Abstract
MicroRNAs (miRNAs) are small, short and noncoding RNAs that regulate gene expression posttranscriptionally. Increasing evidences have demonstrated that deregulated expression of miRNAs is found in osteosarcoma. In this study, we demonstrated that miR-665 was downregulated in osteosarcoma tissues compared to non-tumorous tissues. The overall survival (OS) of osteosarcoma patients with low miR-665 expression was lower than that of these patients with high miR-665 expression. Ectopic expression of miR-665 suppressed the osteosarcoma cell proliferation, EMT and invasion. We identified Rab23 as a direct target gene of miR-665. Rab23 was downregulated in osteosarcoma tissues and cell lines. The expression of miR-665 was inversely associated with the expression of Rab23 in the osteosarcoma tissues. These results suggested that miR-665 acted as a tumor suppressor gene in the development of osteosarcoma.
Keywords: Osteosarcoma, microRNAs, miR-665, Rab23
Introduction
Osteosarcoma, which is the most frequent tumor of bone, is the leading cause of cancer-related death in adolescents and children [1-4]. Despite the development treatment approaches of osteosarcoma such as chemotherapy and curative resection, the 5-year overall survival rate of osteosarcoma patients is still low [5-8]. Therefore, it is important to find new biomarkers for prognosis and diagnosis and a new therapy for osteosarcoma.
miRNAs (MicroRNAs) are non-coding, small, endogenous RNAs that induce mRNA translational inhibition and/or degradation by binding to 3’UTR (3’-untranslated region) of the target mRNAs [9-13]. Increasing evidences showed that play important roles in complicated cell processes including cell metabolism, proliferation, differentiation [14-16]. Moreover, deregualtion expression of miRNA has been found in multiple cancers such as gastric cancer, hepatocellular carcinoma, lung cancer, colorectal cancer, breast cancer and ovarian carcinoma [17-23]. Both the gain and loss of miRNAs expression contribute to tumor development through the downregulation of tumor suppressor genes and upregulation oncogenes [24-27].
In the present study, we demonstrated that miR-665 expression was downregulated in the osteosarcoma tissues compared to non-tumorous tissues. Ectopic expression of miR-665 suppressed the osteosarcoma cell proliferation, EMT and invasion. We identified Rab23 as a direct target gene of miR-665.
Materials and methods
Clinical samples and cell lines
Thirty paired osteosarcoma tissues and matched non-tumorous tissues were obtained from the Third Affiliated Hospital of Third Military Medical University. The tissues were obtained during surgery and frozen in liquid nitrogen immediately. This research was approved by the Ethics Committee of The Third Affiliated Hospital of Third Military Medical University and all patients have written informed consent. Osteosarcoma cell lines (U2OS, SOSP-9607, Saos-2, and MG-63) and osteoblastic cell line (hFOB1.19) were obtained from Institute of Cell Bank of Biological Sciences (Shanghai, China). Cells were cultured in DMEM/F12 medium at 37°C with 5% CO2.
Real-time qRT-PCR
TRIzol reagent (TaKaRa, Japan) was used to extract total RNA. The miR-665 expression was measure by the TaqMan assay (Applied Biosystems, USA) and the expression of Rab23 was detected by the qRT-PCR. U6 or GAPDH was used for miRNA or Rab23 expression control respectively. The primers were used as follow: Rab23, sense 5’-AGCGAGACTCCGTCTTCAAA-3’; antisense, 5’-CACCCCTAAGGTACGCATGT-3’; GAPDH, sense 5’-GCACCGTCAAGGCTG AGA AC-3’; antisense, 5’-TGGTGAAGACGCCAGTGGA-3’.
Plasmid and transfection
MiR-665 mimic and scramble oligonucleotide and pCDNA was synthesize by RiboBio (Guangzhou, China). 20 nM miR-665 mimic or scramble was transfection into cells used Lipofectamine 2000 (Invitrogen) according to manufacturer’s advising.
Cell growth, colony formation assay and invasion assays
The cell proliferation was detected by using CCK-8 (DOJINDO, Japan) following to manufacturer’s advising. The cell proliferation rate was measured at 0, 24, 48 and 72 hours. For colony formation assay, cells were cultured in Petri dish and were maintained with 10% FBS. The colonies were stained with crystal violet after 14 days and counted. For invasion analysis, Transwell chamber was used. The membranes were coated with Matrigel (BD Biosciences, USA) and cells were seeded on the upper chamber with no-FBS medium. 10% FBS medium was put on the lower chamber. After cultured for 24 hours, the invaded cells were stained with crystal violet and counted.
Western blot
Protein was extracted from cell or tissue and was measured using BCA Kit (Thermo Scientific). Protein was separated by 12% SDS-PAGE and transferred onto PVDF membrane. The membrane was probed with primary antibodies (Rab23, GAPDH, abcam, USA). Then membrane was incubated with secondary antibodies and was detected with ECL reagent (PerkinElmer Inc.).
Dual-luciferase reporter assay
MiR-665 mimic, pRL-CMV Renilla luciferase reporter was contransfected into the cells using Lipofectamine 2000 (Invitrogen). After 48 hours, luciferase data was measured by using a luciferase assay kit (Promega, USA).
Statistical analysis
All data are shown as mean ± SD. Student t test was performed for two group’s comparisons and one-way ANOVA was performed for more than two groups’ comparisons. P<0.05 was considered statistically significant.
Results
MiR-665 was downregulated in osteosarcoma tissues
The expression of miR-665 in osteosarcoma tissues and non-tumorous tissues was shown in the Figure 1A and 1B. Furthermore, miR-665 was downregulated in osteosarcoma tissues compared to non-tumorous tissues (Figure 1C). Moreover, the overall survival (OS) of osteosarcoma patients with low miR-665 expression was lower than that of these patients with high miR-665 expression (Figure 1D).
Figure 1.
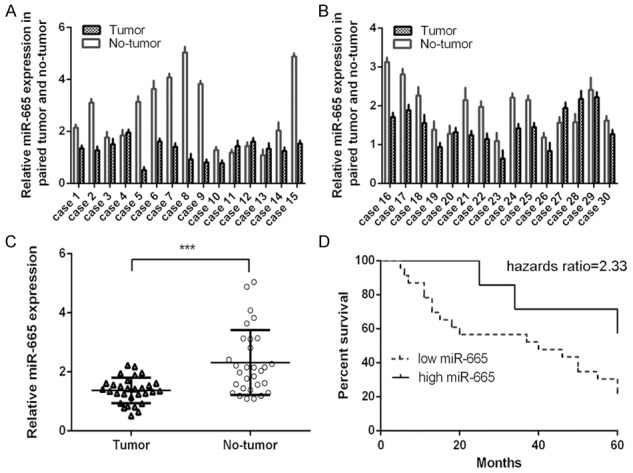
MiR-665 was downregulated in osteosarcoma tissues. A: The expression of miR-665 was detected in 1-15 osteosarcoma tissues and non-tumorous tissues using qRT-PCR. B: The expression of miR-665 was detected in 16-30 osteosarcoma tissues and non-tumorous tissues using qRT-PCR. C: miR-665 was downregulated in osteosarcoma tissues compared to non-tumorous tissues. D: The overall survival (OS) of osteosarcoma patients with low miR-665 expression was lower than that of these patients with high miR-665 expression. ***P<0.001.
MiR-665 was downregulated in osteosarcoma cell lines
We also demonstrated that miR-665 was downregulated in osteosarcoma cell lines (U2OS, SOSP-9607, Saos-2, and MG-63) compared to osteoblastic cell line (hFOB) (Figure 2A). To study the biological function of miR-665, miR-665 mimics and scramble mimics were transfected into both MG-63 and U2OS cells. The ectopic expression of miR-665 was confirmed using qRT-PCR (Figure 2B).
Figure 2.
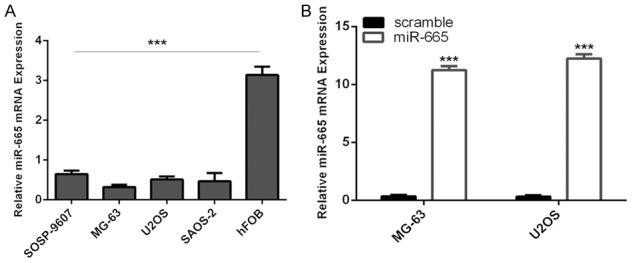
MiR-665 was downregulated in osteosarcoma cell lines. A: The expression of miR-665 in the osteosarcoma cell lines (U2OS, SOSP-9607, Saos-2, and MG-63) and osteoblastic cell line (hFOB) was measured using qRT-PCR. B: The expression of miR-665 in the U2OS and MG-63 after treated with miR-665 mimic was measured using qRT-PCR. ***P<0.001.
MiR-665 overexpression suppressed the osteosarcoma cell proliferation, EMT and invasion
Ectopic expression of miR-665 suppressed osteosarcoma cell (MG-63). Overexpression of miR-665 promoted the E-cadherin mRNA expression and inhibited the expression of N-cadherin and vimentin mRNA in the MG-63 cell (Figure 3B). MiR-665 overexpression increased the protein expression of E-cadherin and suppressed the protein expression of N-cadherin and vimentin in the MG-63 cell (Figure 3C). Overexpression of miR-665 suppressed MG-63 cell invasion (Figure 3D).
Figure 3.
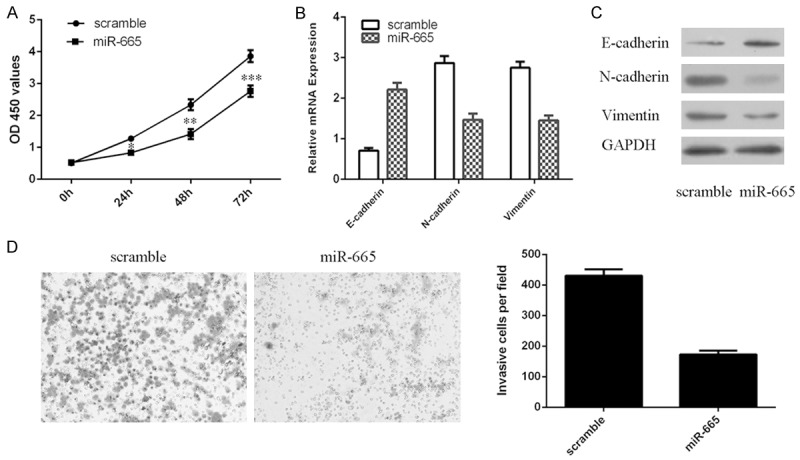
MiR-665 overexpression suppressed the osteosarcoma cell proliferation, EMT and invasion. A: Overexpression of miR-665 suppressed the osteosarcoma cell MG-63 proliferation. B: The mRNA expression of E-cadherin, N-cadherin and vimentin in the MG-63 cells was measured using qRT-PCR. C: The protein expression of E-cadherin, N-cadherin and vimentin in the MG-63 cells was measured using western blot. D: Overexpression of miR-665 suppressed the osteosarcoma cell invasion. The relative invasive cells were shown in the right. *P<0.05, **P<0.01 and ***P<0.001.
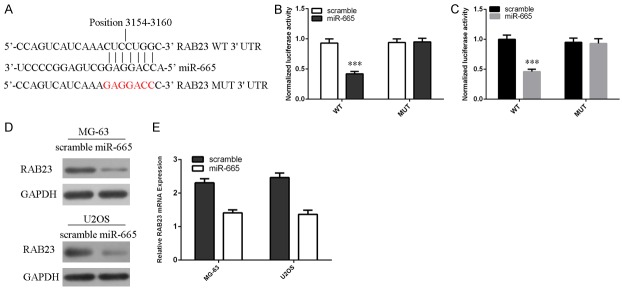
MiR-665 downregulates Rab23 expression through targeting its 3’UTR
Rab23 was found to have a potential binding site of miR-665 within its 3’UTR using bioinformatics (Figure 4A). To confirm whether Rab23 is a direct target gene of miR-665, we performed dual-luciferase reporter containing mutant 3’UTR and wild type of Rab23. Co-transfection with miR-665 suppressed the luciferase activity of the reporter containing the mutant 3’UTR but not the wild type in MG-63 cell (Figure 4B) and U2OS cell (Figure 4C). Overexpression of miR-665 suppressed the expression of Rab23 in both MG-63 cell and U2OS cell (Figure 4D and 4E).
Figure 4.
MiR-665 downregulates Rab23 expression through targeting its 3’UTR. A: Rab23 was predicted to be target gene of miR-665 by TargetScan. B: Dual-luciferase reporter assay proved that miR-665 directly targeted Rab23 by binding its 3’UTR in the MG-63 cells. C: Dual-luciferase reporter assay proved that miR-665 directly targeted Rab23 by binding its 3’UTR in the U2OS cells. D: The protein expression of Rab23 in both MG-63 and U2OS cell was measured by western blot. E: The mRNA expression of Rab23 in both MG-63 and U2OS cell was measured by qRT-PCR. ***P<0.001.
Rab23 was upregualted in osteosarcoma tissues and cell lines
The expression of Rab23 in osteosarcoma tissues and non-tumorous tissues was shown in the Figure 5A and 5B. Furthermore, Rab23 expression was upregulated in the osteosarcoma tissues compared to non-tumorous tissues (Figure 5C). The expression level of miR-665 was inversely associated with the expression level of Rab23 in osteosarcoma tissues (Figure 5D). Rab23 was upregulated in the osteosarcoma cell lines (U2OS, SOSP-9607, Saos-2, and MG-63) compared to osteoblastic cell line (hFOB) (Figure 5E).
Figure 5.
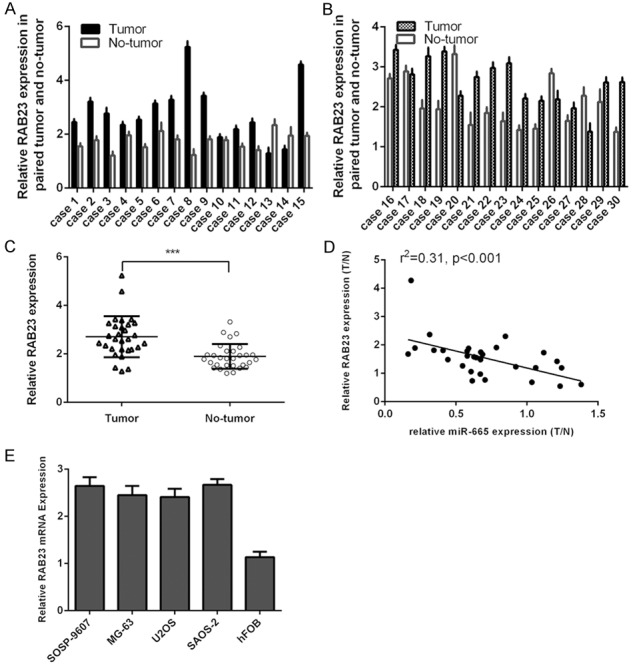
Rab23 was upregualted in osteosarcoma tissues and cell lines. A: The expression of Rab23 was detected in 1-15 osteosarcoma tissues and non-tumorous tissues using qRT-PCR. B: The expression of Rab23 was detected in 16-30 osteosarcoma tissues and non-tumorous tissues using qRT-PCR. C: Rab23 was upregulated in osteosarcoma tissues compared to non-tumorous tissues. D: The expression level of miR-665 was inversely associated with the expression level of Rab23 in osteosarcoma tissues. E: The expression of Rab23 in the osteosarcoma cell lines (U2OS, SOSP-9607, Saos-2, and MG-63) and osteoblastic cell line (hFOB) was measured using qRT-PCR.
Discussion
In this study, we demonstrated that miR-665 was downregulated in osteosarcoma tissues compared to non-tumorous tissues. The OS of osteosarcoma patients with low miR-665 expression was lower than that of these patients with high miR-665 expression. Ectopic expression of miR-665 suppressed the osteosarcoma cell proliferation, EMT and invasion. We identified Rab23 as a direct target gene of miR-665. Rab23 was downregulated in the osteosarcoma tissues and cell lines. The expression of miR-665 was inversely associated with the expression of Rab23 in the osteosarcoma tissues. These results suggested that miR-665 acted as a tumor suppressor gene in the development of osteosarcoma.
miRNAs are small, endogenous and noncoding RNAs that regulate gene expression by posttranscriptionally [10,28-30]. Recently, evidences also have showed that miRNAs acts an important role in the development of osteosarcoma [31-35]. For example, Cheng et al. demonstrated that miR-320 was downregulated in osteosarcoma tissues. Moreover, overexpression of miR-320 suppressed osteosarcoma cell proliferation and tumor growth through targeting fatty acid synthase (FASN) expression [33]. Huang et al. showed that miR-100 was downregulated in osteosarcoma tissues and enforced expression of miR-100 suppressed the osteosarcoma cell proliferation through repressing Cyr61 expression [36]. Li et al. found that miR-145 was downregulated in osteosarcoma tissues and cells. Overexpression of miR-145 repressed the cell invasion, migration and proliferation by inhibiting Rho-associated protein kinase 1 (ROCK1) expression [37]. Shen et al. demonstrated that miR-128 was upregulated in osteosarcoma tissues and overexpression of miR-128 increased the cell proliferation by suppressing the PTEN expression [38]. However, the role of miR-665 in the development of osteosarcoma still remains unknown. In our study, we demonstrated that miR-665 was downregulated in the osteosarcoma tissues compared to non-tumorous tissues. The OS of osteosarcoma patients with low miR-665 expression was lower than that of these patients with high miR-665 expression. Moreover, ectopic expression of miR-665 inhibited the osteosarcoma cell proliferation, EMT and invasion. These results supported that miR-665 played a suppressor miRNA in the development of osteosarcoma.
Furthermore, Rab23 was identified as a direct target gene of miR-665 in osteosarcoma cells. Rab23 was overexpressed and acted an oncogenic role in many tumors such as squamous cell carcinoma, gastric cancer, bladder cancer, breast cancer and pancreatic duct adenocarcinoma [39-43]. Overexpression of Rab23 promoted tumor cell proliferation, invasion, metastasis, and migration [39,40]. In our study, we revealed that Rab23 was upregulated in the osteosarcoma tissues compared to non-tumorous tissues. We also found that the expression of Rab23 was increased in osteosarcoma cell lines. Here, we demonstrated an inverse correlation between miR-665 and Rab23 expression in osteosarcoma tissues. These results suggested that increased Rab23 was involved in osteosarcoma progression. Moreover, Rab23 was regulated by some miRNAs such as miR-367 and miR-200b [43-45]. In line with these results, we demonstrated that miR-665 suppressed osteosarcoma cell proliferation and invasion through targeting Rab23.
In conclusion, we revealed that miR-665 was downregulated in osteosarcoma tissues and cell lines and ectopic expression of miR-665 suppressed osteosarcoma cell proliferation and invasion through targeting Rab23. These results suggested that miR-665 might be a potential target for osteosarcoma therapy.
Disclosure of conflict of interest
None.
References
- 1.Li Z, Yu X, Shen J. Long non-coding RNAs: emerging players in osteosarcoma. Tumour Biol. 2016;37:2811–6. doi: 10.1007/s13277-015-4749-4. [DOI] [PubMed] [Google Scholar]
- 2.Salah Z, Arafeh R, Maximov V, Galasso M, Khawaled S, Abou-Sharieha S, Volinia S, Jones KB, Croce CM, Aqeilan RI. miR-27a and miR-27a* contribute to metastatic properties of osteosarcoma cells. Oncotarget. 2015;6:4920–4935. doi: 10.18632/oncotarget.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu G, Li B, Sun L, An C. MicroRNA-153 Inhibits Osteosarcoma Cells Proliferation and Invasion by Targeting TGF-beta2. PLoS One. 2015;10:e0119225. doi: 10.1371/journal.pone.0119225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Shen L, Wang P, Yang J, Li X. MicroRNA-217 Regulates WASF3 Expression and Suppresses Tumor Growth and Metastasis in Osteosarcoma. PLoS One. 2014;9:e109138. doi: 10.1371/journal.pone.0109138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q, Ma B. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS One. 2012;7:e33778. doi: 10.1371/journal.pone.0033778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao G, Cai C, Yang T, Qiu X, Liao B, Li W, Ji Z, Zhao J, Zhao H, Guo M, Ma Q, Xiao C, Fan Q, Ma B. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLoS One. 2013;8:e53906. doi: 10.1371/journal.pone.0053906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang J, Shen L, Yang Q, Zhang C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014;47:427–434. doi: 10.1111/cpr.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delebinski CI, Georgi S, Kleinsimon S, Twardziok M, Kopp B, Melzig MF, Seifert G. Analysis of proliferation and apoptotic induction by 20 steroid glycosides in 143B osteosarcoma cells in vitro. Cell Prolif. 2015;48:600–610. doi: 10.1111/cpr.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Li Z, Liu J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015;48:271–277. doi: 10.1111/cpr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Li Z. Epigenetic deregulations in chordoma. Cell Prolif. 2015;48:497–502. doi: 10.1111/cpr.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing’s sarcoma. Cell Prolif. 2015;48:1–6. doi: 10.1111/cpr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Zhou L, Cheng Y, Sun L, Fan J, Liang J, Guo M, Liu N, Zhu L. MicroRNA-543 acts as an oncogene by targeting PAQR3 in hepatocellular carcinoma. Am J Cancer Res. 2014;4:897–906. [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Yu M, Liu C, Zhu H, He X, Peng S, Hua J. miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif. 2013;46:223–231. doi: 10.1111/cpr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L, Gong X, Sun L, Yao H, Lu B, Zhu L. miR-454 functions as an oncogene by inhibiting CHD5 in hepatocellular carcinoma. Oncotarget. 2015;6:39225–39234. doi: 10.18632/oncotarget.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L, Ding GF, He C, Sun L, Jiang Y, Zhu L. MicroRNA-424 is down-regulated in hepatocellular carcinoma and suppresses cell migration and invasion through c-Myb. PLoS One. 2014;9:e91661. doi: 10.1371/journal.pone.0091661. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Li J, You T, Jing J. MiR-125b inhibits cell biological progression of Ewing’s sarcoma by suppressing the PI3K/Akt signalling pathway. Cell Prolif. 2014;47:152–160. doi: 10.1111/cpr.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang J, Liu X, Xue H, Qiu B, Wei B, Sun K. MicroRNA-103a inhibits gastric cancer cell proliferation, migration and invasion by targeting c-Myb. Cell Prolif. 2015;48:78–85. doi: 10.1111/cpr.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Han Y, Cheng K, Zhang G, Wang X. miR-99a directly targets the mTOR signalling pathway in breast cancer side population cells. Cell Prolif. 2014;47:587–595. doi: 10.1111/cpr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Li Z, Yu J, Chan MT, Wu WK. MicroRNAs predict and modulate responses to chemotherapy in colorectal cancer. Cell Prolif. 2015;48:503–510. doi: 10.1111/cpr.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Wang X, Li J, Gu K, Lv L, Zhang S, Che D, Cao J, Jin S, Yu Y. miR-34c-3p functions as a tumour suppressor by inhibiting eIF4E expression in non-small cell lung cancer. Cell Prolif. 2015;48:582–592. doi: 10.1111/cpr.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Yan J, Zhou C, Ma Q, Jin Q, Yang Z. miR-1285-3p acts as a potential tumor suppressor miRNA via downregulating JUN expression in hepatocellular carcinoma. Tumour Biol. 2015;36:219–225. doi: 10.1007/s13277-014-2622-5. [DOI] [PubMed] [Google Scholar]
- 22.Ai C, Jiang R, Fu L, Chen Y. MicroRNA-495 mimics delivery inhibits lung tumor progression. Tumour Biol. 2015;36:729–735. doi: 10.1007/s13277-014-2687-1. [DOI] [PubMed] [Google Scholar]
- 23.Creighton CJ, Hernandez-Herrera A, Jacobsen A, Levine DA, Mankoo P, Schultz N, Du Y, Zhang Y, Larsson E, Sheridan R, Xiao W, Spellman PT, Getz G, Wheeler DA, Perou CM, Gibbs RA, Sander C, Hayes DN, Gunaratne PH. Integrated analyses of microRNAs demonstrate their widespread influence on gene expression in high-grade serous ovarian carcinoma. PLoS One. 2012;7:e34546. doi: 10.1371/journal.pone.0034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Zhang SY, Gao YM, Liu YF, Liu YB, Zhao ZG, Yang K. MicroRNAs as oncogenes or tumour suppressors in oesophageal cancer: potential biomarkers and therapeutic targets. Cell Prolif. 2014;47:277–286. doi: 10.1111/cpr.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, Feng F. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–17569. doi: 10.18632/oncotarget.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Yu X, Shen J, Liu Y, Chan MT, Wu WK. MicroRNA dysregulation in rhabdomyosarcoma: a new player enters the game. Cell Prolif. 2015;48:511–516. doi: 10.1111/cpr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Li Z. The role of miRNAs in cutaneous squamous cell carcinoma. J Cell Mol Med. 2016;20:3–9. doi: 10.1111/jcmm.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Yu X, Shen J, Jiang Y. MicroRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget. 2015;6:4562–4568. doi: 10.18632/oncotarget.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, Jee Y, Hong K, Hwang GS, Chun KH. MicroRNA-494, upregulated by tumor necrosis factor-alpha, desensitizes insulin effect in C2C12 muscle cells. PLoS One. 2013;8:e83471. doi: 10.1371/journal.pone.0083471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao J, Wu S, Peng Z, Tania M, Zhang C. MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol. 2013;34:2093–8. doi: 10.1007/s13277-013-0940-7. [DOI] [PubMed] [Google Scholar]
- 32.Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang Y, Zhao W, Wang W. miR-22 inhibits osteosarcoma cell proliferation and migration by targeting HMGB1 and inhibiting HMGB1-mediated autophagy. Tumour Biol. 2014;35:7025–7034. doi: 10.1007/s13277-014-1965-2. [DOI] [PubMed] [Google Scholar]
- 33.Cheng C, Chen ZQ, Shi XT. MicroRNA-320 inhibits osteosarcoma cells proliferation by directly targeting fatty acid synthase. Tumour Biol. 2014;35:4177–4183. doi: 10.1007/s13277-013-1546-9. [DOI] [PubMed] [Google Scholar]
- 34.Sun XH, Geng XL, Zhang J, Zhang C. miRNA-646 suppresses osteosarcoma cell metastasis by downregulating fibroblast growth factor 2 (FGF2) Tumour Biol. 2015;36:2127–2134. doi: 10.1007/s13277-014-2822-z. [DOI] [PubMed] [Google Scholar]
- 35.Han K, Chen X, Bian N, Ma B, Yang T, Cai C, Fan Q, Zhou Y, Zhao TB. MicroRNA profiling identifies MiR-195 suppresses osteosarcoma cell metastasis by targeting CCND1. Oncotarget. 2015;6:8875–8889. doi: 10.18632/oncotarget.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Gao K, Lin J, Wang Q. MicroRNA-100 inhibits osteosarcoma cell proliferation by targeting Cyr61. Tumour Biol. 2014;35:1095–1100. doi: 10.1007/s13277-013-1146-8. [DOI] [PubMed] [Google Scholar]
- 37.Li E, Zhang J, Yuan T, Ma B. miR-145 inhibits osteosarcoma cells proliferation and invasion by targeting ROCK1. Tumour Biol. 2014;35:7645–7650. doi: 10.1007/s13277-014-2031-9. [DOI] [PubMed] [Google Scholar]
- 38.Shen L, Chen XD, Zhang YH. MicroRNA-128 promotes proliferation in osteosarcoma cells by downregulating PTEN. Tumour Biol. 2014;35:2069–2074. doi: 10.1007/s13277-013-1274-1. [DOI] [PubMed] [Google Scholar]
- 39.Jian Q, Miao Y, Tang L, Huang M, Yang Y, Ba W, Liu Y, Chi S, Li C. Rab23 promotes squamous cell carcinoma cell migration and invasion via integrin beta1/Rac1 pathway. Oncotarget. 2016;7:5342–52. doi: 10.18632/oncotarget.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Han Y, Sun C, Han C, Han N, Zhi W, Qiao Q. Rab23 is overexpressed in human bladder cancer and promotes cancer cell proliferation and invasion. Tumour Biol. 2016;37:8131–8. doi: 10.1007/s13277-015-4590-9. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Zeng C, Bao N, Zhao J, Hu Y, Li C, Chi S. Effect of Rab23 on the proliferation and apoptosis in breast cancer. Oncol Rep. 2015;34:1835–1844. doi: 10.3892/or.2015.4152. [DOI] [PubMed] [Google Scholar]
- 42.Cai ZZ, Xu LB, Cai JL, Wang JS, Zhou B, Hu H. Inactivation of Rab23 inhibits the invasion and motility of pancreatic duct adenocarcinoma. Genet Mol Res. 2015;14:2707–2715. doi: 10.4238/2015.March.30.31. [DOI] [PubMed] [Google Scholar]
- 43.Bin Z, Dedong H, Xiangjie F, Hongwei X, Qinghui Y. The microRNA-367 inhibits the invasion and metastasis of gastric cancer by directly repressing Rab23. Genet Test Mol Biomarkers. 2015;19:69–74. doi: 10.1089/gtmb.2014.0210. [DOI] [PubMed] [Google Scholar]
- 44.Kaid C, Silva PB, Cortez BA, Rodini CO, Semedo-Kuriki P, Okamoto OK. miR-367 promotes proliferation and stem-like traits in medulloblastoma cells. Cancer Sci. 2015;106:1188–1195. doi: 10.1111/cas.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q, Tang H, Liu X, Liao Y, Li H, Zhao Z, Yuan X, Jiang W. miR-200b as a prognostic factor targets multiple members of RAB family in glioma. Med Oncol. 2014;31:859. doi: 10.1007/s12032-014-0859-x. [DOI] [PubMed] [Google Scholar]