Abstract
Reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) emerges as a prospective therapeutic angle in regenerative medicine and a tool for drug screening. Although increasing numbers of iPSCs from different sources have been generated, there has been limited progress in yield of iPSC. Here, we show that four Yamanaka factors Oct4, Sox2, Klf4 and c-Myc can convert human embryonic renal cortical cells (hERCCs) to pluripotent stem cells with a roughly 40-fold higher reprogramming efficiency compared with that of adult human dermal fibroblasts. These iPSCs show pluripotency in vitro and in vivo, as evidenced by expression of pluripotency associated genes, differentiation into three embryonic germ layers by teratoma tests, as well as neuronal fate specification by embryoid body formation. Moreover, the four exogenous genes are effectively silenced in these iPSCs. This study highlights the use of hERCCs to generate highly functional human iPSCs which may aid the study of genetic kidney diseases and accelerate the development of cell-based regenerative therapy.
Keywords: Induced pluripotent stem cells (iPSCs), reprogramming, human embryonic renal cortical cells (hERCCs)
Introduction
The cloning of Dolly demonstrated that nuclei from mammalian differentiated cells can be reprogrammed into an undifferentiated state by transacting factors present in the oocyte [1], this discovery led to a search for factors which could mediate similar reprogramming without somatic cell nuclear transfer. Then, Yamanaka and colleagues have shown that overexpression of specific transcription factors (Oct4, Sox2, c-Myc, and Klf4) can reprogram mouse fibroblasts to undifferentiated, pluripotent stem cells. These induced pluripotent stem cells (iPSCs) were similar to human embryonic stem cells (ESCs) in morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity [2]. The iPSCs are deemed equivalent to ESCs especially when Zhou Q et al. produced a viable animal from an iPSC using the tetraploid complementation assay [3]. These findings led people to understand disease mechanisms, to screen effective and safe drugs, and to treat patients of various diseases and injuries directly using iPSCs but not ESCs.
Up to now, iPSCs can be generated from a few cell types, including fetal and adult fibroblasts [2], hepatocytes, stomach cells [4], keratinocytes [5], peripheral blood [6], cord blood [7,8], dental pulp cells [9-11], and even fully differentiated lymphocytes (T and B cells) [12-17]. It is also recognized that stemness facilitates reprogramming, as shown by more efficient reprogramming of progenitor or precursor cells to iPSCs than of differentiated cells [18-22].
However, while the accessibility of these human cell sources provides an advantage in generating iPSCs, reprogramming those samples is not an efficient (0.001-0.1%) process [23,24]. We therefore sought to find a more practical cell type that could be readily isolated and expanded, yet could reprogram quickly and efficiently. Here we report the rapid reprogramming of hERCCs into iPSCs, with efficiency approximately 40-fold higher than human fibroblasts which we used to induce iPSCs under the same conditions. We test the hERCCs-derived iPSCs (ERCC-iPSCs) in morphology, proliferation, gene expression, and teratoma formation, the results demonstrate that there is no difference between ERCC-iPSCs and ESCs. This high-efficiency approach of iPSCs reprogramming described here would further advance the development of model diseases and new drugs test by using quite a few iPSCs.
Materials and methods
Cell culture
Human embryonic kidney was removed from the aborted fetus. The tissue was washed with phosphate-buffered saline (PBS; Invitrogen) containing penicillin and streptomycin (GIBCO) for three times. The cortical part was mechanically disrupted and cut into strips with eye scissors as small as possible, then dissociate the minced tissue pieces with collagenase IV (Sigma) and 0.25% trypsin (Sigma) in a Falcon tube (BD Biosciences) [25,26]. Subsequently, cells were plated in Dulbecco modified Eagle medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS; Invitrogen), 1% nonessential amino acids (NEAA; Invitrogen), 1× GlutaMAX (Invitrogen), and 0.5% penicillin/streptomycin. The medium was changed every other day. Primary renal cortical cells were expanded for three to five passages to remove other types of cells and retained fibroblasts-like only.
Generation of iPSCs clones
Lentiviral vectors carrying the pluripotency genes (Oct4, Sox2, Klf4 and c-Myc) have been used to induce reprogramming of hERCCs as previous reported [27]. hERCCs (105 per 100 mm Petri dish) were maintained in medium supplemented with 5 μg/ml Polybrene (Sigma) with lentivirus (MOI=200) produced by 293FT cells (Invitrogen) at 37°C, 5% CO2 overnight. After 24 h viral infection, cells were incubated with the same medium above for another 24 h to enhance the efficiency of transduction following the method developed by Yamanaka [2]. Then the medium was changed fresh complete DMEM every other day till the 5th day when medium was replaced with KnockOut™ SR XenoFree Feeder-Free medium (1× KnockOut™ DMEM/F-12 supplemented with 20% KnockOut™ SR XenoFree, 2 mM GlutaMAX™-I, 1× KnockOut™ SR-GFC, 20 ng/ml human bFGF, 0.1 mM β-mercaptoethanol (all from Invitrogen) and antibiotics. About 12 days after transduction, clones was observed and mechanically propagated onto plastic dishes (BD Biosciences) which were coated by Mitomycin C (Sigma) treated MEF feeders. The Efficiency of iPSCs Induction was calculated at passage 1.
Alkaline phosphatase staining and immunostaining
Alkaline phosphatase (AP) staining was performed using the Leukocyte Alkaline Phosphatase kit (Sigma), according to the manufacturer’s protocol. For immunostaining, cells cultured on matrigel-coated 10 cm dish were fixed with PBS containing 4% paraformaldehyde (PFA; Sigma) for 10 min at room temperature. After washing with PBS, the cells were treated with PBS containing 5% normal goat or donkey serum (Chemicon), 1% bovine serum albumin (BSA; Nacalai tesque), and 0.1% Triton X-100 (Sigma) for 10 min at room temperature. After rinsed with PBS, cells were incubated with primary antibodies overnight at 4°C, and then labeled with secondary antibodies. The primary antibodies included Vimentin (1:200; Abcam), Fibronectin (1:200; Abcam), cytokeratin 18 (1:250; Abcam), E-Cadherin (1:100; Santa Cruz), Nestin (1:200; Abcam), Nanog (1:100; Santa Cruz), Oct4 (1:200; Abcam), Sox2 (1:200; Abcam), WT1 (1:200; Abcam), LIM1 (1:500; Abcam), SSEA4 (1:250; Abcam), Tuj1 (1:200; Abcam), Map2 (1:100; Abcam) and glial fibrillary acidic protein (GFAP, 1:250; Abcam). Secondary antibodies used were Alexa Fluor 488 donkey anti-mouse/rabbit IgG (1:200; Invitrogen) and Alexa Fluor 555 goat anti-mouse/rabbit IgG (1:200; Invitrogen). Nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI, Invitrogen) in PBS at the room temperature for 5 minutes.
RT-PCR for marker genes
Total RNA of ERCC-iPSCs was isolated by using Trizol (Invitrogen). For each sample, 1 ug of RNA was used for reverse transcription. cDNAs generated from the RNA were diluted 2-, 10-, 20-fold in three replicates to build a calibration curve. The expression value of gene analyzed was normalized to the amount of GAPDH cDNA. PCR products were separated by electrophoresis in 1.5% agarose gel in a tris-acetate buffer and visualized using a uV gel analyzer (Biorad). Primers we used that would amplify transcripts of the endogenous gene but not transcripts of the transgene, which are listed in Table 1. Real-time PCR was performed using SYBR Green RT-PCR Reagents Kit in a two-step cycling protocol on 7700 Fast Real-Time PCR System (Applied Biosystems) according to manufacturer’s instructions. The expression data were averaged upon normalization to GAPDH expression. Data were quantified with the ΔΔCt method.
Table 1.
Primer sequences
| Primer | Sequence | Application |
|---|---|---|
| endo-Oct3/4 | GAC AGG GGG AGG GGA GGA GCT AGG (5’ to 3’) | RT-PCR/qPCR |
| CTT CCC TCC AAC CAG TTG CCC CAA AC (5’ to 3’) | ||
| endo-Sox2 | GGG AAA TGG GAG GGG TGC AAA AGA GG (5’ to 3’) | RT-PCR/qPCR |
| TTG CGT GAG TGT GGA TGG GAT TGG TG (5’ to 3’) | ||
| endo-Klf4 | ACG ATC GTG GCC CCG GAA AAG GAC C (5’ to 3’) | RT-PCR/qPCR |
| TGA TTG TAG TGC TTT CTG GCT GGG CTC C (5’ to 3’) | ||
| endo-c-Myc | GCG TCC TGG GAA GGG AGA TCC GGA GC (5’ to 3’) | RT-PCR/qPCR |
| TTG AGG GGC ATC GTC GCG GGA GGC TG (5’ to 3’) | ||
| endo-c-Nanog | CAG CCC CGA TTC TTC CAC CAG TCC C (5’ to 3’) | RT-PCR/qPCR |
| CGG AAG ATT CCC AGT CGG GTT CAC C (5’ to 3’) | ||
| endo-c-Lin28 | CGG ACC TGG TGG AGT ATT CTG TAT TG (5’ to 3’) | RT-PCR/qPCR |
| GGG TAG GGC TGT GGA TTT CTT CTT C (5’ to 3’) | ||
| endo-c-GAPDH | GGA AAG CCT GCC GGT GAC TAA CCC TGC (5’ to 3’) | RT-PCR/qPCR |
| GCT TCC CGT TCT CAG CCT TGA CGG TG (5’ to 3’) |
Teratoma formation test and karyotype analysis
ERCC-iPSCs were harvested by collagenase IV treatment, collected into tubes, centrifuged, and suspended in DMEM/F12 (Invitrogen). Approximately 5×106 iPSCs were injected subcutaneously to nude immunodeficient mice of 6-8 weeks old. After 6~8 weeks, tumors were dissected, and fixed with 10% formaldehyde in PBS. Parrafin embedded tissue sections were then generated and stained with hemotoxylin and eosin (H&E staining). Chromosomal studies were performed by using standard protocols for high-resolution Giemsa (G)-banding.
In vitro differentiation
Differentiation to kidney progenitor-like cells
ERCC-iPSCs were split onto plates coated with Matrigel, making sure the sub-colonies were of small size (300-500 cells per colony). After 24 h of recovery, the cells were changed to grow in chemically defined media (DMEM/F12, 17.5 mg ml-1 BSA (Sigma Aldrich), 17.5 µg ml-1 insulin human (Sigma), 275 µg ml-1 holo-transferrin human (Sigma), 450 µM monothioglycerol (Sigma Aldrich), 2.25 mM each L-glutamine and non-essential amino acids, 100 unit ml-1 penicillin and 100 µg ml-1 streptomycin supplemented with 50 ng ml-1 bFGF (Invitrogen) and 30 ng ml-1 BMP-4 human (Sigma) for 2 days, and in the basal media supplemented with 1 µM retinoic acid (Sigma), 10 ng ml-1 Activin A human (Sigma) and 100 ng ml-1 BMP-2 human (Sigma) for another 2 days, with the same amount of fresh media changed every other day [28].
Differentiation to other cell lines
For embryoid bodies (EBs) formation, ERCC-iPSCs were harvested by treating with collagenase IV and dispersed into small clumps by scraping and pipetting. The clumps of the cells were added to poly (2-hydroxyrthyl methacrylate)-coated dishes and cultured in suspension in hEB medium consisting of 80% DMEM/F12, 20% knockout serum replacement, 1 mM L-glutamine, 1% non-essential amino acids, 0.1 mM β-mercaptoethanol, and 0.5% penicillin/streptomycin for 5 days. After that, EBs were transferred to gelatin-coated plate and cultured in N3 medium [DMEM/F12, 500 mg/mL transferring (Sigma), 25 mg/mL insulin (Sigma), 30 nM sodium selenite (Sigma), 20 nM progesterone (Sigma), 100 nM putrescine (Sigma), and 0.5% penicillin/streptomycin supplemented with neurotrophic factors, including the brain-derived neurotrophic factor (BDNF), glial cell-derived neuro-trophic factor (GDNF), ciliary neurotrophic factor (CNTF) and neurotrophin-3 (all from Peprotech). EBs were cultured as a floating culture for 5 days, and then transferred to gelatin-coated plate for another 15 days. The medium was refreshed every other day [29].
Electrophysiological recording
The electrophysiological activities of ERCC-iPSCs were analyzed using extracellular electrode recording with an Axopatch 700B amplifier and the pClamp9.2 software (Axon Instruments). The intracellular solution for current-clamp recordings contained (in mM) 140 KCl, 0.5 EGTA, 5 HEPES and 3 Mg-ATP (pH 7.3, 300 mOsm) (all from Sigma). Electrodes had resistances of 2~4 MΩ when filled with this recording solution and cells were hold at -65 mV membrane potential with a stimulation of 0.1~0.5 nA for 5 ms to elicit a response. In voltage-clamp mode, membrane potentials of these cells ranged from -60 mV to -70 mV. For the initiation voltage-gated currents, we used voltage steps from -90 mV to +50 mV in 10 mV increments.
Statistical analysis
Results are shown as mean values ± standard deviation (SD) or standard error of the mean (SEM) as indicated. Student’s t test was used to evaluate significance of PCR data. P values <0.05 were considered statistically significant.
Results
Isolation and culture of hERCCs
Under informed consent, we harvested the kidney from the naturally aborted fetus. After cortical parts were washed, dissociated and centrifuged, cells are plated into the 10 cm2 dish with 5% CO2. Cells were passaged when monolayer cover the dish about 7 days later. After three passages, we checked the morphology of cultured cells to rule out the presence of contaminant cells and performed immunofluorescence characterization, which showed they express markers for Vimentin (a 57,000 molecular weight protein of fibroblast filaments) [30] and Fibronectin (which is secreted from cells, often fibroblasts) [31] (Figure 1A, 1B), but lack epithelial marker cytokeratin 18 (Figure 1C), which confirms purity of the fibroblasts. To eliminate the possibility that the hERCCs cultures might be contaminated with proximal tubular cells, E-Cadherin (which could represent proximal tubular cells) was detected and was negative. Moreover, the absence of neural stem cells (NSCs) markers Nestin [32] and embryo stem cells (ESCs) markers Nanog [33] confirmed that the starting cells contained no other progenitor cells which have pluripotency (Figure 1D-F). Hence, our results demonstrated that hERCCs without stem cells could be obtained and further subcultured for reprogramming.
Figure 1.
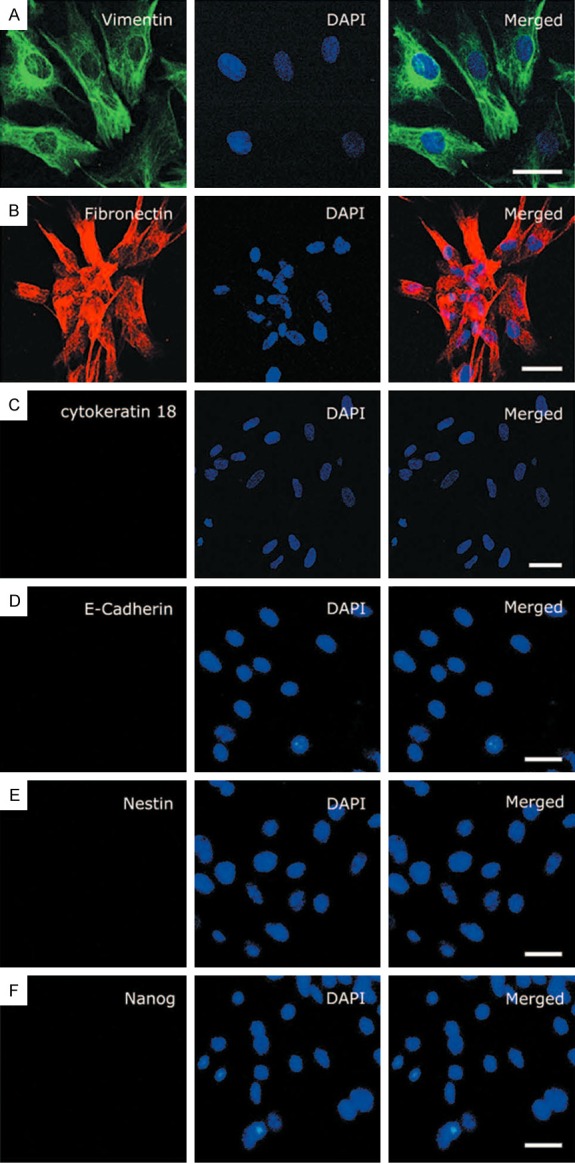
Culture and verification of hERCCs. A: hERCCs exhibited a reliable fibroblast marker Vimentin. B: Positive for fibronectin; C-F: But do not express cytokeratin 18, E-Cadherin, Nestin and Nanog. Scale bars: 50 μm.
Generation and calculation of hERCCs-derived iPSCs
The four classical Yamanaka factors Oct4, Sox2, Klf4 and c-Myc were cloned into the lentiviral vectors respectively (Gifts from Dr. helen L zhang, Boston, Massachusetts), and then 293FT cells were used to produce sufficient lentivirus. To estimate the efficiency of reprogramming, we reprogrammed both hERCCs and human skin fibroblasts (HSFs, got from our previous study) [27] under the same conditions. Twice infection later, medium was refreshed every other day. Approximately 10 days later, some granulated colonies appeared in hERCCs culture, while that were not observed in HSF culture until the 14th day. By contrast, there is no colony formed in iPSCs-condition cultured hERCCs and HSFs without lentivirus infection. The colonies increased in size and new colonies emerged with time especially from day 21, most of them were similar to hESCs in morphology, such as tightly packed and flat, large nuclei and scant cytoplasm (Figure 2A-C).
Figure 2.
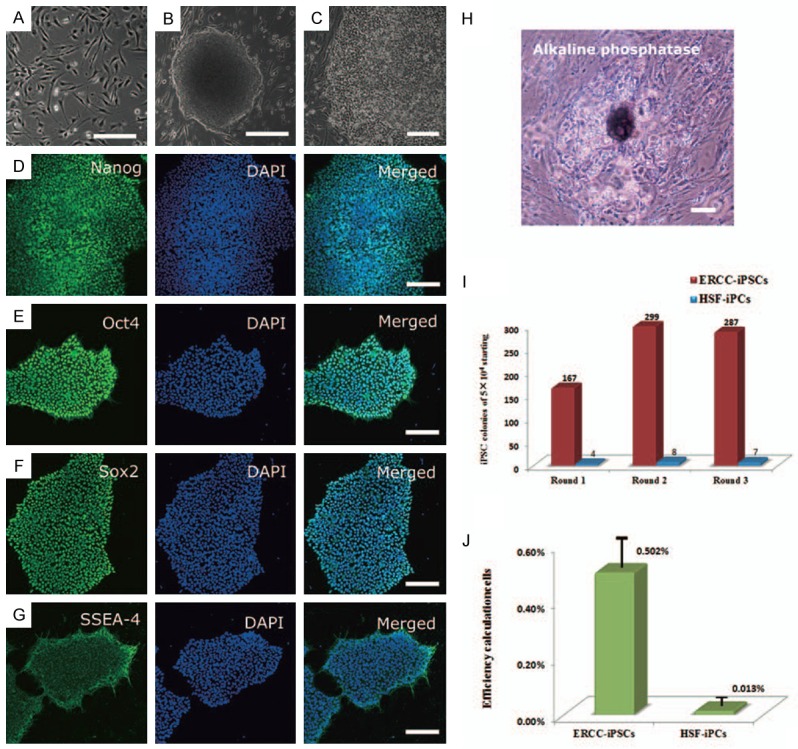
Induction of iPSCs from hERCCs. (A) Morphology of hERCCs. (B) Typical image of iPSC colony. (C) Image of iPSCs with high magnification. (D-G) ERCC-iPSCs were positive Nanog, Oct4, Sox2 and SSEA-4. Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) (blue). (H) ERCC-iPSCs were positive for AP. (I) In three independent experiments, ERCCs and HSFs generated different numbers of typical iPSC colony. (J) ERCCs had a higher reprogramming efficiency compare to HSFs. Scale bars: 200 μm (A, B, J), 50 μm (C), and 100 μm (D-H).
For efficiency calculation, we divided the number of iPSC colonies by the fraction of virus-infected input cells, which we observed 167 hESC-like colonies and 34 granulated colonies in 5×104 hERCCs, and 4 hESC-like colonies in HSFs. The statistic results after three rounds of calculating with similar efficiencies of viral transfection confirmed the difference of reprogramming efficiencies between the two cell sources to generate iPSCs (Figure 2I). Performing reprogramming under hERCCs culture was sufficient to increase the reprogramming efficiency compared to controls in HSFs culture (Figure 2J). Around day 28, iPSCs were handpicked and cultured on Matrigel-coated plates in MEF-conditioned iPSC medium for further verification.
Verification of hES markers in ERCC-iPSC clones
To confirm that the putative iPSCs were pluripotent, we evaluated their similarity to ESCs at the molecular and protein level. The ERCC-iPSC clones were positive for ESC-specific surface antigens, including Nanog, Oct4, Sox2, SSEA-4 and alkaline phosphatase (AP) [34] (Figure 2D-H).
RT-PCR analysis revealed that ERCC-iPSCs expressed some undifferentiated ESC-marker genes, such as Oct3/4, Sox2, Nanog, c-Myc, Klf4 and Lin28 [35] (Figure 3A). However, the transcripts of these genes could not be detected in the initial hERCCs, and primers for RT-PCR are specific for lentiviral transcripts confirmed efficient silencing of all the four lentiviruses. qRT-PCR analysis corroborated these results (normalized to GAPDH in the same samples) (Figure 3B).
Figure 3.
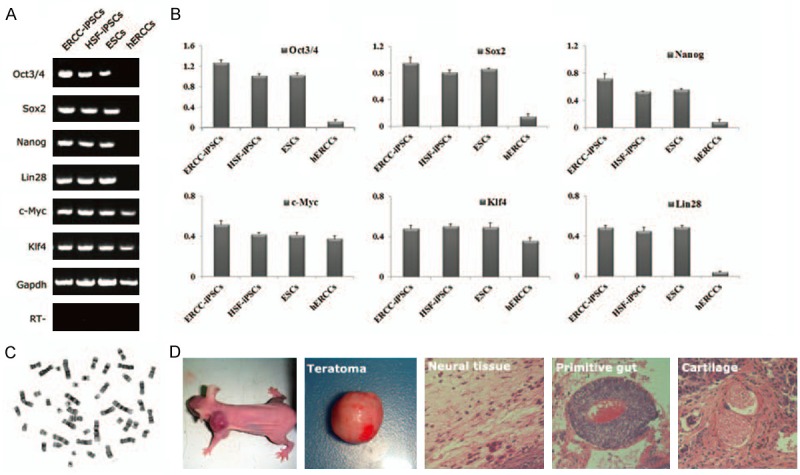
Characterization of hERCCs-derived iPSCs. A: RT-PCR analysis of ES cell-marker genes. Primers used for Oct3/4, Sox2, Klf4, c-Myc, Nanog and Lin28, specifically detect the transcripts from the endogenous genes, but not from the lentiviral transgenes. B: Quantitative PCR analysis of the Oct3/4, Sox2, Nanog, c-Myc, Klf4 and Lin28 compared to HSF-iPSCs, ESCs and hERCCs. C: High-resolution, G-banded karyotype indicating a normal diploid male chromosomal content in the ERCC-iPSCs. D: Teratoma derived from ERCC-iPSCs included neural tissue (ectoderm), primitive gut (endoderm) and cartilage (mesoderm).
In vivo differentiation of ERCC-iPSCs
Teratoma formation is one of the reliable tests to confirm pluripotency of iPSCs in vivo. We injected ERCC-iPSCs which displayed a normal karyotype of 46XY into nude immunedeficient mice (6 weeks old, male, Slac laboratory animal) to assess teratoma-forming capacity (Figure 3C) [36]. About 6 weeks later, tumor with a diameter of 1.5 cm was observed at the injection site. Histological examination showed that the tumor contained three germinal layers, including neural tissue (ectoderm) which presented a layered structure, primitive gut (endoderm) and cartilage (mesoderm) (Figure 3D). All animal experiments were performed according to the guidelines which approved by the Animal Ethics Committee of Fudan University in Shanghai.
In vitro differentiation of ERCC-iPSCs
To directly test the pluripotency of ERCC-iPSCs, we characterized the advantage of ERCC-iPSCs in differentiation into kidney-related cell types. We were able to establish a rapid protocol allowing for the priming of cells towards a kidney progenitor-like cell fate as described previously [28] (Figure 4A). The generated cells demonstrated specific expression of renal progenitor markers WT1 and LIM1 (LHX1) when exposure to defined media conditions (Figure 4B, 4C). These results suggested the possibility of differentiation potential into different renal-like cell types.
Figure 4.
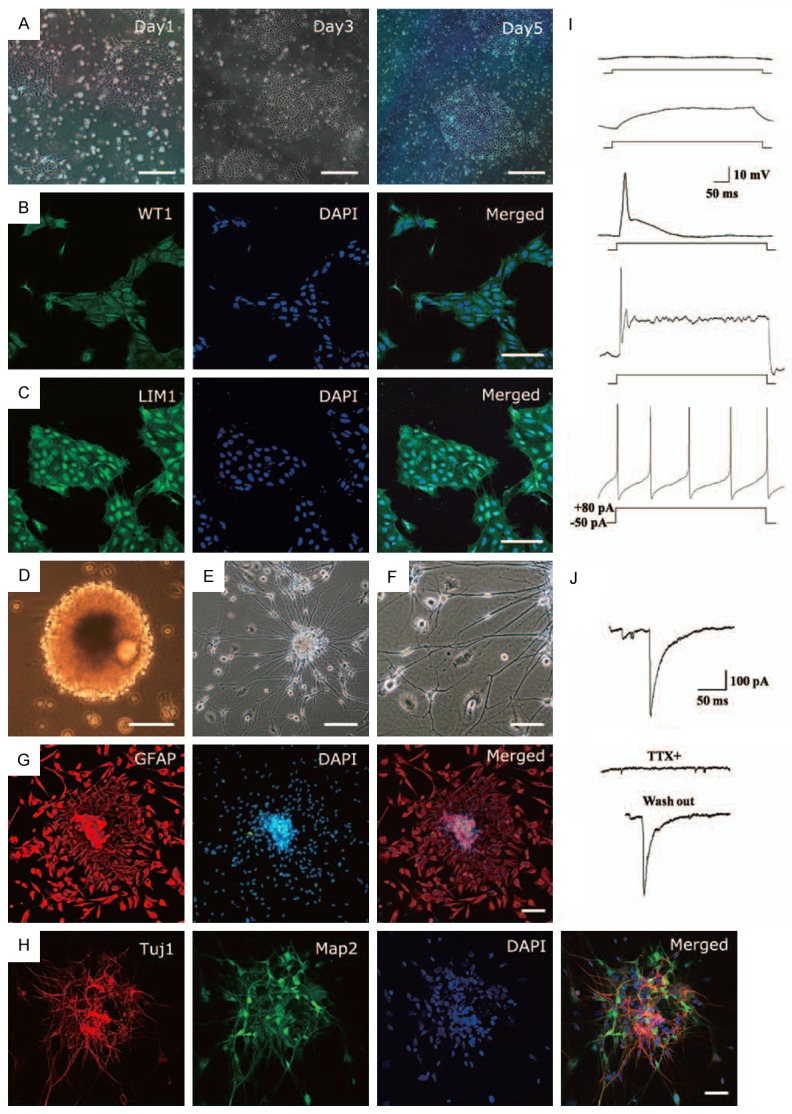
Differentiation of hERCCs-derived iPSCs. (A) Representative bright-field micrographs during the differentiation of ERCC-iPSCs towards renal progenitor-like cells. (B, C) Immunofluorescence analysis demonstrating expression of the indicated renal progenitor markers WT1 and LIM1 after differentiation. (D) Floating culture of EB. (E) Images of differentiated cells. (F) Neuron-like cells. (G) Immunocytochemistry of GFAP. (H) The differentiated cells expressed neuronal markers Tuj1 and Map2. (I) Representative traces of membrane potential (Upper panel) responding to step depolarization by current injection (lower panel), which demonstrated the capability of the neurons derived from ERCC-iPSCs to fire action potentials in response to a series of current injection from a holding potential of -65 mV. (J) The fast inward currents were sensitive to bath application of the Na+ channel blocker tetrodotoxin (TTX, 100 nM). Scale bars: 100 μm (A, D, E, G, H), and 50 μm (B, C, F).
Next, we generated astrocytes and neurons from ERCC-iPSCs in vitro. Traditional embryoid body formation was found to be inefficient for neural differentiation from iPSCs, so we followed a developed protocol as described previously [37]. When EBs were cultured for 3 weeks in the presence of 2% B27 (Gibco) to induce neuron and astrocyte differentiation (Figure 4D-F), GFAP-positive astrocytes and Tuj1- and Map2-positive neurons were found (Figure 4G, 4H). The whole-cell patch-clamp recordings were used to evaluate the electrophysiological phenotype of ERCC-iPSCs, whose results confirmed that ERCC-iPSCs exhibited properties of function mature neurons (Figure 4I, 4J).
Discussion
The derivation of iPSCs from human somatic cells offered a new therapeutic scenario for disease modeling. However, several crucial questions remain to be answered, such as what is the most amenable and efficient cell type to be reprogrammed. Here, we have successfully reprogrammed human embryonic renal cortical cells into iPSCs. The obtained ERCC-iPSCs exhibited several desired pluripotency characteristics and passed the test criteria that have been defined for human pluripotent stem cells. Importantly, our findings demonstrating highly efficient reprogramming of hERCCs are in contrast to some previous report, which showed that KOSM infection of other human somatic cells generated iPSC colonies with a lower reprogramming efficiency [38].
As we know, embryonic kidneys are a heterogenous mix of almost all the types of cells present in the body. This is possible, although most cells derived from an embryonic kidney would be endothelial, epithelial, or fibroblasts, embryonic stem (ES) cell origin is suspected in the starting cell population and they serve as the cellular origin of iPSCs [39]. To exclude this trepidation, we carefully screened the cells with a pack of antibodies against stem cells containing Nestin and Nanog after three passages. The results demonstrated that the starting cell population does not have pluripotency. We speculate that the cell culture environment, including types of culture medium and supplements of growth factor, affects and modifies embryonic kidney cells growth direction to somatic cells.
Human embryonic kidney cells (HEKCs) are also known, more informally, as 293 cells. This particular cell line was initiated by the transformation and culturing of normal HEKCs with sheared adenovirus 5 DNA [40,41]. As an experimentally transformed cell line, 293 cells are extremely easy to work with, being straightforward to culture and to transfect. Typically, these experiments involve transfecting in a gene (or combination of genes) of interest, and then analyzing the expressed protein [42-46]. So, it might be an ideal cell source for transfecting genes to generate and research iPSCs. Here, we reprogrammed hERCCs (one type of HEKCs) and HSFs with the same transcription factors under the same conditions, our data show that reprogramming hERCCs into iPSCs enhanced the efficiency by about 40-fold on iPSCs production from HSFs. Interestingly, the expression levels of pluripotency-specific genes Oct4 and Sox2 in ERCC-iPSCs were higher than the others. Therefore, it is speculated that hERCCs allowing these key transcript genes to produce high levels of protein. Some previous studies showed that reprogramming of somatic cells into iPSCs just by Oct4 or Oct4 and Sox2 [47,48], that means Oct4 and Sox2 are essential for switch on the reprogramming process. Li et al. described that co-expression of Oct4/Sox2 can activate the expression of endogenous Nanog gene [49] which is another key factor for induction of pluripotency [50]. Montserrat et al. proved endogenous c-Myc levels were higher in proximal tubular renal cells and thus could potentially increase the level of transcription [51]. One possible explanation could be that hERCCs already express high endogenous levels of c-Myc, which might confer a more reprogrammable state.
We have demonstrated that additional fate specification of ERCC-iPSCs is possible, such as kidney progenitor-like cell and mature neuron. Differentiation of ERCC-iPSCs into kidney-related cell types was accomplished on exposure to chemically defined media in the absence of feeder layers and EB formation, which showing the possible application for genetic kidney diseases. Also, the neural cells derived from ERCC-iPSCs can express human neuronal cell markers and fire mature action potentials in response to depolarizing current injection. Graham and coworkers provided evidence that 293 cells and some other cell lines generated by adenovirus transformation of human embryonic kidney cells have many properties of immature neurons, suggesting that embryonic kidney cells and neural cells may have a close relationship [52]. Interestingly, our results just give this version a good support, and open an avenue to repair brain injury by implanting ERCC-iPSCs.
In summary, we have successfully reprogrammed hERCCs into iPSCs for the first time. This ERCC-iPSCs exhibited some stringent pluripotency characteristics such as expressed the pluripotency markers, alkaline phosphatase activity and teratoma formation. Notably, the high efficiency of reprogramming and good potential for differentiation suggested hERCCs might be a good resource to generate iPSCs. Next, we may need to evaluate the possibility of obtaining ERCC-iPSCs with less transcription factors such as c-Myc which is known as an oncogenic factor [53-55]. Further studies are essential to reduce the risk of clinical safety of iPSC strain rather than generalizing all the Pisces.
Acknowledgements
We are very grateful to Dr. Ji Xiong (from Huashan Hospital, Fudan University) for pathological processing. This work was supported by grants (134119a8501, 15140902200) from Shanghai Committee of Science and Technology.
Disclosure of conflict of interest
None.
References
- 1.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Guo CL, Ma QW, Wang L, Zeng F, Zhou Q. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 4.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 5.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G, Edel M, Boué S, Izpisúa Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 6.Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, Kim K, Miller JD, Ng K, Daley GQ. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, Maier LS, Han DW, Glage S, Miller K, Fischer P, Schöler HR, Martin U. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Izpisúa Belmonte JC. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat Protoc. 2010;5:811–820. doi: 10.1038/nprot.2010.16. [DOI] [PubMed] [Google Scholar]
- 9.Tamaoki N, Takahashi K, Tanaka T, Ichisaka T, Aoki H, Takeda-Kawaguchi T, Iida K, Kunisada T, Shibata T, Yamanaka S, Tezuka K. Dental pulp cells for induced pluripotent stem cell banking. J Dent Res. 2010;89:773–778. doi: 10.1177/0022034510366846. [DOI] [PubMed] [Google Scholar]
- 10.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19:469–480. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda Y, Yoshimura Y, Ohnishi H, Tadokoro M, Katsube Y, Sasao M, Kubo Y, Hattori K, Saito S, Horimoto K, Yuba S, Ohgushi H. Induction of pluripotent stem cells from human third molar mesenchymal stromal cells. J Biol Chem. 2010;285:29270–29278. doi: 10.1074/jbc.M109.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira CF, Terranova R, Ryan NK, Santos J, Morris KJ, Cui W, Merkenschlager M, Fisher AG. Heterokaryon-based reprogramming of human B lymphocytes for pluripotency requires Oct4 but not Sox2. PLoS Genet. 2008;4:e1000170. doi: 10.1371/journal.pgen.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, Okada Y, Seimiya H, Fusaki N, Hasegawa M, Fukuda K. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, Urbach A, Heffner GC, Grskovic M, Vigneault F, Lensch MW, Park IH, Agarwal S, Church GM, Collins JJ, Irion S, Daley GQ. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown ME, Rondon E, Rajesh D, Mack A, Lewis R, Feng X, Zitur LJ, Learish RD, Nuwaysir EF. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One. 2010;5:e11373. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, Hock H, Hochedlinger K. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 20.Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Araúzo-Bravo MJ, Ruau D, Han DW, Zenke M, Schöler HR. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 21.Giorgetti A, Montserrat N, Rodriguez-Piza I, Azqueta C, Veiga A, Izpisúa Belmonte JC. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat Protoc. 2010;5:811–820. doi: 10.1038/nprot.2010.16. [DOI] [PubMed] [Google Scholar]
- 22.Xie LQ, Sun HP, Wang T, Tang HL, Wang P, Zhu JH, Yao ZW, Feng XY. Reprogramming of adult human neural stem cells into induced pluripotent stem cells. Chin Med J (Engl) 2013;126:1138–1143. [PubMed] [Google Scholar]
- 23.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodemann HP, Müller GA, Knecht A, Norman JT, Fine LG. Fibroblasts of rabbit kidney in culture. I. Characterization and identification of cell-specific markers. Am J Physiol. 1991;261:F283–91. doi: 10.1152/ajprenal.1991.261.2.F283. [DOI] [PubMed] [Google Scholar]
- 26.Grupp C, Müller GA. Renal fibroblast culture. Exp Nephrol. 1999;7:377–385. doi: 10.1159/000020635. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Zhang HL, Li W, Sha H, Xu C, Yao L, Tang Q, Tang H, Chen L, Zhu J. Generation of patient-specific induced neuronal cells using a direct reprogramming strategy. Stem Cells Dev. 2014;23:16–23. doi: 10.1089/scd.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wernig M, Tucker KL, Gornik V, Schneiders A, Buschwald R, Wiestler OD, Barde YA, Brüstle O. Tau EGFP embryonic stem cells: an efficient tool for neuronal lineage selection and transplantation. J Neurosci Res. 2002;69:918–924. doi: 10.1002/jnr.10395. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived mibraindopaminergic neurons in the brain of a primate model of parkinson’s disease. J Parkinson Dis. 2011;1:395–412. doi: 10.3233/JPD-2011-11070. [DOI] [PubMed] [Google Scholar]
- 30.Dahl D, Rueger DC, Bignami A, Weber K, Osborn M. Vimentin, the 57 000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia. Eur J Cell Biol. 1981;24:191–196. [PubMed] [Google Scholar]
- 31.Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- 32.Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 34.International Stem Cell Initiative. Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo AB, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz-Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RD, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O’Brien CM, Oh SK, Olsson C, Otonkoski T, Park KY, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RA, Reubinoff BE, Robins AJ, Rossant J, Rugg-Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, van den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 36.Cao H, Yang P, Pu Y, Sun X, Yin H, Zhang Y, Zhang Y, Li Y, Liu Y, Fang F, Zhang Z, Tao Y, Zhang X. Characterization of bovine induced pluripotent stem cells by lentiviral transduction of reprogramming factor fusion proteins. Int J Biol Sci. 2012;8:498–511. doi: 10.7150/ijbs.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebert AD, Yu J, Rose FF Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagarkova MA, Shutova MV, Bogomazova AN, Vassina EM, Glazov EA, Zhang P, Rizvanov AA, Chestkov IV, Kiselev SL. Induction of pluripotency in human endothelial cells resets epigenetic profile on genome scale. Cell Cycle. 2010;9:937–946. doi: 10.4161/cc.9.5.10869. [DOI] [PubMed] [Google Scholar]
- 39.Sareen D, Svendsen CN. Stem cell biologists sure play a mean pinball. Nat Biotechnol. 2010;28:333–335. doi: 10.1038/nbt0410-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 41.Harrison T, Graham F, Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977;77:319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- 42.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006;148:16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amar L, Desclaux M, Faucon-Biguet N, Mallet J, Vogel R. Control of small inhibitory RNA levels and RNA interference by doxycycline induced activation of a minimal RNA polymerase III promoter. Nucleic Acids Res. 2006;34:e37. doi: 10.1093/nar/gkl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanno T, Yamamoto H, Yaguchi T, Hi R, Mukasa T, Fujikawa H, Nagata T, Yamamoto S, Tanaka A, Nishizaki T. The linoleic acid derivative DCP-LA selectively activates PKC-epsilon, possibly binding to the phosphatidylserine binding site. J Lipid Res. 2006;47:1146–1156. doi: 10.1194/jlr.M500329-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Li T, Paudel HK. Glycogen synthase kinase 3beta phosphorylates Alzheimer’s disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism. Biochemistry. 2006;45:3125–3133. doi: 10.1021/bi051634r. [DOI] [PubMed] [Google Scholar]
- 46.Mustafa H, Strasser B, Rauth S, Irving RA, Wark KL. Identification of a functional nuclear export signal in the green fluorescent protein asFP499. Biochem Biophys Res Commun. 2006;342:1178–1182. doi: 10.1016/j.bbrc.2006.02.077. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P, Ge J, Liu C, Zhang W, Zhang X, Wu Y, Li H, Liu K, Wu C, Song Z, Zhao Y, Shi Y, Deng H. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montserrat N, Ramírez-Bajo MJ, Xia Y, Sancho-Martinez I, Moya-Rull D, Miquel-Serra L, Yang S, Nivet E, Cortina C, González F, Izpisua Belmonte JC, Campistol JM. Generation of induced pluripotent stem cells from human renal proximal tubular cells with only two transcription factors, OCT4 and SOX2. J Biol Chem. 2012;287:24131–24138. doi: 10.1074/jbc.M112.350413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li ZZ, Cheng D, Gao Y, Wang HY. Co-expression of OCT4/SOX2 Activates Endogenous NANOG Expression in Human Embryonic Kidney Cells. Chinese Journal of Biochemistry and Molecular Biology. 2011;27:833–840. [Google Scholar]
- 50.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montserrat N, Ramírez-Bajo MJ, Xia Y, Sancho-Martinez I, Moya-Rull D, Miquel-Serra L, Yang S, Nivet E, Cortina C, González F, Izpisua Belmonte JC, Campistol JM. Generation of induced pluripotent stem cells from human renal proximal tubular cells with only two transcription factors, OCT4 and SOX2. J Biol Chem. 2012;287:24131–24138. doi: 10.1074/jbc.M112.350413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 53.Clavería C, Giovinazzo G, Sierra R, Torres M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature. 2013;500:39–44. doi: 10.1038/nature12389. [DOI] [PubMed] [Google Scholar]
- 54.Denis N, Kitzis A, Kruh J, Dautry F, Corcos D. Stimulation of methotrexate resistance and dihydrofolate reductase gene amplification by c-myc. Oncogene. 1991;6:1453–1457. [PubMed] [Google Scholar]
- 55.Gearhart J, Pashos EE, Prasad MK. Pluripotency Redeux-advances in stem-cell research. N Engl J Med. 2007;357:1469–1472. doi: 10.1056/NEJMp078126. [DOI] [PubMed] [Google Scholar]


