Abstract
CarboxypeptidaseA4 (CPA4) is a zinc-containing exopeptidases, and its aberrant expression has been implicated in cancer development and progression. However, few studies have investigated the association between CPA4 over-expression and clinical significance in gastric cancer (GC). In this study, we employed immunohistochemistry to evaluate the expression of CPA4 in gastric cancer tissues, and found that elevated CPA4 expression was detected in 64% (n=100) of primary GCs, but was weak or no staining in the normal mucosa. Clinical relevance analysis showed that positive staining for CPA4 was significantly associated with Tumor size, Stage, Lymph node metastasis, Depth of invasion and Distant metastasis. As tumor markers p53 and Ki67 are closely associated with tumor progression, we further analyzed the correlations between CPA4 levels and these two factors. We found that abnormal expression of CPA4 was positively associated with Ki67 (P=0.002) and reversely correlated with p53 (P=0.035) in GC. In Kaplan-Meier survival analysis, high levels of CPA4 were significantly associated with unfavorable survival in GC patients (P<0.001). Multivariate Cox regression model showed that high expression of CPA4 was an independent prognostic factor for GC patients. In conclusion, our results suggested that CPA4 was highly expressed in gastric cancer tissues. Overexpression of CPA4 can be used as an independent poor prognostic factor in gastric cancer.
Keywords: CPA4, gastric cancer, marker, prognosis
Introduction
Gastric cancer is the second leading cause of cancer-related death worldwide [1]. Although great improvements have been made in the treatment of gastric cancer, 5-year survival rates have remained very low due to the tendency of early invasion and metastasis [2]. Therefore, it is necessary to identify the key modulators of gastric cancer progression, which would be potential therapeutic targets for gastric cancer therapy.
CarboxypeptidaseA4 (CPA4) belongs to a family of Zn-containing metallocarboxypeptidases specific to catalyze the release of carboxy-terminal amino acids. CPA4 was speculated to be involved in creating tumor microenvironment to facilitate cancer progression. It has been reported that CPA4 may be a strong candidate gene for prostate cancer aggressiveness [3]. Our previous studies also indicated that CPA4 was significantly overexpressed in pancreatic cancer tissues and serum samples, which was closely associated with tumor progression and poor prognosis [4]. However, the expression of CPA4 in gastric cancer tissues and its clinical significance still remains unclear.
In this study, we evaluated the expression of CPA4 in gastric cancer tissues by IHC analysis, and investigated the possible association of CPA4 expression with clinicopathologic parameters.
Materials and methods
Tissue microarray and immunohistochemistry
The commercial tissue microarrays were constructed by Shanghai Biochip Co. Ltd., as described previously [5]. Briefly, the tissue microarrays including 100 gastric cancer patients and 80 adjacent normal tissues were prepared from archival formalin-fixed, paraffin embedded tissue blocks. A representative tumor area was carefully selected from a H&E-stain section. For all the specimens, clinicopathological information (age, gender, and pathology, differentiation, and TNM stage) and Follow-up information were available. Standard Avidin-biotin complex peroxidase immunohistochemical staining was performed. Briefly, after deparaffinizationin xylene and graded alcohols, heated antigen retrieval was done in citrate buffer (10 mmol/L pH 6.0) by water-bath kettle heating for 30 min. Endogenous peroxidase was blocked in 0.3% hydrogen peroxide for 10 min. Nonspecific binding was blocked by incubation in 10% normal animal serum for 10 min. Sections were incubated at 4°C for 24 h with primary antibodies including polyclonal antibody against anti-CPA4 (HPA021030, Sigma-Aldrich), Anti-p53 antibody (ab28, Abcam) and anti-Ki67 (ab833, Abcam). Next, biotinylated secondary antibodies and horseradish peroxidase labeled avidin were incubated with samples. Color was developed using the DAB method.
Immunostaining analysis
Slides were independently evaluated by 2 two pathologists who were blinded to patients’ clinical data. The levels of CPA4, P53 and Ki67 were scored by staining intensity and the percentage of immunoreactive cancer cells. Staining intensity was arbitrarily scored on a scale of four grades: 0 (no staining of cancer cells), 1 (weak staining), 2 (moderate staining), and 3 (strong staining), and the percentage of positive cells was scored as follows: 0 (0%), 1 (1% to 30%), 2 (31% to 50%), and 3 (>50%). The staining positivity was determined using the following formula: overall score=positive percentage score x intensity score. For CPA4, a score of 0 to ≤2 was defined as “0”, and >2 as “1”. For Ki67 or p53, a score of 0 to ≤1 was defined as “0”, and >1 as “1”.
Statistical analysis
The SPSS 15 software package (SPSS, Inc., Chicago, IL) was used for statistical analysis. The association between the immunoreactive markers and clinicopathologic features was analyzed using χ2-test or two-sided t-test as appropriate. The survival rates were assessed by the Kaplan-Meier method and compared by the log-rank test. To investigate the prognostic significance, we included all variables having P<0.05 in multivariate survival analysis using a Cox regression model. Spearman’s rank correlation coefficient and Fisher’s exact test were used to explore the association among CPA4, p53 and Ki67 expression. All comparisons were two-tailed, and P<0.05 was considered significant.
Results
Expression of CPA4 in primary GC tissues
The levels of CPA4 in GC and adjacent normal tissues were examined by immunohistochemical analysis. As showed in the Figure 1A, the levels of CPA4 were evaluated in 64% (64/100) GC samples, but found weak or no staining of CPA4 in normal gastric mucosa. Furthermore, we also found that the CPA4 was highly expressed at the invasive edge of GC tissues. Statistical analysis also indicated that high levels of CPA4 was significantly associated with Tumor size (P=0.029), Stage (P=0.024), Lymph node metastasis (P=0.022), Depth of invasion (P=0.017) and Distant metastasis (P=0.027). While there was no significant correlation between CPA4 expression and patient age, gender and grade (Table 1).
Figure 1.
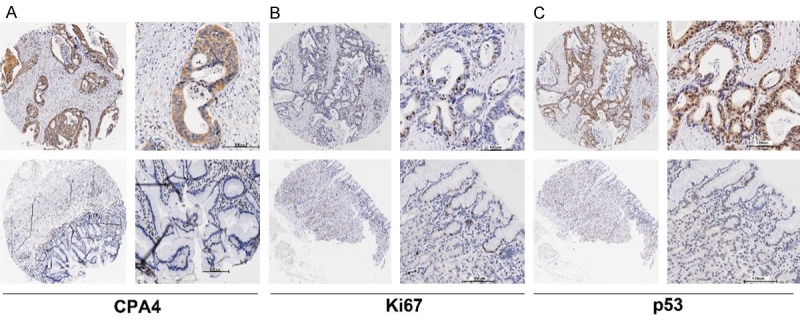
CPA4, Ki67 and p53 expression in gastric cancer tissues were determined by immunochemistry. A. The expression of CPA4 in gastric cancer tissue and corresponding normal gastric tissue. B. The expression of Ki67 in gastric cancer tissue and corresponding normal gastric tissue. C. The expression of p53 in gastric cancer tissue and corresponding normal gastric tissue.
Table 1.
Correlation between CPA4 expression and clinicopathological characteristics in 100 gastric cancer cases
| CPA4 | |||
|---|---|---|---|
|
| |||
| Negative | Positive | P-value | |
| Gender (Male: Female) | 25:11 | 39:25 | 0.395 |
| Age | 63.74 ± 9.027 | 64.84 ± 10.941 | 0.161 |
| Tumor size (cm) | 0.029 | ||
| <7 cm3 | 30 | 40 | |
| >7 cm3 | 6 | 24 | |
| Grade | 0.192 | ||
| 1+2+3 | 34 | 55 | |
| 4 | 2 | 9 | |
| Depth of invasion | 0.017 | ||
| T1 | 6 | 2 | |
| T2+T2+T3 | 30 | 62 | |
| Lymph node involvement | 0.022 | ||
| N0 | 15 | 13 | |
| N1+N2+N3 | 21 | 51 | |
| Stage | 0.024 | ||
| I | 7 | 2 | |
| II | 14 | 22 | |
| III | 15 | 32 | |
| IV | 0 | 7 | |
| M | 0.027 | ||
| M0 | 36 | 56 | |
| M1 | 0 | 8 | |
| Ki67 | 0.000 | ||
| Negative | 28 | 49 | |
| Positive | 8 | 14 | |
| P53 | 0.019 | ||
| Negative | 23 | 40 | |
| Positive | 13 | 24 | |
Correlation between CPA4 and Ki67, p53 in GC
It has reported that Ki67 as well as p53 are particularly important markers for cancer progression [6]. Then, we evaluated their expression in the same tissue array by immunohistochemistry. The results showed that staining of Ki67 and p53 was mainly nucleus-positive, and Ki67 expression was detected in 22% (22/100) GC samples, and positive stain of p53 was found in 37% (37/100) specimens. In addition, we examined the correlations among CPA4 and p53 or Ki67 expression by using Spearman’s rank correlation. The results revealed that aberrant expression of CPA4 was positively associated with Ki67 (P=0.002) and inversely correlated with p53 (P=0.032) in GC (Table 2). Taken together, these observations demonstrated that over-expression of CPA4 was significantly associated with cancer invasion and progression.
Table 2.
Correlations between the Expression of CPA4, Ki67 and p53
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
CPA4 expression was associated with overall survival in GC patients
Based on the association between CPA4 and other cancer marker (ki67 and p53), we analyzed their impact on GC patient’s survival. Using Kaplan-Meier survival analysis, we found that overexpression of CPA4 was significantly correlated with poor overall survival of GC patients (P<0.001), while high levels of ki67 or p53 had no statistically significant association with poor survival (Figure 2). Next, we performed the multivariate survival analysis by using Cox multivariate regression model. The results revealed that CPA4 level (HR=1.965; 95% CI: 1.072-3.600; P=0.029), distant metastasis (HR=3.875; 95% CI: 1.656-9.071; P=0.002) and tumor size (HR=1.842; 95% CI: 1.056-3.215; P=0.031) were statistically independent predictive factors of poorer prognosis for gastric cancer (Table 3).
Figure 2.
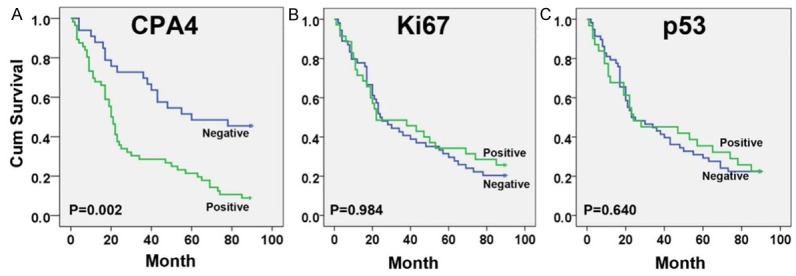
Survival curves for GC using the Kaplan-Meier method and the log-rank test. A. Overall survival curves for patients with negative CPA4 expression (blue line) and patients with positive CPA4 (green line); B. Overall survival curves for patients with negative Ki67 expression (blue line) and patients with positive Ki67 (green line); C. Overall survival curves for patients with negative p53 expression (green line) and patients with positive p53 (blue line).
Table 3.
Multivariate analysis of Cox Proportional Hazards Model for Gastric Cancer
| Characteristics | B | SE | Wald | DF | Sig. | Exp (B) | 95.0% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower | Upper | |||||||
| CPA4 | .675 | .309 | 4.780 | 1 | .029 | 1.965 | 1.072 | 3.600 |
| Grade | .505 | .378 | 1.782 | 1 | .182 | 1.657 | .789 | 3.479 |
| Tumor Size (cm3) | .611 | .284 | 4.628 | 1 | .031 | 1.842 | 1.056 | 3.215 |
| Depth of invasion | 1.254 | 1.045 | 1.440 | 1 | .230 | 3.504 | .452 | 27.177 |
| Lymph node metastasis | -.270 | .422 | .410 | 1 | .522 | .763 | .334 | 1.745 |
| Distant metastasis | 1.355 | .434 | 9.746 | 1 | .002 | 3.875 | 1.656 | 9.071 |
| Stage | .250 | .386 | .419 | 1 | .517 | 1.284 | .603 | 2.733 |
| KI67 level | .160 | .314 | .261 | 1 | .610 | 1.174 | .634 | 2.173 |
| P53 level | -.132 | .267 | .247 | 1 | .619 | .876 | .519 | 1.477 |
Discussion
Gastric cancer (GC) is a clinically aggressive malignancy. Despite advancements in the understanding and treatment of GC, local invasion and distant metastasis still remains the major cause of mortality [7,8]. Therefore, identification of the genes involving in GC progression may contribute to develop novel therapeutic and diagnostic agents.
Carboxypeptidase A4 (CPA4) is a zinc-containing exopeptidase that catalyze the release of carboxy-terminal amino acids [9]. Several studies have indicated that the aberrant expression of CPA4 was highly correlated to GC invasion and progression. In prostate cancer, CPA4 gene is imprinted and may become a strong candidate gene for prostate cancer-aggressiveness [10]. Our previous study also indicated that CPA4 level was significantly elevated in pancreatic cancer tissues as well as serum samples, and was closely associated with tumor progression [4].
In this study, we detected the CPA4 levels in gastric cancer tissues by immunohistochemistry, and studied the correlation between CPA4 and clinical significance. Our studies indicated that CPA4 was specifically overexpressed in gastric cancer tissues with 64% positive rate, but found weak or no staining of CPA4 in normal gastric mucosa. Further analysis revealed that positive staining for CPA4 was significantly associated with Tumor size, Stage, Lymph node metastasis, Depth of invasion and Distant metastasis. To our knowledge, this was the first study showing that CPA4 was highly expressed in gastric cancer tissues, and indicating CPA4 as a poor prognostic marker for gastric cancer patients.
It has reported that tumor markers Ki67 as well as p53 are particularly important markers for cancer progression [6]. Ki67 is a general marker for cancer cell proliferation, and p53 is a tumor suppressor protein that regulates cell apoptosis and genomic stability. Therefore, we evaluated the association between CPA4 expression and Ki67, p53 in GC tissue samples. The results demonstrated that Ki67 and p53 were positive in 22 (22%) and 37 (37%) GC samples, respectively. We found that abnormal expression of CPA4 was positively associated with Ki67 and inversely correlated with p53 in GC. It is possible that CPA4 might enhance cancer progression by inhibiting p53 function. Based on the association between CPA4 and cancer markers (ki67 and p53), we analyzed their impact on GC patient’s survival. Kaplan-Meier survival analysis revealed a correlation between high levels of CPA4 and shorter overall survival times, but the expression of Ki67 or p53 have not found this correlation. Multivariate Cox regression model demonstrated that CPA4 level was a statistically independent predictive factor of poor prognosis for gastric cancer.
In conclusion, our studies for the first time suggested that CPA4 was highly expressed in gastric cancer tissues. Overexpression of CPA4 can be used as an independent poor prognostic factor in gastric cancer.
Acknowledgements
Supported by National High-tech R&D Program of China for Young Scholars (No: 2014AA020537), Beijing Talents Fund (No: 2015000021223ZK23).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Zhou Y. The role of surgery in the treatment of gastric cancer. J Surg Oncol. 2010;101:687–692. doi: 10.1002/jso.21455. [DOI] [PubMed] [Google Scholar]
- 3.Ross PL, Cheng I, Liu X, Cicek MS, Carroll PR, Casey G, Witte JS. Carboxypeptidase 4 gene variants and early-onset intermediate-to-high risk prostate cancer. BMC Cancer. 2009;9:69–69. doi: 10.1186/1471-2407-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Burnett J, Guo C, Xie Y, Pan J, Yang Z, Ran Y, Sun D. CPA4 is a promising diagnostic serum biomarker for pancreatic cancer. Am J Cancer Res. 2016;6:91–96. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Wang J, Ji D, Wang C, Liu R, Wu Z, Liu L, Zhu D, Chang J, Geng R, Xiong L, Fang Q, Li J. Functional Genetic Approach Identifies MET, HER3, IGF1R, INSR Pathways as Determinants of Lapatinib Unresponsiveness in HER2-Positive Gastric Cancer. Clin Cancer Res. 2014;20:4559–4573. doi: 10.1158/1078-0432.CCR-13-3396. [DOI] [PubMed] [Google Scholar]
- 6.Tzanakis NE, Peros G, Karakitsos P, Giannopoulos GA, Efstathiou SP, Rallis G, Tsigris C, Kostakis A, Nikiteas NI. Prognostic significance of p53 and Ki67 proteins expression in Greek gastric cancer patients. Acta Chir Belg. 2009;109:606–611. doi: 10.1080/00015458.2009.11680496. [DOI] [PubMed] [Google Scholar]
- 7.Ma M, Chen S, Zhu BY, Zhao BW, Wang HS, Xiang J, Wu XB, Lin YJ, Zhou ZW, Peng JS, Chen YB. The clinical significance and risk factors of solitary lymph node metastasis in gastric cancer. PLoS One. 2015;10:e0114939. doi: 10.1371/journal.pone.0114939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue L, Chen XL, Lin PP, Xu YW, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Impact of capillary invasion on the prognosis of gastric adenocarcinoma patients: A retrospective cohort study. Oncotarget. 2016;7:31215–25. doi: 10.18632/oncotarget.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanco S, Zhang X, Morano C, Avilés FX, Lorenzo J, Fricker LD. Characterization of the Substrate Specificity of Human Carboxypeptidase A4 and Implications for a Role in Extracellular Peptide Processing. J Biol Chem. 2010;285:18385–18396. doi: 10.1074/jbc.M109.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayashima T, Yamasaki K, Yamada T, Sakai H, Miwa N, Ohta T, Yoshiura K, Matsumoto N, Nakane Y, Kanetake H, Ishino F, Niikawa N, Kishino T. The novel imprinted carboxypeptidase A4 gene ( CPA4) in the 7q32 imprinting domain. Hum Genet. 2003;112:220–226. doi: 10.1007/s00439-002-0891-3. [DOI] [PubMed] [Google Scholar]


