
miRNA-22-3p directly targets SP1, and SP1 regulates CCND1 and BCL2 expression. A: A sequence alignment of miRNA-22-3p and its conserved target site in the SP1 3’-UTR. Luciferase activity was measured in the indicated cells with the Dual Luciferase Reporter Assay System. Firefly luciferase activity was normalized to Renilla luciferase activity, and the value for the empty pMIR vector was used as the control. B: Knockdown of SP1 had no effect on miRNA-22-3p expression, determined with qRT-PCR, in HepG2 cells. C: SP1, CCND1, and BCL2 protein expression in HepG2 cells was analyzed with western blotting after the cells were transfected with miRNA-22-3p or nature control (NC) for 48 h.
Knockdown of SP1 reduces downstream CCND1 and BCL2 expression
To confirm that SP1 is a functional target of miR-22-3p, we knocked down SP1 expression using a specific siRNA, and then evaluated miR-22-3P expression and the effects on the regulation of CCND1 and BCL2 expression. SP1 protein expression was reduced to approximately 60% after the knockdown of SP1 by siRNA in HepG2 cells (Figure 5C), and the knockdown of SP1 markedly reduced CCND1 and BCL2 expression (Figure 5C). These results suggest that the antitumor effects of miR-22-3P act through SP1 and its downstream targets, CCND1 and BCL2, in HepG2 cells.
Discussion
Drug-induced miRNAs have emerged as key regulators of cancer development and progression. Previous studies have shown that treatment with berberine upregulates miR-21-3p expression in hepatoma cells [34,35]. In the present study, we examined whether berberine treatment also alters miR-22-3p expression in a time- and concentration-dependent manner in HCC. Our results show that the expression of miR-22-3p increased after HepG2 cells were treated with berberine. To improve miRNA-based cancer treatments, it is essential to identify functionally important target genes of specific miRNAs and to understand the mechanisms of the actions of these miRNAs to determine their biological functions. However, the functions and specific mechanisms of miR-22-3p in HCC are not currently well understood. Here, we have demonstrated that berberine treatment increases the expression of miR-22-3p and reduces that of SP1 and its downstream targets CCND1 and BCL2 in HepG2 cells. SP1 was recently reported to play a vital role in cell proliferation in gastric cancer, breast cancer, and HCC, and is therefore a valid target of antineoplastic therapies [36-42]. Although SP1 is the predicted target of several miRNAs, no study has experimentally demonstrated a direct relationship between miR-22-3p and SP1 in HCC. In this study, we have shown that miR-22-3p directly targets the 3’-UTR of SP1, and thus also regulates the expression of the downstream proteins CCND1 and BCL2, thus exerting antitumor effects in HepG2 cells. Consistent with these data, the knockdown of SP1 expression by siRNA in hepatoma cells increased intracellular CCND1 and BCL2 expression. Therefore, we propose that the antitumor effects of miR-22-3p in HCC are mediated by its direct targeting of SP1, and the consequent regulation of CCND1 and BCL2 expression. The miR-22-3p/SP1 axis plays a vital role in HCC cell growth and may be an important therapeutic target for HCC. However, the clinical relevance of miR-22-3p in HCC remains unknown. Further studies are required to determine the treatment efficacy of berberine in other cancers, in animal models in vivo, and finally in human interventional trials. Future studies should also define the mechanism by which berberine treatment enhances the expression of miR-22-3p. Finally, because HCC is characterized by its rapid growth, relentless invasion, and redundant microvessel growth, future studies must focus on identifying the prognostic value of miR-22-3p and other miRNAs in HCC patients. In conclusion, our data indicate that berberine exerts a significant antitumor effect by upregulating miR-22-3p in HCC. miR-22-3p then functions as a tumor suppressor by directly targeting SP1, thus causing the downregulation of CCND1 and BCL2 expression in HCC. We have identified miR-22-3p as a tumor suppressor that directly targets SP1 to inhibit cell proliferation and induce cell-cycle arrest and apoptosis in HCC.
Conclusions
This study suggests that berberine has antitumor effects on HCC by inducing cell-cycle arrest and apoptosis. Furthermore, berberine upregulates miR-22-3p and downregulates SP1 expression in HepG2 cells. We have also demonstrated that SP1 is a direct target of miR-22-3p. These data suggest that berberine acts by regulating miR-22-3p/SP1 and the downstream proteins, CCDN1 and BCL2. Therefore, berberine has potential utility in the treatment of HCC, and both miR-22-3p and SP1 may be candidate anticancer therapies for HCC patients.
Acknowledgements
This study was supported by Guangxi Zhuang Autonomous Region Department of Health and Family Planning Commission Health (Grant No. Z.2015570).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chen KW, Ou TM, Hsu CW, Horng CT, Lee CC, Tsai YY, Tsai CC, Liou YS, Yang CC, Hsueh CW, Kuo WH. Current systemic treatment of hepatocellular carcinoma: A review of the literature. World J Hepatol. 2015;7:1412–1420. doi: 10.4254/wjh.v7.i10.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tejeda-Maldonado J, García-Juárez I, Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M, Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-ávila JF, Carrillo-Pérez DL. Diagnosis and treatment of hepatocellular carcinoma: An update. World J Hepatol. 2015;7:362–376. doi: 10.4254/wjh.v7.i3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126–136. doi: 10.1159/000367735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Xun K, Wang Y, Chen X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs. 2009;20:757–769. doi: 10.1097/CAD.0b013e328330d95b. [DOI] [PubMed] [Google Scholar]
- 7.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014:289264. doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JP, Yang JS, Lee JH, Hsieh WT, Chung JG. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol. 2006;12:21–28. doi: 10.3748/wjg.v12.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F, Yang Q, Gao G, Gong Y, Shao C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res. 2009;662:75–83. doi: 10.1016/j.mrfmmm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Chai YS, Hu J, Lei F, Wang YG, Yuan ZY, Lu X, Wang XP, Du F, Zhang D, Xing DM, Du LJ. Effect of berberine on cell cycle arrest and cell survival during cerebral ischemia and reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. Eur J Pharmacol. 2013;708:44–55. doi: 10.1016/j.ejphar.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y, Peng JY. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine. 2008;15:1062–1068. doi: 10.1016/j.phymed.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Feng Y, Zhu M, Tsang CM, Man K, Tong Y, Tsao SW. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem. 2010;111:1426–1436. doi: 10.1002/jcb.22869. [DOI] [PubMed] [Google Scholar]
- 14.Ma C, Tang K, Liu Q, Zhu R, Cao Z. Calmodulin as a potential target by which berberine induces cell cycle arrest in human hepatoma Bel7402 cells. Chem Biol Drug Des. 2013;81:775–783. doi: 10.1111/cbdd.12124. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Huang N. Berberine induces selective apoptosis through the AMPK mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol Med Rep. 2013;8:505–510. doi: 10.3892/mmr.2013.1506. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Zhu M, Wang X, Tan HY, Tsao SW, Feng Y. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of MIR-23a in hepatocellular carcinoma. Biochim Biophys Acta. 2014;1839:849–857. doi: 10.1016/j.bbagrm.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Tsang CM, Cheung KC, Cheung YC, Man K, Lui VW, Tsao SW, Feng Y. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim Biophys Acta. 2015;1852:541–551. doi: 10.1016/j.bbadis.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Li O, Kan M, Zhang M, Shao D, Pan Y, Zheng H, Zhang X, Chen L, Liu S. Berberine induces apoptosis by suppressing the arachidonic acid metabolic pathway in hepatocellular carcinoma. Mol Med Rep. 2015;12:4572–4577. doi: 10.3892/mmr.2015.3926. [DOI] [PubMed] [Google Scholar]
- 19.Morishita A, Masaki T. miRNA in hepatocellular carcinoma. Hepatol Res. 2015;45:128–141. doi: 10.1111/hepr.12386. [DOI] [PubMed] [Google Scholar]
- 20.Hong SH, Kim KS, Oh IH. Concise review: Exploring miRNAs--toward a better understanding of hematopoiesis. Stem Cells. 2015;33:1–7. doi: 10.1002/stem.1810. [DOI] [PubMed] [Google Scholar]
- 21.Dang Y, Zhao S, Qin Y, Han T, Li W, Chen ZJ. MicroRNA-22-3p is down-regulated in the plasma of Han Chinese patients with premature ovarian failure. Fertil Steril. 2015;103:802–7. e1. doi: 10.1016/j.fertnstert.2014.12.106. [DOI] [PubMed] [Google Scholar]
- 22.Kaur K, Vig S, Srivastava R, Mishra A, Singh VP, Srivastava AK, Datta M. Elevated Hepatic miR-22-3p Expression Impairs Gluconeogenesis by Silencing the Wnt-Responsive Transcription Factor Tcf7. Diabetes. 2015;64:3659–3669. doi: 10.2337/db14-1924. [DOI] [PubMed] [Google Scholar]
- 23.Guo MM, Hu LH, Wang YQ, Chen P, Huang JG, Lu N, He JH, Liao CG. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1. Med Oncol. 2013;30:542. doi: 10.1007/s12032-013-0542-7. [DOI] [PubMed] [Google Scholar]
- 24.Xiong J, Du Q, Liang Z. Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene. 2010;29:4980–4988. doi: 10.1038/onc.2010.241. [DOI] [PubMed] [Google Scholar]
- 25.Song SJ, Pandolfi PP. miR-22 in tumorigenesis. Cell Cycle. 2014;13:11–12. doi: 10.4161/cc.27027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey AK, Zhang Y, Zhang S, Li Y, Tucker-Kellogg G, Yang H, Jha S. TIP60-miR-22 axis as a prognostic marker of breast cancer progression. Oncotarget. 2015;6:41290–41306. doi: 10.18632/oncotarget.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koufaris C, Valbuena GN, Pomyen Y, Tredwell GD, Nevedomskaya E, Lau CH, Yang T, Benito A, Ellis JK, Keun HC. Systematic integration of molecular profiles identifies miR-22 as a regulator of lipid and folate metabolism in breast cancer cells. Oncogene. 2016;35:2766–76. doi: 10.1038/onc.2015.333. [DOI] [PubMed] [Google Scholar]
- 28.Kong LM, Liao CG, Zhang Y, Xu J, Li Y, Huang W, Zhang Y, Bian H, Chen ZN. A regulatory loop involving miR-22, Sp1, and c-Myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014;74:3764–3778. doi: 10.1158/0008-5472.CAN-13-3555. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad HM, Muiwo P, Ramachandran SS, Pandey P, Gupta YK, Kumar L, Kulshreshtha R, Bhattacharya A. miR-22 regulates expression of oncogenic neuro-epithelial transforming gene 1, NET1. FEBS J. 2014;281:3904–3919. doi: 10.1111/febs.12926. [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Ning S, Li Z, Qin X, Xu W. miR-22 is down-regulated in esophageal squamous cell carcinoma and inhibits cell migration and invasion. Cancer Cell Int. 2014;14:138. doi: 10.1186/s12935-014-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Yu H, Lu X, Zhang P, Wang M, Hu Y. MiR-22 suppresses the proliferation and invasion of gastric cancer cells by inhibiting CD151. Biochem Biophys Res Commun. 2014;445:175–179. doi: 10.1016/j.bbrc.2014.01.160. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi RU, Takata T, Shimamoto A, Ochiya T, Tahara H. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 34.Cho WC. Great potential of miRNAs as predictive and prognostic markers for cancer. Expert Rev Mol Diagn. 2012;12:315–318. doi: 10.1586/erm.12.21. [DOI] [PubMed] [Google Scholar]
- 35.Lo TF, Tsai WC, Chen ST. MicroRNA-21-3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One. 2013;8:e75628. doi: 10.1371/journal.pone.0075628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Huang Y, Huang Y, Xia X, Zhang J, Zhou Y, Tan Y, He S, Qiang F, Li A, Re OD, Li G, Zhou J. JWA suppresses tumor angiogenesis via Sp1-activated matrix metalloproteinase-2 and its prognostic significance in human gastric cancer. Carcinogenesis. 2014;35:442–451. doi: 10.1093/carcin/bgt311. [DOI] [PubMed] [Google Scholar]
- 37.Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang Z, Zhu W, Shu Y, Liu P. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014;588:1168–1177. doi: 10.1016/j.febslet.2014.02.054. [DOI] [PubMed] [Google Scholar]
- 38.Xu Q, Liu M, Xu N, Zhu H. Variation in Sp1 binding sites correlates with expression of survivin in breast cancer. Mol Med Rep. 2014;10:1395–1399. doi: 10.3892/mmr.2014.2371. [DOI] [PubMed] [Google Scholar]
- 39.Xu TP, Liu XX, Xia R, Yin L, Kong R, Chen WM, Huang MD, Shu YQ. SP1-induced upregulation of the long noncoding RNA TINCR regulates cell proliferation and apoptosis by affecting KLF2 mRNA stability in gastric cancer. Oncogene. 2015;34:5648–5661. doi: 10.1038/onc.2015.18. [DOI] [PubMed] [Google Scholar]
- 40.Yao Y, Shen Z, Yao R, Liu S, Li Y, Cong H, Wang X, Qiu W, Yue L. MiR-200b expression in breast cancer: a prognostic marker and act on cell proliferation and apoptosis by targeting Sp1. J Cell Mol Med. 2015;19:760–769. doi: 10.1111/jcmm.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y, Li N, Qi J, Wang L, Shi Y, Qiu S, Fan J, Zha X. Sp1 is involved in regulation of cystathionine gamma-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal. 2012;24:1229–1240. doi: 10.1016/j.cellsig.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Cao L, Xie B, Yang X, Liang H, Jiang X, Zhang D, Xue P, Chen D, Shao Z. MiR-324-5p Suppresses Hepatocellular Carcinoma Cell Invasion by Counteracting ECM Degradation through Post-Transcriptionally Downregulating ETS1 and SP1. PLoS One. 2015;10:e0133074. doi: 10.1371/journal.pone.0133074. [DOI] [PMC free article] [PubMed] [Google Scholar]


