Abstract
Background: The β2-Adrenergic receptor (β2-AR) is associated with tumor growth and progression. However, the clinical significance of β2-AR expression in patients with non-small cell lung cancer (NSCLC) remains unclear. Methods: Three hundred twenty-eight patients with surgically resected NSCLC were retrospectively investigated. Tumor sections were stained by immunohistochemistry for assessing β2-AR and Ki-67 expression and microvessel density (MVD), which was using CD34 levels. Results: β2-AR was positively expressed in 27% of all patients, in 29% of adenocarcinoma (AC) patients, and in 24% of non-AC patients. In AC patients, β2-AR expression was significantly correlated with lymphatic permeation (r=0.240; P<0.001), vascular invasion (r=0.239; P<0.001), and Ki-67 expression (r=0.175; P=0.009). However, this correlation was not observed in non-AC patients. Positive β2-AR expression was identified as a negative predictor for worse outcomes in AC patients, particularly in those with stage I tumors. Multivariate analysis confirmed that β2-AR expression was an independent factor for predicting poor progression-free survival in stage I AC patients (HR=2.220; 95% CI, 1.077-4.573; P=0.031). Conclusion: β2-AR expression is an independent prognostic factor for early-stage AC patients.
Keywords: Lung cancer, adenocarcinoma, stage I, β2-adrenergic receptor, prognostic factor, immunohistochemistry
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. Despite years of research, the prognosis for patients with lung cancers remains dismal, with a 5-year survival rate of 14% [1]. In addition, patients with stage I non-small cell lung cancer (NSCLC) have a risk for disease recurrence even if the tumor is completely resected via surgery [2]. Thus, assessing the potential of established biomarkers for predicting the outcome and response to specific anti-cancer therapies is vital for improving the prognosis in NSCLC patients. Tumor staging and performance status are currently the most powerful prognostic predictors in patients with NSCLC [3]. Recent large-scale studies have demonstrated that sex, smoking history, and histology may also affect the treatment outcome in patients with NSCLCs [4-6].
The β2-adrenergic receptor (β2-AR), a member of the transmembrane G protein-coupled receptors (GPCRs) family, initiates multiple signaling cascades and regulates cell proliferation through a classical cyclic-adenosine-monophosphate (cAMP)/protein kinase A (PKA) pathway [7,8]. Tumor recurrence is a multifactorial process that includes tumor cell migration, invasion, and metastasis. Neurotransmitters and chemokines are ligands that bind to GPCRs and act as prominent regulatory factors in tumor recurrence [9,10]. It has recently been reported that β-adrenergic activation of the cAMP-PKA signaling pathway via β2-AR could affect angiogenesis followed by cell growth and proliferation in ovarian cancer cells [11].
β2-AR overexpression has been reported in various human cancers, including breast cancer, oral cancer, prostate cancer, melanoma, and hepatocellular carcinoma, and it can lead to poor clinicopathological features such as tumor recurrence, metastasis, and reduced survival [12-16]. The results of these studies suggest that β2-AR may be a cancer-relevant biomarker in carcinogenic processes. An in vitro study indicated that β2-AR could be used as a potential target for cancer therapy [17]. Moreover, it has been reported that the use of β-blockers, particularly propranolol, prolongs survival in breast cancer patients [18,19]. β-blockers are also generally administered to lung cancer patients with coexisting heart diseases. However, little is known about the clinicopathological significance of β2-AR expression in lung cancer patients and of the relationship between β-blocker use and lung cancer prognosis. Considering previous data on β2-AR expression within cancer cells, β2-AR could be an attractive therapeutic target in various human neoplasms, including lung cancer.
Considering this, we conducted a clinicopathological study to evaluate the expression of β2-AR in patients with NSCLCs. We investigated whether β2-AR expression was closely associated with post-treatment outcomes and explored the relationship between β2-AR and various clinicopathological characteristics. Furthermore, we assessed the correlation between β2-AR expression and both the Ki-67 labeling index (LI), as well as microvessel density (MVD; as determined by CD34).
Materials and methods
Patients
Our study cohort was composed of 347 consecutive patients with NSCLCs (stages I-III), who had undergone resection by lobectomy or pneumonectomy with mediastinal lymph-node dissection at Gunma University Hospital (Maebashi, Gunma, Japan) between June 2003 and December 2010. Of 347 patients, 19 were excluded from further analysis owing to unavailable tissue specimens; thus, 328 patients were enrolled. Our approach to the evaluation and resection of these tumors was identical to the process outlined in our previous study [20]. One patient received platinum-based chemotherapy before surgery as neoadjuvant therapy. In our cohort, 79 patients were administered postoperative adjuvant chemotherapy, with 15 patients receiving platinum-based regimens and 64 patients receiving oral tegafur (a fluorouracil derivative drug). The study protocol was approved by the institutional review board. The tumor specimens were histologically classified according to the World Health Organization criteria. The stages of pathological tumor node metastasis were determined using the International System for Staging Lung Cancer adopted by the American Joint Committee on Cancer and Union Internationale Contre le Cancer [21]. The day of surgery was considered as the first day after the operation. The follow-up duration ranged from 23 to 3,821 days (median, 1,438 days).
Immunohistochemical staining
β2-AR expression was determined by immunohistochemical staining using a rabbit anti-human β2-AR monoclonal antibodies (1:100 dilution; Abcam, Inc., Cambridge, UK) raised against a C-terminal peptide of the human β2-AR. Immunohistochemical staining was performed on paraffin sections using the polymer peroxidase method (Histofine Simple Stain MAX PO (MULTI) kit; Nichirei Corporation, Tokyo, Japan). Briefly, deparaffinized rehydrated sections were treated with 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity. To expose the antigens, sections were autoclaved in 10 mmol/L sodium citrate buffer (pH 6.0) for 5 min, and cooled for 30 min. After rinsing with phosphate-buffered saline, sections were incubated with the anti-β2-AR antibody (1:100) overnight. Thereafter, they were incubated using the Histofine Simple Stain MAX PO (MULTI) kit (Nichirei Corporation). The peroxidase reaction was performed using 0.02% of 3,3-diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide in 0.05 M Tris-HCl buffer, pH 7.6. Negative control tissue sections were prepared by omitting the primary antibody. The expression of β2-AR was considered as positive only if distinct membrane and cytoplasm staining was present. The β2-AR expression scores were assessed based on the extent of the staining as follows: 1, ≤10% of the tumor area stained; 2, 11%-25% of the tumor area stained; 3, 26%-50% of the tumor area stained; and 4, ≥51% of the tumor area stained. Tumors in which the stained tumor cells were scored as ≥2 were defined as showing positive expression.
Mouse monoclonal antibodies against CD34 (1:800 dilution; Nichirei Corporation) and Ki-67 (1:40; Dako, Glostrup, Denmark) were also used. The number of CD34-positive vessels was counted in four randomly selected regions in a 400× field (0.26 mm2 field area). The MVD was defined as the mean number of microvessels per 0.26 mm2 field area and tumors in which the number of stained tumor cells was greater than the median were defined as areas of high expression. For Ki-67, epithelial cells with nuclear staining of any intensity were considered as positive. Approximately 1,000 nuclei were counted on each tumor slide, and their proliferative activity was assessed as the percentage of Ki-67-stained nuclei (Ki-67 LI) in each sample. The median Ki-67 LI was evaluated, and tumors with an LI greater than the median were considered to show high level of expression. All sections were independently assessed using light microscopy in a blinded manner by at least two of the authors.
Statistical analysis
P-values of less than 0.05 were used to indicate a statistically significant difference. Chi-squared or Fisher’s exact tests were used to examine the association between the two categorical variables. The correlation between different variables was analyzed using the non-parametrical Spearman’s rank test.
Elderly patients were defined as those older than 65 years, and smokers were defined as those who had smoked at least 100 cigarettes in their lifetimes. Disease staging was divided into two groups: stages I group and stage II/III group. The Kaplan-Meier method was used to estimate survival as a function of time, and differences in survival were analyzed using the log-rank test. Overall survival (OS) was determined as the time from tumor resection to death from any cause. Progression-free survival (PFS) was defined as the time between tumor resection and the first disease progression or death. Multivariate analysis was performed using a stepwise Cox proportional hazards model to identify the independent prognostic factors. Statistical analysis was performed using IBM SPSS Statistics version 21 (IBM corp., NY, USA) for Windows.
Results
Immunohistochemical analysis and patient demographics
Three hundred twenty-eight primary NSCLC lesions were analyzed using immunohistochemical analysis. The β2-AR expression was predominantly localized to the plasma membrane (Figure 1). All positive cells showed strong membranous and cytoplasmic staining. Positive expression of β2-AR was identified in 27% (89/328) of all patients; in 29% (64/222) of adenocarcinoma (AC) patients; and in 24% (25/106) of non-AC patients.
Figure 1.
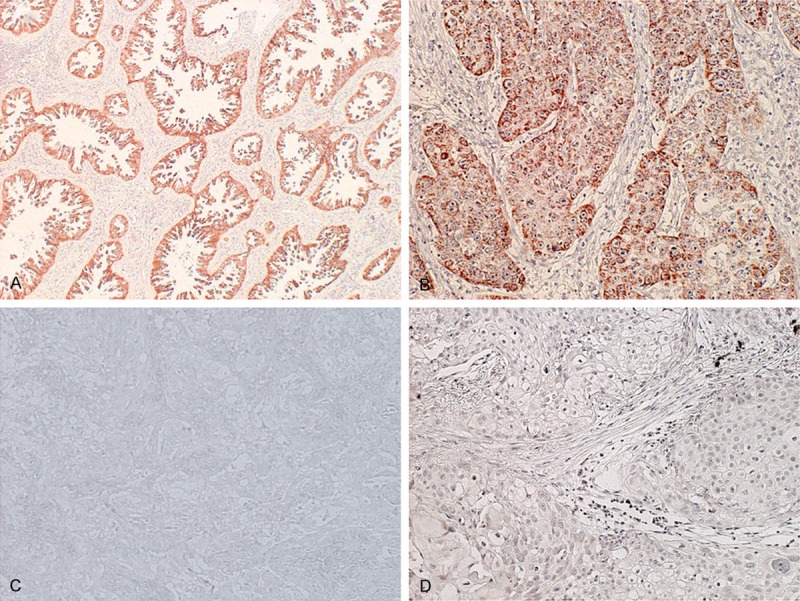
Immunohistochemical staining of tumor tissues. Immunohistochemical staining sections showed positive or negative β2 adrenergic receptor (β2-AR) expression in non-small cell lung cancer. A: Positive β2-AR expression in adenocarcinoma (AC); B: Positive β2-AR in squamous cell carcinoma (SCC); C: Negative β2-AR in AC; D: Negative β2-AR in SCC.
The median number of CD34-positive vessels was 12 (range, 1-45) and was chosen as a cutoff point. The median Ki-67 LI was 14% (range, 1-92), and was, likewise, used as a cutoff point. High expressions of CD34 and Ki-67 LI was detected in 51% (168/328) and 52% (172/328) of all patients, respectively.
Non-AC tumors consisted of squamous cell carcinoma (82%, 87/106), adenosquamous carcinoma (4%, 4/106), large-cell carcinoma (3%, 3/106), and large-cell neuroendocrine carcinoma (11%, 12/106).
Table 1 represents patient characteristics based on the β2-AR expression. In all patients, positive β2-AR expression was significantly associated with disease stage, T factor, N factor, lymphatic permeation, vascular invasion, and Ki-67. This was mirrored in AC patients, where similar association could be seen in between β2-AR expression and almost all of the aforementioned factors (with the exception of vascular invasion). However, no significant association between β2-AR and any of the tested variables was observed in non-AC patients. Furthermore, in stage I AC patients, positive β2-AR expression was found to be significantly associated with pleural invasion, lymphatic permeation, vascular invasion, and Ki-67 expression (Supplementary Table 1).
Table 1.
Patient characteristics according to β2-AR expression
| Variables | All (n = 328) | AC (n = 222) | non-AC (n = 106) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Total (n = 328) | Positive (n = 89) | Negative (n = 239) | P-value | Positive (n = 64) | Negative (n = 158) | P-value | Positive (n = 25) | Negative (n = 81) | P-value | |
| Age | ||||||||||
| <65 years/≥65 years | 96/232 | 23/66 | 73/166 | 0.405 | 17/47 | 59/99 | 0.125 | 6/19 | 14/67 | 0.559 |
| Sex | ||||||||||
| Male/Female | 198/130 | 50/39 | 148/91 | 0.344 | 30/34 | 74/84 | 0.996 | 20/5 | 74/7 | 0.149 |
| Smoking | ||||||||||
| Yes/No | 212/116 | 57/32 | 155/84 | 0.892 | 32/32 | 75/83 | 0.732 | 25/0 | 80/1 | >0.999 |
| p-Stage | ||||||||||
| I/II-III | 221/107 | 52/37 | 169/70 | 0.035 | 40/24 | 124/34 | 0.014 | 12/13 | 45/36 | 0.647 |
| Histology | ||||||||||
| AC/non-AC | 222/106 | 64/25 | 158/81 | 0.318 | - | - | - | - | - | - |
| T factor | ||||||||||
| T1/T2-4 | 151/177 | 33/56 | 118/121 | 0.047 | 28/36 | 92/66 | 0.050 | 5/20 | 26/55 | 0.245 |
| N factor | ||||||||||
| N0/N1-2 | 248/80 | 59/30 | 189/50 | 0.016 | 42/22 | 132/26 | 0.003 | 17/8 | 57/24 | 0.821 |
| Pleural invasion | ||||||||||
| Positive/Negative | 116/212 | 37/52 | 79/160 | 0.151 | 23/41 | 40/118 | 0.112 | 14/11 | 39/42 | 0.492 |
| Lymphatic permeation | ||||||||||
| Positive/Negative | 138/190 | 47/42 | 91/148 | 0.016 | 34/30 | 44/114 | <0.001 | 13/12 | 47/34 | 0.595 |
| Vascular invasion | ||||||||||
| Positive/Negative | 124/204 | 44/45 | 80/159 | 0.008 | 31/33 | 38/120 | <0.001 | 13/12 | 42/39 | 0.990 |
| Ki-67 | ||||||||||
| High/Low | 172/156 | 55/34 | 117/122 | 0.038 | 34/30 | 54/104 | 0.009 | 21/4 | 63/18 | 0.502 |
| CD34 | ||||||||||
| High/Low | 168/160 | 38/51 | 130/109 | 0.060 | 24/40 | 73/85 | 0.236 | 14/11 | 57/24 | 0.182 |
Abbreviations: AC = adenocarcinoma; β2-AR = β2 adrenergic receptor; p-stage = pathological stage. The bold entries show a statistically significant difference.
Correlation between β2-AR and different variables
Using Spearman’s rank correlation, positive β2-AR expression in all patients was significantly correlated with lymphatic permeation, vascular invasion, and cell proliferation, based on Ki-67 expression levels. When assessing tumors based on their histological type, a statistically significant correlation was observed in AC patients, but not in non-AC patients. In stage I AC patients, a significant correlation with pleural invasion was also identified (Table 2).
Table 2.
Spearman’s rank correlation according to β2-AR
| Variables | Total (n = 328) | AC (n = 222) | non-AC (n = 106) | stage I AC (n = 164) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| |r| | P-value | |r| | P-value | |r| | P-value | |r| | P-value | |
| Pleural invasion | 0.079 | 0.152 | 0.107 | 0.113 | 0.067 | 0.497 | 0.183 | 0.019 |
| Lymphatic permeation | 0.133 | 0.016 | 0.240 | <0.001 | 0.052 | 0.599 | 0.211 | 0.007 |
| Vascular invasion | 0.146 | 0.008 | 0.239 | <0.001 | 0.001 | 0.990 | 0.270 | <0.001 |
| Ki-67 | 0.114 | 0.038 | 0.175 | 0.009 | 0.065 | 0.507 | 0.267 | 0.001 |
| CD34 | 0.104 | 0.060 | 0.079 | 0.238 | 0.130 | 0.185 | 0.047 | 0.551 |
Abbreviatoins: AC = adenocarcinoma; β2-AR = β2 adrenergic receptor. The bold entries show a statistically significant difference.
Patient mortality
The 5-year survival rates were 67% for all patients, 73% for AC patients, and 54% for non-AC patients. Table 3 shows the results of univariate and multivariate analyses for each group. Figure 2 shows the Kaplan-Meier survival curve for patients displaying either positive or negative β2-AR expression. In all patients, univariate analysis showed that age, sex, smoking history, disease stage, pleural invasion, lymphatic permeation, vascular invasion, β2-AR, Ki-67, and MVD were significant predictors for worse OS and PFS. Sex was also identified as a significant prognostic factor for OS.
Table 3.
Univariate and multivariate analysis of overall survival and progression-free survival
| Overall survival | Progression-free survival | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Variables | Total (n = 328) | AC (n = 222) | Multivariate analysis | non-AC (n = 106) | Total (n = 328) | Multivariate analysis | AC (n = 222) | Multivariate analysis | non-AC (n = 106) | ||||||
|
| |||||||||||||||
| 5-year survival rate (%) | P-value | 5-year survival rate (%) | P-value | HR 95% CI P-value | 5-year survival rate (%) | P-value | 5-year survival rate (%) | P-value | HR 95% CI P-value | 5-year survival rate (%) | P-value | HR 95% CI P-value | 5-year survival rate (%) | P-value | |
| Age | |||||||||||||||
| <65 years/≥65 years | 82/61 | <0.001 | 90/65 | <0.001 | 57/54 | 0.422 | 75/55 | 0.001 | 81/58 | 0.001 | 52/50 | 0.579 | |||
| Sex | |||||||||||||||
| Male/Female | 62/75 | 0.003 | 68/78 | 0.026 | 1.282 | 55/49 | 0.869 | 58/65 | 0.051 | 0.784 | 64/68 | 0.224 | 0.752 | 52/40 | 0.613 |
| 0.593-2.773 | 0.471-1.305 | 0.390-1.451 | |||||||||||||
| 0.527 | 0.349 | 0.395 | |||||||||||||
| Smoking | |||||||||||||||
| Yes/No | 60/79 | <0.001 | 66/80 | 0.005 | 1.605 | 55/0 | 0.012 | 54/74 | <0.001 | 2.195 | 56/74 | 0.004 | 2.215 | 51/0 | 0.013 |
| 0.736-3.501 | 1.244-3.870 | 1.129-4.345 | |||||||||||||
| 0.234 | 0.007 | 0.021 | |||||||||||||
| p-Stage | |||||||||||||||
| I/II-III | 79/44 | <0.001 | 85/44 | <0.001 | 4.113 | 62/44 | 0.017 | 74/33 | <0.001 | 3.685 | 80/30 | <0.001 | 4.370 | 60/38 | 0.001 |
| 2.932-7.701 | 2.568-5.287 | 2.710-7.046 | |||||||||||||
| <0.001 | <0.001 | <0.001 | |||||||||||||
| Pleural invasion | |||||||||||||||
| Positive/Negative | 47/79 | <0.001 | 49/84 | <0.001 | 46/63 | 0.021 | 43/71 | <0.001 | 40/77 | <0.001 | 46/55 | 0.068 | |||
| Lymphatic permeation | |||||||||||||||
| Positive/Negative | 50/80 | <0.001 | 48/87 | <0.001 | 51/58 | 0.432 | 42/75 | <0.001 | 39/81 | <0.001 | 46/56 | 0.216 | |||
| Vascular invasion | |||||||||||||||
| Positive/Negative | 49/78 | <0.001 | 47/86 | <0.001 | 52/56 | 0.274 | 44/72 | <0.001 | 37/79 | <0.001 | 52/50 | 0.479 | |||
| β2-AR | |||||||||||||||
| Positive/Negative | 56/71 | 0.062 | 60/79 | 0.045 | 1.125 | 47/57 | 0.414 | 47/66 | 0.005 | 1.427 | 47/74 | 0.001 | 1.548 | 48/52 | 0.660 |
| 0.651-1.943 | 0.982-2.073 | 0.955-2.508 | |||||||||||||
| 0.674 | 0.062 | 0.076 | |||||||||||||
| Ki-67 | |||||||||||||||
| High/Low | 58/76 | 0.001 | 60/80 | 0.004 | 55/50 | 0.871 | 50/72 | <0.001 | 49/75 | 0.001 | 50/55 | 0.730 | |||
| CD34 | |||||||||||||||
| High/Low | 62/73 | 0.028 | 66/79 | 0.013 | 56/51 | 0.479 | 52/70 | 0.008 | 52/76 | 0.006 | 50/50 | 0.831 | |||
Abbreviations: AC = adenocarcinoma; β2-AR = β2 adrenergic receptor; 95% CI = 95% confidence interval; HR = hazard ratio; p-stage = pathological stage. The bold entries show a statistically significant difference.
Figure 2.
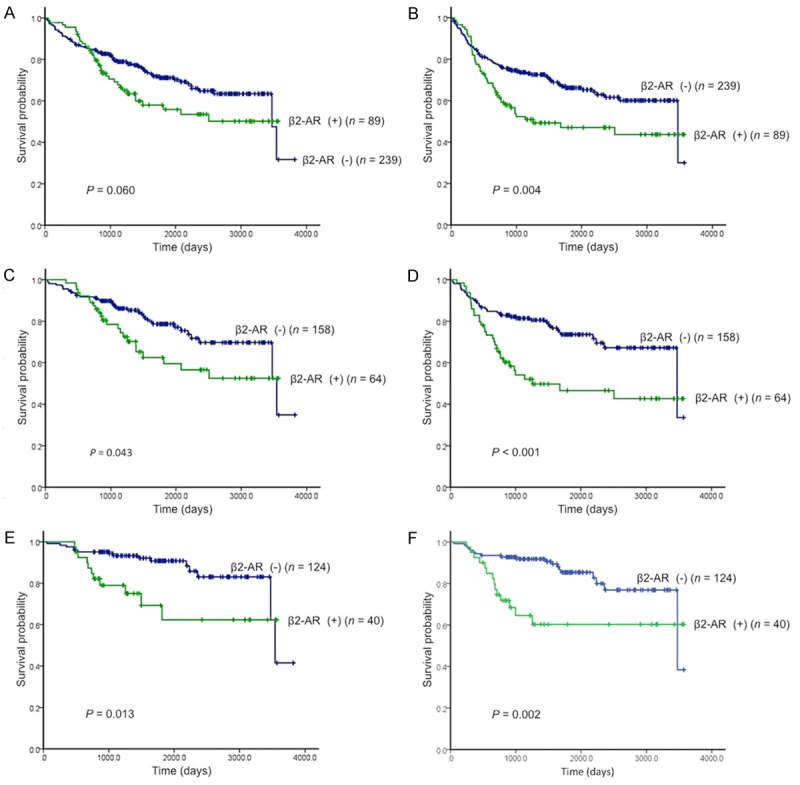
Kaplan-Meier analysis of overall survival (OS) and progression-free survival (PFS) according to β2-adrenergic receptor (β2-AR) expression. There was no statistically difference in OS (A), whereas there was a statistically difference in PFS (B) in NSCLC patients with positive β2-AR expression. A statistically significant difference in OS and PFS was observed in the adenocarcinoma patients with positive β2-AR expression of stage I-III (OS, C; PFS, D); stage I (OS, E; PFS, F), respectively.
Next, a survival analysis of OS and PFS was performed according to the histological type of the tumor. Both AC patients, as well as the overall patient cohort, showed identical tendencies for OS and PFS. However, in non-AC patients, disease stage and pleural invasion were significantly associated with poor OS, while smoking and disease stage were significantly associated with poor PFS.
Next, we examined the relationship between β2-AR and survival in patients with stage I AC. Poor OS was significantly associated with age, sex, smoking, pleural invasion, lymphatic permeation, vascular invasion, β2-AR expression, Ki-67 expression, and MVD. These factors were also associated with poor PFS, as was disease stage (Table 4).
Table 4.
Univariate and multivariate analysis in overall survival and progression-free survival of stage I AC patients
| Variables | Overall survival | Progression-free survival | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Univariate | Multivariate | Univariate | Multivariate | |||
|
| ||||||
| 5-year survival rate (%) | P-value | HR 95% CI P-value | 5-year survival rate (%) | P-value | HR 95% CI P-value | |
| Age | ||||||
| <65 years/≥65 years | 100/77 | <0.001 | 100/69 | 0.012 | ||
| Sex | ||||||
| Male/Female | 76/93 | 0.001 | 3.238 | 77/83 | 0.032 | 1.089 |
| 1.055-9.944 | 0.447-2.651 | |||||
| 0.040 | 0.851 | |||||
| Smoking | ||||||
| Yes/No | 77/92 | 0.003 | 1.669 | 71/87 | 0.003 | 2.371 |
| 0.596-4.678 | 0.924-6.087 | |||||
| 0.330 | 0.073 | |||||
| p-Stage | ||||||
| IA/IB | 92/71 | 0.057 | 1.703 | 88/62 | 0.002 | 2.480 |
| 0.764-3.794 | 1.229-5.005 | |||||
| 0.193 | 0.011 | |||||
| Pleural invasion | ||||||
| Positive/Negative | 59/91 | 0.001 | 50/86 | <0.001 | ||
| Lymphatic permeation | ||||||
| Positive/Negative | 65/90 | 0.001 | 53/86 | <0.001 | ||
| Vascular invasion | ||||||
| Positive/Negative | 45/94 | <0.001 | 46/87 | <0.001 | ||
| β2-AR | ||||||
| Positive/Negative | 62/91 | 0.017 | 1.889 | 60/85 | 0.003 | 2.220 |
| 0.839-4.254 | 1.077-4.573 | |||||
| 0.125 | 0.031 | |||||
| Ki-67 | ||||||
| High/Low | 69/90 | 0.016 | 63/86 | 0.002 | ||
| CD34 | ||||||
| High/Low | 76/90 | 0.016 | 64/88 | 0.004 | ||
Abbreviations: AC = adenocarcinoma; β2-AR = β2 adrenergic receptor; 95% CI = 95% confidence interval; HR = hazard ratio; p-Stage = pathological stage. The bold entries show a statistically significant difference.
Finally, we performed multivariate analyses for each group (Tables 3 and 4) and β2-AR was identified as an independent prognostic marker for predicting a worse PFS in AC patients with stage I disease.
Discussion
This is the first clinicopathological study to evaluate the prognostic significance of β2-AR expression in patients with NSCLC. Our results demonstrate that a positive β2-AR expression is significantly correlated with lymphatic permeation, vascular invasion, and tumor cell proliferation, and is a promising predictor related to poor outcome in patients with AC. This was particularly true for patients with early-stage AC but was not observed in patients with non-AC. Furthermore, multivariate analyses also identified positive β2-AR expression as an independent prognostic factor for predicting poor PFS in patients with stage I AC. Considering our observations, β2-AR expression seems to play an important role in tumor progression and metastasis. Thus, β2-AR could be a potential biomarker for early-stage lung adenocarcinoma. However, the reason for the variation in β2-AR protein expression between AC and non-AC remains unclear. Therefore, further study is warranted to clarify the biological mechanisms underpinning β2-AR expression in histologically varying tumors.
Previous clinicopathological studies have demonstrated an enhanced expression of β2-AR in pancreatic cancer, hepatic cellular carcinoma, and oral squamous cell carcinoma [12,14,22,23]. These studies have indicated a close relationship between the enhanced expression of β2-AR and poor prognosis in pancreatic cancer and hepatic cellular carcinoma, due to a significant correlation between β2-AR and tumor size and vascular invasion. However, some controversy still remains on whether such a correlation exists in oral squamous cell carcinoma. In our study, the expression level of β2-AR was significantly correlated with progression and metastasis in AC patients, but no such relationship was found in non-AC patients. The clinicopathological parameters involved in the varying expression levels of β2-AR in different histological types, such as adenocarcinoma and squamous cell carcinoma, remain unknown. Therefore, further study is needed to investigate β2-AR expression due to possible mechanical difference based on the histological type of tumor.
β2-AR increases vascularization and enhances the expression of vascular endothelial growth factor (VEGF), MMP2, and MMP9 in ovarian carcinoma [11]. In pancreatic cancer, amplification of the β2-AR signaling pathway might augment the expression of EGFR, VEGF, and MMP2 as a result of variable downstream pathways of β2-AR, such as EGFR pathways and angiogenesis, resulting in the promotion of lymph node metastasis [23]. Other studies have indicated that stimulation of β2-AR induces VEGF, MMP2, and MMP9, followed by the promotion of angiogenesis, cell proliferation, and cell adhesion [24,25]. Several in vitro studies have revealed that the β2-AR agonist isoproterenol promotes the growth of human cancer cells in vitro via β2-AR-mediated activation of cAMP/PKA, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1/2 (ERK1/2), and PI3-kinase (PI3 K)/protein kinase B (AKT) signaling pathways. Furthermore, isopreterenol activates MAPK/ERK1/2 through a β2-AR-mediated and vascular endothelial growth factor (VEGF)-independent mechanism [26-29]. Thus, β2-AR has recently received attention as a potential therapeutic target for the treatment of cancer.
In vitro studies have demonstrated that norepinephrine-induced stimulation of cell proliferation and migration is significantly inhibited by propranolol, a non-cardioselective β2-AR antagonist, in human prostate, colorectal, ovarian, and breast cancer cell lines [30-33]. In addition, propranolol has been shown to suppress prostate cancer cell growth in a nude mouse xenograft model [34]. Moreover, a recent experimental study using melanoma cells has documented that propranolol inhibits cell proliferation and induces apoptosis as a result of regulating the expression of different genes involved in tumor angiogenesis, cell death, or proliferation, such as TP53, Akt3, HIF1a, and PiK3R5. It is intriguing, however, that metoprolol, which is a cardioselective β-blocker, hardly affects cell survival or proliferation [35]. Furthermore, other experimental studies have shown that, after encouraging their proliferation, lung cancer cell lines produced noradrenaline, and propranolol inhibited their growth due to restricting the activity of p-CREB and p-ERK1/2 downstream signaling pathways [36]. Other in vivo studies have revealed that β-blockers could augment the effectiveness of chemotherapy in certain conditions [37].
Clinical reports have shown that the β-blocker propranolol, but not atenolol, which is a β1-adrenergic receptor-selective antagonist, improves the outcome for breast cancer patients by reducing metastasis, recurrence, and mortality. Reports also indicate that angiotensin receptor blockers and angiotensin converting enzyme inhibitors do not affect patient prognosis [18,19]. On the other hand, it has been reported that β-blocker usage did not affect prognosis in breast and prostate cancer patients [38,39]. However, in those studies, the effect of β2-AR expression on patient prognosis was not adequately examined. For example, it has been demonstrated that strong β2-AR expression in breast cancer patients can be an indicator good early prognosis due to effects of tamoxifen, an estrogen antagonist, but after 60 months, this trend is reversed [40]. Therefore, it is also important to evaluate the β2-AR expression in lung adenocarcinoma patients.
Although adjuvant chemotherapy including cisplatin-based combination regimens after surgery is the current standard of care based on the results of phase III trials in patients with stage II/III NSCLCs, the use of adjuvant chemotherapy with cisplatin-based regimens in AC patients with stage I disease remains controversial [41-43]. Therefore, an investigation of the possible markers capable of determining the candidates for adjuvant therapy is extremely relevant for treating patients with stage I lung adenocarcinoma. In light of our data, propranolol may potentially be used as a novel post-surgery adjuvant therapy for early-stage lung adenocarcinoma patients showing positive β2-AR expression.
However, there were several limitations to our study. First, we used a single-center cohort, which may have biased our results. Second, it remains unclear what precisely is the optimal cutoff point for the expression level of β2-AR. Further study is warranted to investigate and identify the appropriate cutoff level for β2-AR. Third, the detailed mechanism responsible for β2-AR expression in various neoplasms still remains unclear. To the best of our knowledge, our investigation is the first study to examine the expression of β2-AR in patients with lung cancer. Keeping this in mind, future studies should aim to clarify the clinicopathological problems involved, such as the appropriate cut off level for β2-AR, before β2-AR inhibition would be truly applicable in clinical settings.
In conclusion, the expression of β2-AR is closely related to tumor cell proliferation, angiogenesis, and metastasis, and is a significant independent marker for predicting a worse outcome in patients with AC, particularly those with stage I disease. Our results suggest that β2-AR can play a crucial role in the development of tumor progression and metastasis. Therefore, the inhibition of β2-AR by antagonists such as propranolol may be a future potential target for anticancer therapy in early-stage lung adenocarcinoma.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Spira A, Ettinger DS. Multidiciplinary management of lung cancer. N Engl J Med. 2004;350:379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of prognosis. Chest. 2002;122:1037–1057. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, Saito R, Kawahara M, Maruyama Y. Gender, histology, and time of diagnosis are important factors for prognosis: analysis of 1499 never-smokers with advanced non-small cell lung cancer in Japan. J Thorac Oncol. 2010;5:1011–1017. doi: 10.1097/JTO.0b013e3181dc213e. [DOI] [PubMed] [Google Scholar]
- 5.Kogure Y, Ando M, Saka H, Chiba Y, Yamamoto N, Asami K, Hirashima T, Seto T, Nagase S, Otsuka K, Yanagihara K, Takeda K, Okamoto I, Aoki T, Takayama K, Yamasaki M, Kudoh S, Katakami N, Miyazaki M, Nakagawa K. Histology and smoking status predict survival of patients with advanced non-small-cell lung cancer. Results of West Japan Oncology Group (WJCOG) Study 3906L. J Thorac Oncol. 2013;8:753–758. doi: 10.1097/JTO.0b013e31828b51f5. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura H, Ando K, Shinmyo T, Morita K, Mochizuki A, Kurimoto N, Tatsunami S. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg. 2011;17:469–480. doi: 10.5761/atcs.oa.10.01637. [DOI] [PubMed] [Google Scholar]
- 7.Maki T, Kontula K, Harkonen M. The beta-adrenergic system in man: physiological and pathophysiological response. Regulation of receptor density and functioning. Scand J Clin Lab Invest Suppl. 1990;201:25–43. [PubMed] [Google Scholar]
- 8.Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 9.Entschladen F, Drell TL 4th, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 2004;5:254–258. doi: 10.1016/S1470-2045(04)01431-7. [DOI] [PubMed] [Google Scholar]
- 10.Entschladen F, Drell TL 4th, Lang K, Joseph J, Zaenker KS. Neurotransmitters and chemokines regulate tumor cell migration: potential for a new pharmacological approach to inhibit invasion and metastasis development. Curr Pharm Des. 2005;11:403–411. doi: 10.2174/1381612053382197. [DOI] [PubMed] [Google Scholar]
- 11.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Xing W, Hong J, Wang M, Huang Y, Zhu C, Yuan Y, Zeng W. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2012;19:3556–3565. doi: 10.1245/s10434-012-2396-1. [DOI] [PubMed] [Google Scholar]
- 13.Ramberg H, Eide T, Krobert KA, Levy FO, Dizeyi N, Bjartell AS, Abrahamsson PA, Taskén KA. Hormonal regulation of beta2-adrenergic receptor level in prostate cancer. Prostate. 2008;68:1133–1142. doi: 10.1002/pros.20778. [DOI] [PubMed] [Google Scholar]
- 14.Shang ZJ, Liu K, Liang de F. Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med. 2009;38:371–376. doi: 10.1111/j.1600-0714.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 15.Shi M, Liu D, Duan H, Qian L, Wang L, Niu L, Zhang H, Yong Z, Gong Z, Song L, Yu M, Hu M, Xia Q, Shen B, Guo N. The beta2-adrenergic receptor and Her2 compriise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125:351–362. doi: 10.1007/s10549-010-0822-2. [DOI] [PubMed] [Google Scholar]
- 16.Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, Barsky SH, Glaser R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuller HM. Beta-adrenergic signaling, a novel target for cancer therapy? Oncotarget. 2010;1:466–469. doi: 10.18632/oncotarget.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J. Clin. Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 19.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Yamada M, Oyama T, Takeyoshi I. ASC-amino acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer. 2014;110:2030–2039. doi: 10.1038/bjc.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mountain CF. Revision in the international system for staging lung cancer. Chest. 1997;11:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 22.Bravo-Calderón DM, Oliveira DT, Marana AN, Onogaki S, Carvalho AL, Kowalski LP. Prognostic significance of beta-2 adrenergic receptor in oral squamous cell carcinoma. Cancer Biomark. 2011-2012;10:51–59. doi: 10.3233/CBM-2012-0228. [DOI] [PubMed] [Google Scholar]
- 23.Wenjuan Y, Yujun L, Ceng Y. Association of single nucleotide polymorphism of β2-adrenergic receptor gene with clinicopathological features of pancreatic carcinoma. Acta Histochem. 2013;115:198–203. doi: 10.1016/j.acthis.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Bos JL. Linking rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 26.Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D, Zhang M. Norepinephrine-induced invasion by pancreatic cancer cell is inhibited by propranolol. Oncol Rep. 2009;22:825–830. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Wu WK, Yu L, Li ZJ, Sung JJ, Zhang ST, Cho CH. Epidermal growth factor-induced esophageal cancer cell proliferation requires transactivation of beta-adrenoceptors. J Pharmacol Exp Ther. 2008;326:69–75. doi: 10.1124/jpet.107.134528. [DOI] [PubMed] [Google Scholar]
- 28.Pullar CE, Isseroff RR. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci. 2006;119:592–602. doi: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 29.Yuan A, Li Z, Li X, Yi S, Wang S, Cai Y, Cao H. The mitogenic effectors of isoproterenol in human hepatocellular carcinoma cells. Oncol Rep. 2010;23:151–157. [PubMed] [Google Scholar]
- 30.Drell TL 4th, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 31.Lang K, Drell TL 4th, Lindecke A, Niggemann B, Kaltschmidt C, Zaenker KS, Entschladen F. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer. 2004;112:231–238. doi: 10.1002/ijc.20410. [DOI] [PubMed] [Google Scholar]
- 32.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 33.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palm D, Lang K, Niggemann B, Drell TL 4th, Masur K, Zaenker KS, Entschladen F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 35.Wrobel LJ, Le Gal FA. Inhibition of human melanoma cell growth by a non-cardioselective β-blocker. J Invest Dermatol. 2015;135:525–531. doi: 10.1038/jid.2014.373. [DOI] [PubMed] [Google Scholar]
- 36.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, Hylander BL. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat Commun. 2015;6:6426. doi: 10.1038/ncomms7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardwell CR, Coleman HG, Murray LJ, Entschladen F, Powe DG. Beta-blocker usage and breast cancer survival: a nested case-control study within a UK clinical practice research datalink cohort. Int J Epidemiol. 2013;42:1852–1861. doi: 10.1093/ije/dyt196. [DOI] [PubMed] [Google Scholar]
- 39.Cardwell CR, Coleman HG, Murray LJ, O’Sullivan JM, Powe DG. Beta-blocker usage and prostate cancer survival: a nested case-control study in the UK Clinical Practice Research Datalink cohort. Cancer Epidemiol. 2014;38:279–285. doi: 10.1016/j.canep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Powe DG, Voss MJ, Habashy HO, Zänker KS, Green AR, Ellis IO, Entschladen F. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat. 2011;130:457–463. doi: 10.1007/s10549-011-1371-z. [DOI] [PubMed] [Google Scholar]
- 41.Douillard JY, Tribodet H, Aubert D, Shepherd FA, Rosell R, Ding K, Veillard AS, Seymour L, Le Chevalier T, Spiro S, Stephens R, Pignon JP LACE Collaborative Group. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5:220–228. doi: 10.1097/JTO.0b013e3181c814e7. [DOI] [PubMed] [Google Scholar]
- 42.NSCLC Meta-analyses Collaborative Group. Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, Le Pechoux C, Parmar MK, Pignon JP, Souhami RL, Stephens RJ, Stewart LA, Tierney JF, Tribodet H, van Meerbeeck J. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le Chevalier T LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


