Abstract
It is well demonstrated that the high mobility group box 1 (HMGB1) mediated inflammation has been implicated as one of the important causes for brain damage induced by cerebral ischemia/reperfusion (I/R). In the present study, we assessed the neuro-protective and anti-inflammation effects of the ethanol extracts from Portulaca oleracea L. (EEPO) against cerebral I/R injury in the rat transient middle cerebral artery occlusion (tMCAO) model. Rats were administrated with their respective treatment for 7 days before the MCA occlusion. After that, rats were intraperitoneal injection with chloral hydrate and sacrificed by decapitation, then the serum and brain tissue were collected. The neurological deficit score, infarct size and brain edema were tested. The levels of serum cytokine as TNF-α, IL-1β, INF-γ, IL-6, and HMGB1 and LDH were detected. The protein level of tissue or nucleus HMGB1, IκB and p-p65 were tested, too. The results showed that pretreatment with EEPO significantly decreased the neurological deficit score, infarct size and brain edema. Moreover, EEPO decreased rat serum cytokine level and rat right cortices p-p65 and IκB protein level. In conclusion all these results suggested that pretreatment with EEFPO provided significant protection against cerebral I/R injury in rats might by virtue of its anti-inflammation property through inhibition of increase of neuleus HMGB1.
Keywords: Portulaca oleracea L, high mobility group box 1, ischemia/reperfusion, brain
Introduction
Cerebral I/R is characterized by an initial restriction of blood supply to the brain, followed by subsequent restoration of blood flow and concomitant reoxygenation [1,2]. Cerebral I/R induced cerebrovascular dysfunction is thought to be an important contributor to neurological damage in diseases such as ischemia stroke. So, it is significant to find effective measures to prevent the injury induced by cerebral I/R for prevention of ischemia stroke.
Increasing evidence supports the idea that inflammation is detrimental in ischemia-reperfusion induced injury. As in stroke, inflammatory cascades are triggered by cerebral ischemia-reperfusion and subsequently influence secondary brain injury due to cytotoxic neuronal cell death [3]. It has been found that inflammatory reaction accompanying brain injury is mostly dependent on Toll-like receptor (TLR) 2 and TLR4. The endogenous TLR ligand, high mobility group box 1 (HMGB1), is involved in the activation of inflammatory cytokine expression in infiltrating macrophages.
HMGB1, a well-known damage-associated molecular pattern molecule, was first described as a non-histone chromosomal protein with high electrophoretic mobility and was found to stimulate inflammatory response and activate macrophages upon release into the extracellular milieu from necrotic cells [4-7]. In another study, HMGB1 was released and exacerbated neuronal damage by triggering inflammatory processes during N-methyl-D-aspartate (NMDA)-induced acute damage in the post-ischemic brain [6,7]. Consistently, administration of HMGB1 monoclonal antibody suppressed infarct formation and protected the blood-brain barrier in the post-ischemic brain [8,9]. Furthermore, both siRNA-mediated HMGB1 knockdown and HMGB1 A box-mediated HMGB1 inhibition markedly reduced brain infarct volume in a rat middle cerebral artery occlusion (MCAO) model [10-12]. Mechanically, HMGB1 release resulted in activation of nuclear factor kappa B (NF-κB), a transcription factor often leads to the up-regulation of pro-inflammatory cytokines, chemokines and adhesion molecules and intensifies cellular oxidative stress [13-17]. It is reasonable that reducing of HMGB1 can inhibit NF-κB activation. So, to decrease the extracellular level of HMGB1 and subsequently inhibit inflammatory necrotic cells, reactive oxygen species (ROS) and inflammatory cytokines activity remains an important potent method for effective treatment of brain I/R injury.
Portulaca oleracea L. is a warm-climate annual with a cosmopolitan distribution. A wide range of pharmacological effects of Portulaca oleracea L., such as antibacterial, analgesic, anti-inflammatory and wound-healing activities, have been reported [18,19]. In our previous study we found that mice pretreated with the ethanol extracts from Portulaca oleracea L. (EEPO) for one week showed decreased hypoxia induced brain damage [20,21]. Based on all these mentioned above, we speculated whether EEPO could reduce the brain ischemia reperfusion injury. Studies on this question might provide a new candidate for prevention or treatment of cerebral I/R injury.
In present experiments, using the rat transient middle cerebral artery occlusion (tMCAO) model, we demonstrated the protective effects of EEPO against cerebral I/R injury as EEPO pretreatment significantly reduced cerebral injury, improved recovery of neurological function and reduced brain tissue water content. The results of mechanism research showed that EEPO pre-administration significantly decreased the rat serum HMGB1 level in a time-dependent manner and down regulated the expression of inflammatory cytokine. In addition, EEPO pretreatment attenuated the increase of nucleus p-P65 and degradation of cytoplasm IκB, indicating inhibition of HMGB1 release and following activation of inflammation response might be involved in the neuro-protective effects of EEPO against cerebral I/R injury.
Material and methods
Animals
This study has been approved by the Ethics Committee of The First Affiliated Hospital of Bengbu Medical College. Male Sprague-Dawley rats (weight 220±10 g) were provided by the Research Animal Center of the Second Military Medical University. Rats were housed with food and water ad libitum. All of the procedures were performed under the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23), and all the experiments were carried out with the approval of the Experimental Animal Administration Committee of the Second Military Medical University.
Induction of cerebral ischemia
Animals were fasted overnight but allowed free access to water before surgical procedure. A heating lamp and a heating pad were used to maintain the rat rectal temperature between 36.5°C and 37.5°C. Through a ventral midline incision, the right common carotid artery, internal carotid artery, and external carotid artery were surgically exposed. The external carotid artery was then isolated and coagulated. A 6-0 nylon suture with silicon coating (Doccol Corporation, Redlands, USA) was inserted into the internal carotid artery through the external carotid artery stump and gently advanced to occlude the middle cerebral artery. One hour after of MCA occlusion (MCAO), the suture was carefully removed to restore blood flow (reperfusion), the neck incision was closed, and the rats were allowed to recover [22]. Animal body temperature was carefully monitored during the post-operation period and until the complete recovery of the animal from the anesthetic. Animals were housed individually with free access to food and water throughout the experiment.
Evaluation of cerebral infarct and edema
Twenty-four hours after reperfusion, some animals were anesthetized with intraperitoneal (i.p.) injection of 4% choral hydrate, brains were dissected and sliced onto a plastic module (Harvard Apparatus, Holliston, USA), sections of 1.5 mm thickness were made and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 30 min at 37°C and then fixed with 4% paraformaldehyde.
Some animals were anesthetized with intraperitoneal (i.p.) injection of 4% choral hydrate, brains were quantified with electronic scale (wet weight) and dried to constant weight (dry weight) at 105°C in a desiccating oven. The total brain water was calculated using the formula [(wet weight-dry weight)/wet weight]*100% [23].
Evaluation of neurological function
The neurologic deficits were determined using the Longa’s score [24] and were evaluated by an examiner who were blinded to the animal group and treatment to determine global neurological function according to the following scoring system: 0, no deficit; 1, forelimb flexion; 2, decreased resistance to lateral push; 3, unidirectional circling; 4, longitudinal spinning; and 5, no movement.
Plant materials
The preparation of plan materials were as previously described [21]. Briefly, the powdered aerial parts of PO were refluxed with 80% ethanol solution for one hour. The extract was concentrated under reduced pressure and then centrifuged at 5000 rpm for 4 min. The precipitation part was used as testing materials.
Animal treatment with EEPO
The male Sprague-Dawley rats were pretreated with phosphate PBS or different dose of EEPO (50, 100 and 200 mg/kg body weight defined as l-EEPO, m-EEPO and H-EEPO, respectively) once a day for seven days, one hour after the last administration, the animals were operated as described for following experiments.
Measurement of serum IL-1β, IL-6, TNF-α and INF-γ
Serum IL-1β, IL-6, TNF-α and INF-γ were assessed using commercial ELISA kits as described by the manufacturer (eBIOSCIENCE, San Diego, USA). Results were expressed in pg/ml for IL-1β, IL-6, and TNF-α, and μg/ml for INF-γ.
Western blotting analysis of p-p65, and IκB protein expression level
Expression of p-p65, and IκB were investigated by western blotting analysis. Protein was extracted in RIPA buffer with protease inhibitor (complete protease inhibitor cocktail, Roche), and protein concentration was determined by a BCA protein assay kit (Beyotime Institute of Biotechnology, Nantong, China), equal amount of proteins (40 μg) were resolved by using 10% SDS-PAGE, which were then transferred to nitrocellulose membranes. The blots were blocked with 2% BSA, 0.1% Tween-20 in Tris-NaCl buffer for one hour, and were incubated overnight at 4°C with the primary antibody (Santa Cruz Biotechnology Inc) at a dilution of 1:500~1:1000. After extensive washing, the blots were incubated with the secondary horseradish peroxidase-conjugated antibody (Santa Cruz Biotechnology Inc, 1:1000) for 2 hours at 37°C. Immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Amersham Life Science, Arlington Heights, IL). The β-actin expression was used as loading control.
Statistical analysis
Data were presented as means ± S.E.M. When the variance was equal, the data were compared using the independent t-test. When the variance was unequal, the data were compared by Welch’s test. Multiple group comparisons for the infarct volumes and protein levels were performed by one-way analysis of variance (ANOVA), followed by Turkey’s test. P<0.05 was considered statistically significant.
Results
EEPO decreased ischemia-reperfusion induced rat cerebral injury
After ischemia-reperfusion, brain tissues changed edema and the brain water content increased. The results showed that brain water content was significantly reduced in EEPO administrated mice compared with those in control mice (Figure 1A). Twenty-four hours after MCAO, the serum lactate dehydrogenase (LDH) level in EEPO administrated mice was significantly decreased compared with that in control mice (Figure 1B). The Longa’s score assessing global neurological function was significantly better in EEPO administrated mice than that in control mice at 24 h after MCAO (Figure 1C). Furthermore, TTC staining results showed that EEPO pretreatment decreased the infarct size (Figure 1D).
Figure 1.
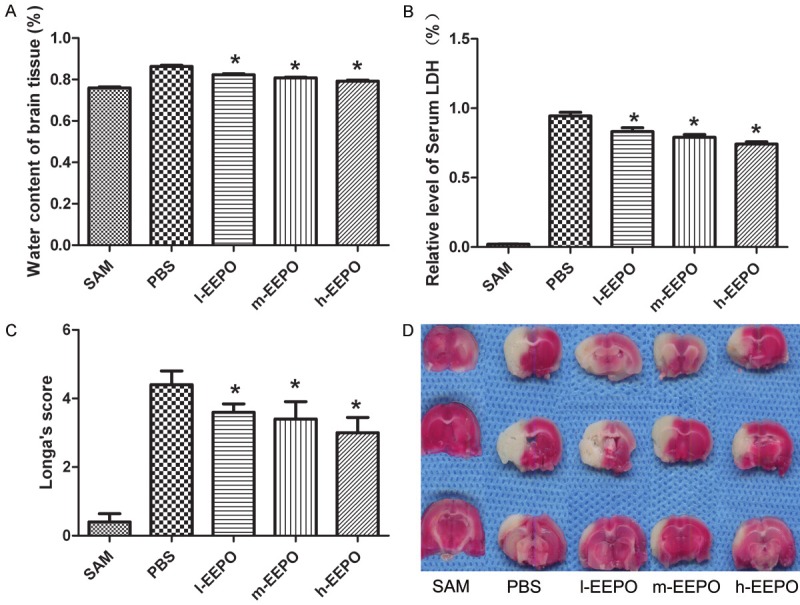
The EEPO decreased rat cerebral ischemia-reperfusion injury. The male Sprague-Dawley rats were pretreated with phosphate PBS or different dose of EEPO (50, 100 and 200 mg/kg body weight defined as l-EEPO, m-EEPO and H-EEPO, respectively) once a day for seven days. one hour after the last administration, all rats were occluded the middle cerebral artery (MCA occlusion, MCAO) and after one hour all rats were carefully restored blood flow for 24 hours and used for experiments. The untreated rats which operated same as MACO group but not occluded the middle cerebral artery used as SAM group. Rats were sacrificed after over dose of chloral hydrate. The water level of rat brain tissue (A), the serum lactate dehydrogenase (LDH) (B) and the neurologic deficits (C) were determined (n=5, *P<0.05 vs PBS gourp). The represent pictures of the infarct area of brain tissue were shown in (D).
EEPO reduced the increase of inflammatory cytokines in serum
ELISA results showed that administration of EEPO attenuated the increase of serum cytokine including TNF-α (Figure 2A), IL-6 (Figure 2B), IL-1β (Figure 2C) and INF-γ (Figure 2D) in a dose dependent manner.
Figure 2.
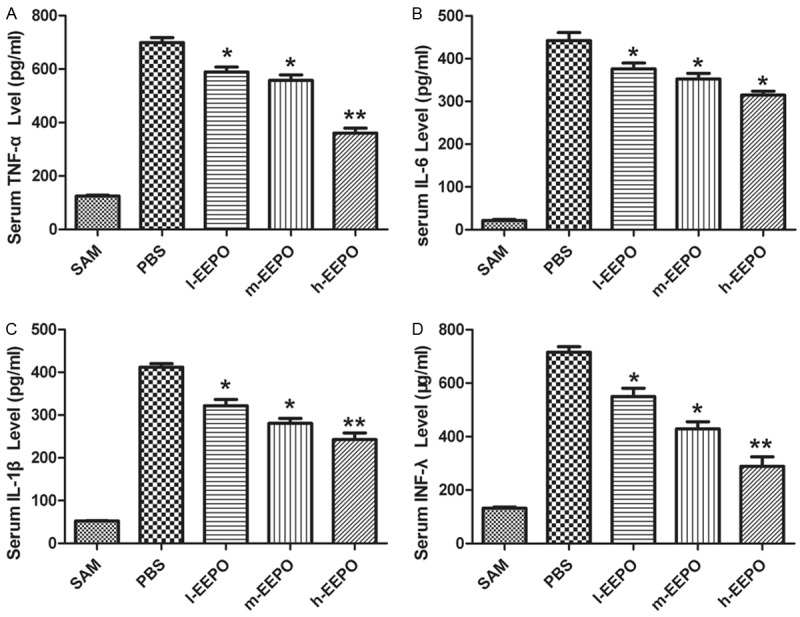
The EEPO reduced the increase of serum cytokines increase. The male Sprague-Dawley rats were pretreated with phosphate PBS or different dose of EEPO (50, 100 and 200 mg/kg body weight defined as l-EEPO, m-EEPO and H-EEPO, respectively) once a day for seven days, one hour after the last administration, all rats were occluded the middle cerebral artery (MCA occlusion, MCAO) and after one hour all rats were carefully restored blood flow for 24 hours and used for experiments. The untreated rats which operated same as MACO group but not occluded the middle cerebral artery used as SAM group. Rats were sacrificed after over dose of chloral hydrate. The serum level of cytokines including as TNF-α (A), IL-6 (B), IL-1β (C) and INF-γ (D) were detected tested (n=5, *P<0.05, **P<0.01, vs PBS group).
EEPO attenuated increase of nucleus phosphorylated P65 and degradation of cytoplasm IκB
Using western blotting, we tested the level of p-p65 in nucleus and IκB in cytoplasm. The results showed that cerebral I/R significantly increased the nucleus level of p-P65 and decreased the cytoplasm level of IκB in control group, while pretreatment of EEPO significantly attenuated the increase of nucleus p-P65 level (Figure 3A) and decrease of cytoplasm IκB level (Figure 3B) in a dose dependent manner.
Figure 3.
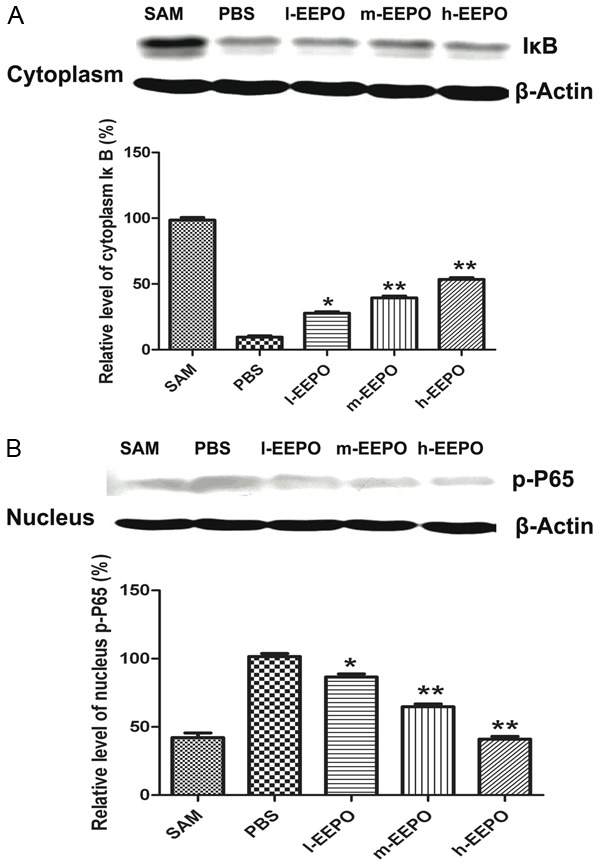
The EEPO attenuated increase of nucleus P65 and cytoplasm IκB degradation. The male Sprague-Dawley rats were pretreated with phosphate PBS or different dose of EEPO (50, 100 and 200 mg/kg body weight defined as l-EEPO, m-EEPO and H-EEPO, respectively) once a day for seven days, one hour after the last administration, all rats were occluded the middle cerebral artery (MCA occlusion, MCAO) and after one hour all rats were carefully restored blood flow for 24 hours and used for experiments. The untreated rats which operated same as MACO group but not occluded the middle cerebral artery used as SAM group. Rats were sacrificed after over dose of chloral hydrate. The opposite side cortices were used and the cytoplasm IκB level (A) and nucleus p-P65 (B) level were assessed (n=4, *P<0.05, **P<0.01 vs PBS group).
EEPO inhibited HMGB1 release
With western blot, our results (Figure 4A) showed that cerebral I/R induced the release of HMGB1 from the nucleus. Twenty-four hours after MCAO, the level of cortices nucleus HMGB1 of PBS group decreased about 77.5% of that of SAM group. EEPO pretreatment significantly attenuated the decrease of HMGB1 level (63.8%, 56.1% and 42.5% in l-EEPO, m-EEPO and h-EEPO group, respectively). In addition, EEPO pretreatment decreased the level of HMGB1 release into serum during the reperfusion process (Figure 4B) and reduced the release of LDH (Figure 4C), which indicating the neuro-injury induced by cerebral I/R. All these results suggested that EEPO decrease the cerebral I/R induced neuro-damage by inhibition of HMGB1 release.
Figure 4.

The EEPO inhibited HMGB1 release. The male Sprague-Dawley rats were pretreated with phosphate PBS or different dose of EEPO (50, 100 and 200 mg/kg body weight defined as l-EEPO, m-EEPO and H-EEPO, respectively) once a day for seven days, one hour after the last administration, all rats were occluded the middle cerebral artery (MCA occlusion, MCAO) and after one hour all rats were carefully restored blood flow for 24 hours and used for experiments. The untreated rats which operated same as MACO group but not occluded the middle cerebral artery used as SAM group. Rats were sacrificed after over dose of chloral hydrate. The serum level of HMGB1 (A) were tested (n=5, *P<0.05, **P<0.01, vs PBS group). In another experiments, the male Sprague-Dawley rats were pretreated with phosphate PBS or 100 mg/kg body weight once a day for seven days, one hour after the last administration, all rats were occluded the middle cerebral artery (MCA occlusion, MCAO) for one hour or reperfusion for three hour, six hour, 12 hour or 24 hour, rats were sacrificed at indicated time and the serum level of HMGB1 (B) and lactate dehydrogenase (LDH) (C) were tested (n=5, *P<0.05, **P<0.01, vs PBS group).
Discussion
In present study, using MCAO, a classical model of cerebral ischemia reperfusion, we demonstrated the protective effects of EEPO against I/R induced cerebral injury. EEPO pre-administration significantly reduced cerebral injury, improved recovery of neurological function and reduced brain tissue water content. Further study showed that EEPO pre-administration significantly decreased the rat serum HMGB1 level in a time-dependent manner and down regulated the expression of inflammatory cytokine. In addition, EEPO pretreatment attenuated the increase of nucleus p-P65 and degradation of cytoplasm IκB.
Cerebral I/R injury produces core infarct tissue and further brings damage to the surrounding tissue, the peri-infarct region, through activation of secondary inflammatory and neurodegenerative cascades [12]. Cerebral I/R injury can cause brain edema and neurological deficit, which is accompanied with serum LDH increase [25]. It has been demonstrated that EEPO had anti inflammation and anti hypoxia function [21]. So, we test whether EEPO had protective effects against cerebral I/R injury. The results showed that EEPO significantly decreased the brain water content (Figure 1A), reduced infarct area (Figure 1D) and attenuated the decease of the rat motor-neuro function (Figure 1C). All these results indicated the significant neuro protective effects of EEPO against cerebral I/R injury.
It has been reported that inflammation plays a vital role in the pathogenesis of cerebral I/R injury [26,27] and inflammatory and immunological reactions are involved in the pathogenesis of cerebral ischemia following blood reperfusion to the surrounding tissue [28]. In this way, the inhibition of inflammatory cytokine activities at the early stage of ischemia might constitute an attractive therapeutic strategy. Based on the anti inflammation and anti hypoxia function of EEPO, we further tested the effect of EEPO on inflammation reaction during I/R induced brain injury. As expected, the results demonstrated that pretreatment of EEPO attenuated the increase of serum TNF-α (Figure 2A), IL-6 (Figure 2B), IL-1β (Figure 2C) and INF-γ (Figure 2D) in a dose-dependent manner.
It has been found that cerebral I/R injury resulted in IκB-α degradation, which then increased nucleus p-P65, activated nuclear factor κB (NF-κB), and stimulated subsequent cytokines production [29-31]. Consistent with these results, in our study cerebral I/R also increased the nucleus level of p-P65 and decreased the cytoplasm level of IκB in control group, whereas pretreatment of EEPO significantly attenuated the increase of nucleus p-P65 level (Figure 3A) and decrease of cytoplasm IκB level (Figure 3B) in a dose dependent manner, indicating that inhibition of the activation of NF-κB may be involved the function of EEPO.
Necrotic cell death lead to the damage of cellular membrane and resulted in passive leakage of HMGB1 from the impaired cells. After being released extracellularly, HMGB1 then activated immune-related cells and evoked inflammatory reactions [32,33], which further lead to activation of the transcription NF-κB. Based on these data and our results, we hypothesized that EEPO might inhibit HMGB1 release to inhibit the inflammation. The western blotting results showed that 24 hours after MCAO, the nucleus HMGB1 level was increased in EEPO group compared to that of PBS group in a dose-dependent manner (Figure 4A), indicating the inhibition of HMGB1 release from nucleus by EEPO. At the same time, EEPO pretreatment decreased the serum HMGB1 level at the indicated time point (Figure 4B) and accompany with a delay of inhibition of EEPO on LDH release, which indicated the degree of neuro-injury (Figure 4C). So these data demonstrated our hypothesis that EEPO might inhibit HMGB1 release to inhibit the inflammation.
Conclusions
Collectively, our results demonstrated the protective effects of EEPO against cerebral I/R injury and the effects were, at least partly, associated with inhibition of HMGB1 release, attenuation of NF-κB activation and the following activation of inflammation response.
Acknowledgements
The authors thank Yue Tan and Xiao Ming for their helpful assistant in animal experiments. This work was supported in part by research grants from the Natural Science Research of Educational Department Anhui Province (No. KJ2015ZD31), the Scientific Research Foundation of Shanghai third People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. syz2015-014), and scientific research project of Science and Technology Committee of Shanghai (No. 16ZR1444400, No. 201440381).
Disclosure of conflict of interest
None.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, Bauer H, Sommer C, Veltkamp R. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS One. 2011;6:e21312. doi: 10.1371/journal.pone.0021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll G, Kleinschnitz C, Nieswandt B. Combating innate inflammation: a new paradigm for acute treatment of stroke? Ann N Y Acad Sci. 2010;1207:149–154. doi: 10.1111/j.1749-6632.2010.05730.x. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 6.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 8.Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX. HMGB1 activates nuclear factor-kappaB signaling by RAGE and increases the production of TNF-alpha in human umbilical vein endothelial cells. Immunobiology. 2010;215:956–962. doi: 10.1016/j.imbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi M, Takino J, Yamagishi S. Involvement of the toxic AGEs (TAGE)-RAGE system in the pathogenesis of diabetic vascular complications: a novel therapeutic strategy. Curr Drug Targets. 2010;11:1468–1482. doi: 10.2174/1389450111009011468. [DOI] [PubMed] [Google Scholar]
- 10.Jin YC, Kim SW, Cheng F, Shin JH, Park JK, Lee S, Lee JE, Han PL, Lee M, Kim KK, Choi H, Lee JK. The effect of biodegradable gelatin microspheres on the neuroprotective effects of high mobility group box 1 A box in the postischemic brain. Biomaterials. 2011;32:899–908. doi: 10.1016/j.biomaterials.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 11.Kim ID, Shin JH, Kim SW, Choi S, Ahn J, Han PL, Park JS, Lee JK. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20:829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SW, Jin Y, Shin JH, Kim ID, Lee HK, Park S, Han PL, Lee JK. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol Dis. 2012;46:147–156. doi: 10.1016/j.nbd.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 13.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 14.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, Andersson U, Yang H, Tracey KJ, Andersson J, Palmblad JE. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 16.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S, Nishibori M. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42:1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- 18.Xiang L, Xing D, Wang W, Wang R, Ding Y, Du L. Alkaloids from Portulaca oleracea L. Phytochemistry. 2005;66:2595–2601. doi: 10.1016/j.phytochem.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Rashed AN, Afifi FU, Disi AM. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J Ethnopharmacol. 2003;88:131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen CJ, Wang WY, Wang XL, Dong LW, Yue YT, Xin HL, Ling CQ, Li M. Anti-hypoxic activity of the ethanol extract from Portulaca oleracea in mice. J Ethnopharmacol. 2009;124:246–250. doi: 10.1016/j.jep.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Wanyin W, Liwei D, Lin J, Hailiang X, Changquan L, Min L. Ethanol extract of Portulaca oleracea L. protects against hypoxia-induced neuro damage through modulating endogenous erythropoietin expression. J Nutr Biochem. 2012;23:385–391. doi: 10.1016/j.jnutbio.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- 23.Mdzinarishvili A, Kiewert C, Kumar V, Hillert M, Klein J. Bilobalide prevents ischemia-induced edema formation in vitro and in vivo. Neuroscience. 2007;144:217–222. doi: 10.1016/j.neuroscience.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Wu X, Yu S, Lin X, Wu J, Li L, Zhao J, Zhao Y. Neuroprotection of tanshinone IIA against cerebral ischemia/reperfusion injury through inhibition of macrophage migration inhibitory factor in rats. PLoS One. 2012;7:e40165. doi: 10.1371/journal.pone.0040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choe CU, Lardong K, Gelderblom M, Ludewig P, Leypoldt F, Koch-Nolte F, Gerloff C, Magnus T. CD38 exacerbates focal cytokine production, postischemic inflammation and brain injury after focal cerebral ischemia. PLoS One. 2011;6:e19046. doi: 10.1371/journal.pone.0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvoriantchikova G, Hernandez E, Grant J, Santos AR, Yang H, Ivanov D. The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Invest Ophthalmol Vis Sci. 2011;52:7187–7194. doi: 10.1167/iovs.11-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripathy D, Grammas P. Acetaminophen inhibits neuronal inflammation and protects neurons from oxidative stress. J Neuroinflammation. 2009;6:10. doi: 10.1186/1742-2094-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elango C, Devaraj SN. Immunomodulatory effect of Hawthorn extract in an experimental stroke model. J Neuroinflammation. 2010;7:97. doi: 10.1186/1742-2094-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu JH, Ge JB, Li M, Wu F, Zhang W, Qin ZH. Inhibition of NF-kappaB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci. 2012;47:652–660. doi: 10.1016/j.ejps.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim SW, Lim CM, Kim JB, Shin JH, Lee S, Lee M, Lee JK. Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox Res. 2011;20:159–169. doi: 10.1007/s12640-010-9231-x. [DOI] [PubMed] [Google Scholar]


