Abstract
Naringin is an active compound extracted from Rhizoma Drynariae, and studies have revealed that naringin can promote proliferation and osteogenic differentiation of bone marrow stromal cells (BMSCs). In this study, we explored whether naringin could promote osteogenic differentiation of BMSCs by upregulating Foxc2 expression via the Indian hedgehog (IHH) signaling pathway. BMSCs were cultured in basal medium, basal medium with naringin, osteogenic induction medium, osteogenic induction medium with naringin and osteogenic induction medium with naringin in the presence of the IHH inhibitor cyclopamine (CPE). We examined cell proliferation by using a WST-8 assay, and differentiation by Alizarin Red S staining (for mineralization) and alkaline phosphatase (ALP) activity. In addition, we detected core-binding factor α1 (Cbfα1), osteocalcin (OCN), bone sialoprotein (BSP), peroxisome proliferation-activated receptor gamma 2 (PPARγ2) and Foxc2 expression by using RT-PCR. We also determined Foxc2 and IHH protein levels by western blotting. Naringin increased the mineralization of BMSCs, as shown by Alizarin red S assays, and induced ALP activity. In addition, naringin significantly increased the mRNA levels of Foxc2, Cbfα1, OCN, and BSP, while decreasing PPARγ2 mRNA levels. Furthermore, the IHH inhibitor CPE inhibited the osteogenesis-potentiating effects of naringin. Naringin increased Foxc2 and stimulated the activation of IHH, as evidenced by increased expression of proteins that were inhibited by CPE. Our findings indicate that naringin promotes osteogenic differentiation of BMSCs by up-regulating Foxc2 expression via the IHH signaling pathway.
Keywords: Naringin, osteogenic differentiation, bone marrow stromal cells (BMSCs), forkhead box C2 (Foxc2), indian hedgehog (IHH), osteoporosis
Introduction
Osteoporosis develops as a result of an imbalance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation [1,2]. It is characterized by bone microarchitectural deterioration and loss of bone mass, with a resultant increase in bone fracture risk, posing a major public health challenge. An acute loss of bone mineral density (BMD) occurs in the perimenopausal period coinciding with a dramatic decline in serum estrogen in perimenopausal women. This and a subsequent more gradual and progressive decline in BMD help explain why the prevalence of primary osteoporosis peaks among postmenopausal women [3-5]. Currently, novel therapeutic options are being pursued to combat osteoporosis, aiming at inhibiting bone resorption by osteoclasts and/or increasing bone formation by osteoblasts [6]. Drugs currently in use for treating osteoporosis, including bisphosphonates, vitamin D analogues, estrogen, calcitonin, and ipriflavone, are all bone absorption-inhibitor drugs that rescue bone mass by inhibiting osteoclast activity. However, they only demonstrate modest effects in increasing or recovering bone mass, which is less than 2% per year [7]. Development of therapeutic agents with an anabolic effect on the osteoporotic bone could provide a new alternative to or complement these agents for treating osteoporosis [3].
BMSCs are noted for their capacity to differentiate into osteoblasts, chondrocytes, adipocytes, myocytes and fibroblasts. Of these, the osteogenic and adipogenic lineages derived from BMSCs are inverse related, such that differentiation towards an osteoblast phenotype accompany with decreasing an adipocytic phenotype [8,9]. An imbalance between osteoblasts and adipocytes is present in osteoporotic bones, especially in age-related osteoporotic bone cases that are accompanied by an increase in the number of adipocytes in the bone marrow [10]. Specific inhibition of bone marrow adipogenesis, and a concomitant enhancement of osteogenesis of BMSCs may provide a novel therapeutic approach for the treatment of osteogenic disorders, such as osteoporosis.
Naringin is an active compound extracted from the Chinese medicinal herb Rhizoma Drynariae [11]. Naringin possesses many pharmacological properties, such as the promotion of human mesenchymal stem cell differentiation [11,12] and upregulation of bone morphogenetic protein (BMP) in osteoblasts. The latter involves, at least partially, phosphoinositide 3-kinase (PI3K), Akt, c-Fos/c-Jun and the AP-1-dependent signaling pathway [13]. However, the underlying signaling pathway(s) whereby naringin promotes proliferation and osteogenic differentiation of BMSCs remains uncertain.
Forkhead box C2/mesenchymal forkhead-1 (Foxc2/MFH-1) is a member of the family of winged helix/forkhead transcription factors [14]. Foxc2 is expressed during early skeletal cell condensation and in C1 mesodermal cells [15]. Foxc2 also stimulates osteoblast differentiation of mesenchymal cells and preosteoblasts by activating the canonical Wnt/β-catenin signaling pathway [16]. Park et al. found that Foxc2 plays an important role in osteoblastogenesis by promoting osteoblast proliferation, survival and differentiation through up-regulation of integrin β1 in response to bone formation stimuli [17]. Moreover, Foxc2 over-expression in BMSCs stimulates osteogenic differentiation and inhibits adipogenic differentiation [18].
The Indian hedgehog (IHH) protein, a member of the hedgehog family, plays an important role in the regulation of tissue patterning, skeletogenesis, and cellular proliferation [19,20]. Mice deficient in the IHH gene show impaired proliferation and maturation of chondrocytes, as well as failure of osteoblast development in endochondral bones [21,22]. Loss of IHH leads to diminished ossification of the secondary hard palate [23]. In addition, IHH regulates osteoblast differentiation of mesenchymal cells through Gli2-mediated up-regulation of Runx2 expression and function [24]. We hypothesized that naringin promotes the differentiation of the osteoblastic lineage of BMSCs by up-regulating Foxc2 expression via the IHH signaling pathway.
Materials and methods
Chemicals and reagents
Naringin (chemical purity ≥98.0%) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in dimethyl sulfoxide (DMSO). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Shanghai, China). Culture flasks and plates were purchased from Corning (Lowell, MA, USA). The ALP colorimetric assay kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China). The Bradford Protein Assay Kit was purchased from Generay Biotech. Co., Ltd (Shanghai, China). Alizarin Red S, cetylpyridinium chloride, β-glycerophosphate, ascorbic acid, and dexamethasone were all purchased from Sigma-Aldrich (St. Louis, MO).
Isolation and culture of rat BMSCs
All animal protocols were in accord with the Guidelines of the Animal Care and Use Committee of Shantou University Medical College. BMSCs were isolated from 4- to 6-week-old female Sprague-Dawley rats (Experimental Animal Center, Shantou University Medical College, Shantou, China) as previously reported [25]. The femurs were dissected, the ends of the bones were cut, and the marrow was flushed out with 5 mL DMEM containing 10% (v/v) FBS by using a 10 mL syringe. A single bone marrow cell suspension was obtained by repeated aspirations and blowing of the dropper. Cells were seeded into a 60-mm Petri dish at a density of 1×106 cells/mL, and cultured in DMEM supplemented with 10% (v/v) FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (basal medium, as control), and cultured in a humidified incubator at 37°C, with 5% CO2 and 95% air. Non-adherent cells were removed and the medium was refreshed three or four times a week. Cells at passages three to six were used for experiments.
CCK-8 assays
Cells were inoculated into 96 well plates, at a density of 1000 cells per well, and treated with basal medium containing 1, 10, 50, or 100 μg/mL naringin, and cell proliferation was determined nine days post treatment using the CCK-8 assay according to the manufacturer’s instructions (Xingzhi Biotechnology Co., Ltd, Guangdong, China). Briefly, cells were incubated basal medium containing 10% (v/v) CCK-8 for 1 hour. Absorbance was recorded at 450 nm using a microplate reader (Thermo Scientific, Shanghai, China). Proliferation of BMSCs was presented as the optical density (OD) value.
Osteogenic differentiation and cell treatments
For osteogenic differentiation, BMSCs were inoculated at approximately 1×104 cells/cm2 into 12-well plates and induced in an osteogenic induction medium (OIM, 0.1 μM dexamethasone, 50 μM ascorbic acid, and 10 mM β-glycerophosphate) containing 0, 1, 10, or 50 μg/mL naringin. BMSCs were grown under 5% CO2 at 37°C in a humidified atmosphere. Osteogenic differentiation was evaluated by measuring alkaline phosphatase (ALP) activity and mineralization assays. Furthermore, BMSCs in OIM were treated with 50 μg/mL naringin in the presence of the IHH inhibitor CPE (0.5 μM).
ALP activity determination
Intracellular ALP assays were performed in quadruplicate on days 1, 3, 5, 7, and 9 post naringin treatment, as depicted previously [26]. Absorbance was measured at 520 nm using a microplate reader (Thermo Scientific, Shanghai, China). Total protein concentrations were determined by the Bradford method, and ALP activity was normalized against total protein.
Alizarin Red S staining
At day 21 post naringin treatment, cells were fixed with ice-cold 70% ethanol (v/v) for 10 min and then stained with 40 mM Alizarin Red S in deionized water (pH 4.2) for 10 min at room temperature. After rinses with distilled water and drying at room temperature, cells were digitally photographed under a microscope. Calcium deposits were evaluated using the cetylpyridinium chloride method. Stained cultures were photographed, followed by a quantitative analysis using 10% cetylpyridinium chloride. Alizarin Red S concentrations were calculated through comparison with an Alizarin Red S dye standard curve and presented as nmol/mL [27].
Real-time quantitative PCR
Total cellular RNA was isolated from BMSCs using Trizol reagent (Invitrogen, CA, USA) Total RNA was then converted to cDNA synthesis using a PrimeScriptTM RT reagent Kit (TaKaRa, Dalian, China). Real-Time quantitative PCR was then performed using the SYBR@ PreMix Ex TaqTM (TaKaRa) on the CFX96TM Real-time PCR Detection System (Applied Biosystems). The PCR conditions and the sequences of the primers are listed in Table 1 for the genes encoding the following proteins: rat ALP, BSP, Cbfα1, Foxc2, PPARγ2, and β-actin. Gene expression was calculated using the 2-ΔΔCt method, and β-actin was used as an internal control to normalize the mRNA levels. PCR was performed at 95°C for 30 s, followed by 40 cycles of 5 s at 95°C, and 30 s at 56°C. At least three independent experiments for each reaction were performed in triplicate.
Table 1.
PCR primer sequences for real-time PCR
| Gene and GenBank accession No. | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| OCN (NM_001137) | 5’-TGAGGACCCTCTCTCTGCTC-3’ | 5’-GGTAGCGCCGGAGTCTATTC-3’ |
| BSP (NM_012587) | 5’-GCCACACTCTCAGGGGTAAC-3’ | 5’-CCTTGCCCTCTGCATCTCC-3’ |
| Cbfα1 (NM_00127) | 5’-AACCACAGAACCACAAGTGC-3’ | 5’-TCACTGACTCGGTTGGTCTC-3’ |
| FoxC2 (NM_001101) | 5’-AAGGTAGCTACTGGACGCTC-3’ | 5’-TCCTCCTTATCCTTGGGCAC-3’ |
| PPARγ2 (NM_013124) | 5’-ATCCCGTTCACAAGAGCTGA-3’ | 5’-GCAGGCTCTACTTTGATCGC-3’ |
| β-Actin (NM_001307) | 5’-ATCGTGGGCCGCCCTAGGCA-3’ | 5’-TGGCCTTAGGGTTCAGAGGGG-3’ |
Western blotting assays
Cells were harvested by scraping in ice-cold RIPA lysis buffer (Boster, Wuhan, China). Western blotting was performed as described previously [28] and blots were probed with anti-Foxc2 antibodies (Proteintech) and anti-IHH antibodies (Abcam, UK). Protein bands were detected using an enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL). The intensity of the bands was quantified by Quantity One Software v4.62 (Bio-Rad, Hercules, CA). As an internal control, GAPDH was used to normalize the protein levels.
Statistical analyses
Experiments were performed in triplicate and repeated more than three times independently. Data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL), and were presented as mean ± standard deviation (SD). All data were analyzed for statistically significant differences by using Student’s t test (two-tailed). In this study, a probability value (P) <0.05 was regarded as statistically significant.
Results
Naringin promotes the proliferation and mineralization of BMSCs
Primary BMSCs were isolated from the femora and tibia of rats and cultured under basal conditions. We first investigated the effects of naringin on proliferation of BMSCs in vitro. Our CCK-8 assays showed that 1, 10, and 50 μg/mL naringin caused a dose and time-dependent increase in the proliferation of BMSCs. At 50 μg/mL, naringin caused a statistically significant increase in the growth of BMSCs on days 5 to 9 post treatment, compared to controls (P<0.01 or 0.05). Naringin at a higher dose (100 μg/mL), by contrast, markedly depressed the proliferation of BMSCs (Figure 1). ALP assays revealed that 1 to 50 μg/mL naringin caused a significant dose- and time-dependent increase in ALP activity (P<0.01) (Figure 2A). Furthermore, we found that naringin resulted in a statistically significant dose-dependent increase in mineralization, which peaked at 50 μg/mL naringin (Figure 2B and 2C). The above findings indicated that 50 μg/mL naringin was the optimal dose for potentiating osteogenic differentiation and was used for subsequent experiments.
Figure 1.
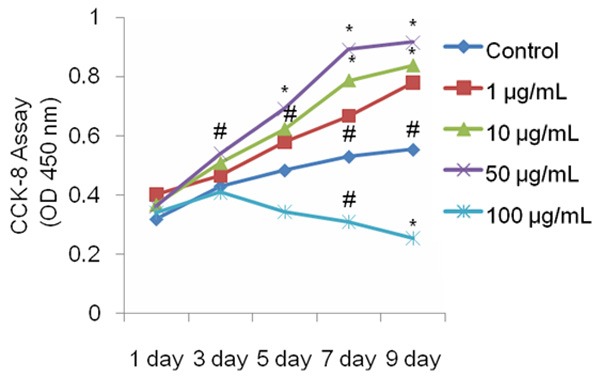
Naringin enhances the proliferation of BMSCs. Naringin promoted the proliferation of BMSCs in a dose-dependent manner. BMSCs were treated for 1-9 days, and the proliferation rate was assessed by CCK-8 assays. The proliferation of BMSCs was enhanced by naringin. Data is expressed as mean ± SD and the experiments were done in quadruplicate (n=4). *P<0.05 versus controls at the same time point; #P<0.05 versus controls at the same time points.
Figure 2.
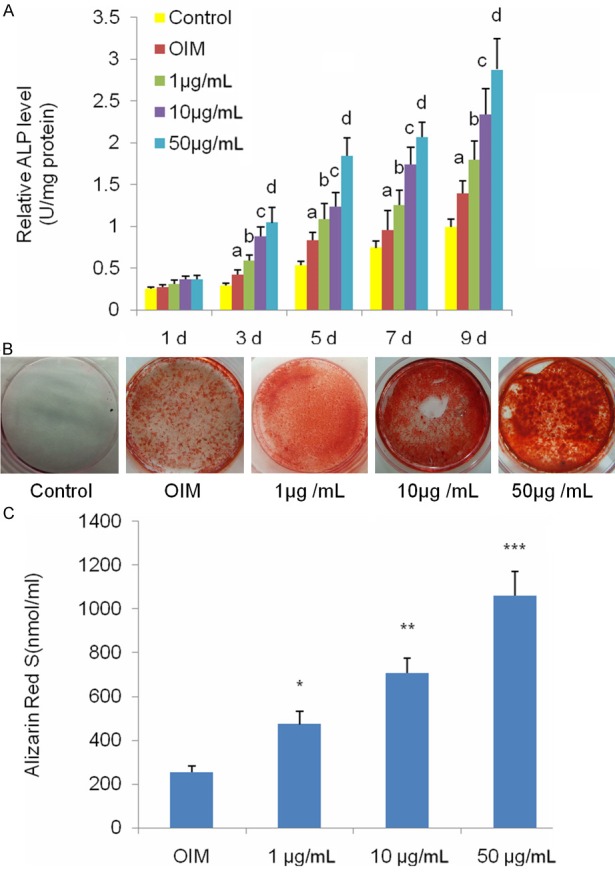
Naringin enhances osteogenic differentiation of BMSCs. A. Naringin enhanced ALP activities of BMSCs. ALP activities were measured by conventional methods and the data represent the mean ± SD of quadruplicate samples (n=4). aP<0.05 versus the control group; bP<0.01 versus the OIM group; cP<0.01 versus the 1 μg/mL group; dP<0.01 versus the 10 μg/mL group. B. Naringin potentiated the mineralization of BMSCs under OIM. BMSCs underwent OIM or OIM with naringin at 1, 10, or 50 μg /mL for 21 days. C. Mineralization of BMSCs was quantified by Alizarin Red S assays. Alizarin Red S showed that naringin enhanced the staining of BMSCs in a dose-dependent manner. Data represents mean ± SD of experiments done in quintuplicate (n=5). *P<0.01 versus the OIM group; **P<0.01 versus the 1 μg/mL group; ***P<0.01 versus the 10 μg/mL group.
Naringin enhances osteogenesis of BMSCs by up-regulating Foxc2 expression via the IHH signaling pathway
The IHH pathway has been shown to be intimately involved in the proliferation and osteogenic differentiation of BMSCs [29]. We investigated whether naringin promoted osteogenesis by up-regulating Foxc2 expression via the IHH signaling pathway. ALP assays showed that CPE significantly inhibited naringin-mediated increase in ALP activity (Figure 3A). We further investigated the effect of naringin on the expression of genes involved in osteogenesis of BMSCs. Real-time quantitative PCR assays showed that, compared to controls or BMSCs under OIM, 50 μg/mL naringin treatment for 14 days caused a significant increase in the mRNA transcript levels for Cbfα1, OCN and BSP, and a noticeable reduction in the mRNA transcript levels for PPARγ2 (Figure 3B). However, CPE effectively abolished the effects of naringin on the mRNA transcript levels of the above genes. We treated BMSCs with IHH inhibitor CPE (0.5 μM), followed by 50 μg/mL naringin for 21 days. Alizarin Red S assays showed that CPE markedly suppressed the naringin-mediated increase in calcium deposition (Figure 3C and 3D).
Figure 3.
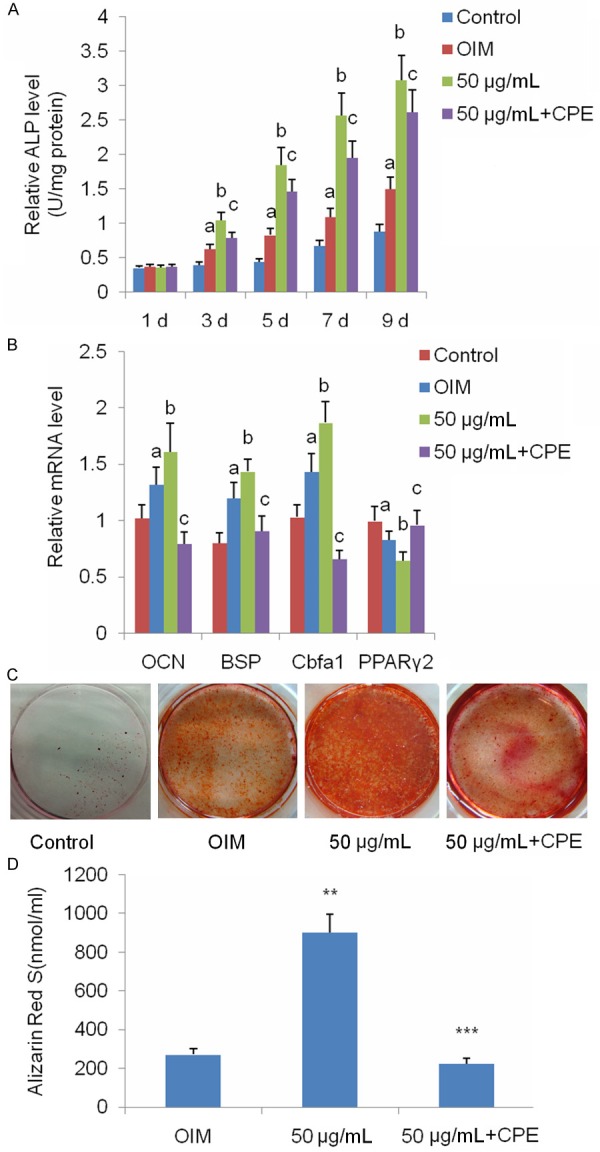
Naringin potentiates osteogenesis of BMSCs via the IHH signaling pathway. A. BMSCs were cultured in basal medium, OIM or OIM with 50 μg/mL naringin in the presence or absence of CPE for 1, 3, 5, 7, and 9 days, then subjected to intracellular ALP assays. Data represent the mean ± SD of five independent experiments (n=5). aP<0.01 versus the control group; bP<0.01 versus the OIM group; cP<0.05 versus the 50 μg/mL group. B. Expression of genes involved in osteogenesis of BMSCs was quantified by real-time quantitative RT-PCR. BMSCs were grown in basal medium, OIM, or OIM with 50 μg/mL naringin in the absence or presence of CPE for 14 days. aP<0.05 versus the control group; bP<0.05 versus the OIM group. cP<0.01 versus the 50 μg/mL group. Alizarin Red S assay of BMSCs cultured in basal medium, OIM or OIM with 50 μg/mL naringin, in the presence or absence of CPE, for 21 days. C. Alizarin Red S assays show that CPE inhibits naringin-potentiated mineralization of BMSCs. D. The amount of calcium deposit was quantified and statistically analyzed (n=5). **P<0.01 versus the OIM group; ***P<0.01 versus the 50 μg/mL group.
We then investigated whether naringin potentiated osteogenesis of BMSCs via the IHH signaling pathway by examining the protein levels of Foxc2 and IHH by immunoblotting assays. We found that Foxc2 expression was up-regulated, and IHH was activated in BMSCs under OIM. Naringin further enhanced the expression of Foxc2 and the activation of IHH in BMSCs (Figure 4A-E). By contrast, CPE significantly attenuated the naringin-induced increase in Foxc2 expression and IHH activation (Figure 4A-E). These results suggest that naringin potentiated osteogenesis of BMSCs by increasing the expression of Foxc2 via activation of the IHH signaling pathway.
Figure 4.
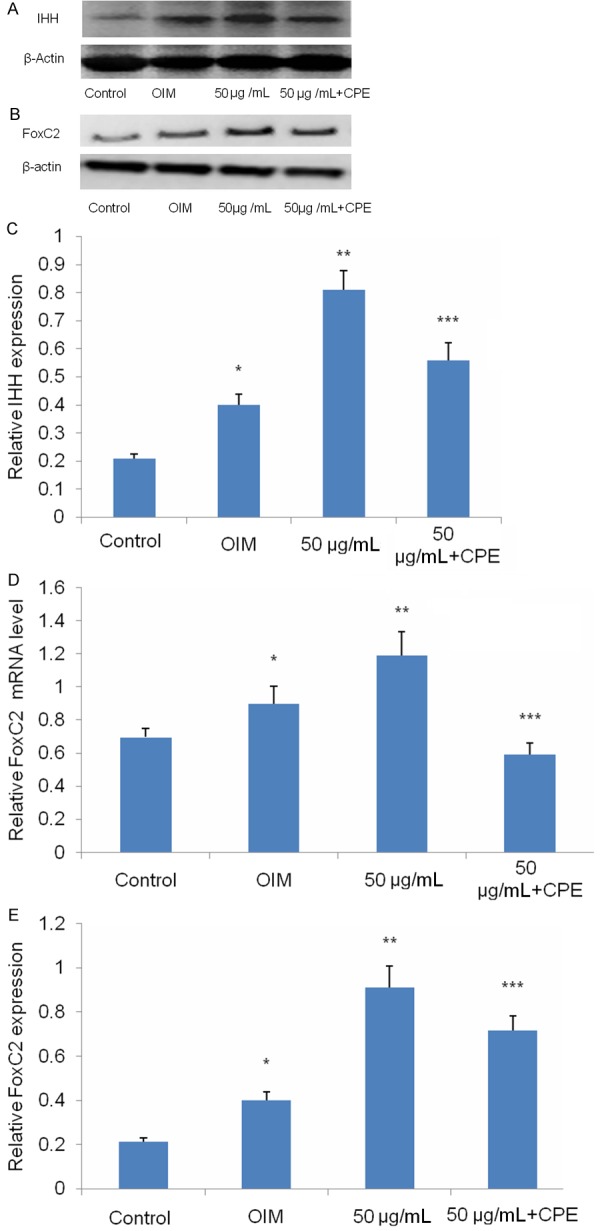
Naringin potentiates osteogenesis of BMSCs through up-regulating Foxc2 expression via the IHH signaling pathway. BMSCs were cultured in basal medium, OIM, OIM with 50 μg/mL naringin, and OIM with 50 μg/mL naringin in the absence or presence of 0.5 μM CPE for 14 days. A, B. Western blotting analysis of Foxc2 and IHH. C. Relative expression of FoxC2 in BMSCs was checked using real time PCR. D, E. Densitometric quantification of activated Foxc2 and IHH was performed. C. *P<0.05 versus the control group; **P<0.01 versus the OIM group; ***P<0.01 versus the 50 μg/mL group. D. *P<0.05 versus the control group; **P<0.01 versus the OIM group; ***P<0.05 versus the 50 μg/mL group.
Discussion
Osteoporosis poses an increasing public health challenge given the rapid aging of the population and the inadequacy of currently available therapies that are hampered by a myriad of side effects. An early study showed naringin, a polymethoxylated flavonoid, improves BMD in a rat model of osteoporosis [30]. Naringin was also found to possess estrogen-like activities and prevent loss of bone mass in ovariectomized mice and rats [31,32]. Subsequent studies demonstrated that naringin stimulates osteogenic differentiation of bone marrow mesenchymal stem cells [12]. Consistently, in this study, we have found that naringin enhances osteogenesis of BMSCs in vitro. Fan et al. have recently found that naringin potentiates osteogenic differentiation by upregulating microRNA 20a and downregulating PPARγ in BMSCs [33]. On the other hand, Wang et al. have demonstrated that naringin facilitates bone formation in ovariectomized mice by stimulating the canonical Wnt/β-catenin signaling pathway by activated Akt and AMPK signaling [34]. Foxc2 also stimulates osteoblast differentiation of mesenchymal cells by activating the canonical Wnt/β-catenin signaling pathway [16]. Here, we demonstrate that naringin increases mRNA transcript and protein levels of Foxc2 in BMSCs undergoing osteogenic differentiation. More importantly, inhibition of IHH signaling by CPE significantly attenuates naringin-induced increase in Foxc2, suggesting an intimate link between Foxc2 and IHH signaling in naringin-potentiated osteogenic differentiation of BMSCs. The mechanisms whereby naringin promotes osteogenesis of BMSCs have hitherto remained largely undefined. Our study provides the first direct piece of experimental evidence that naringin-potentiated osteogenesis may involve Foxc2 and IHH signaling.
Osteoblast commitment and differentiation are controlled by complex activities involving signal transduction and transcriptional regulation of gene expression [35]. Cbfα1 (Runx2) is considered as an early marker of osteogenic differentiation, whereas OCN and BSP are mid and late markers [36,37]. In the present study, we found that naringin markedly increased the mRNA transcript levels of Cbfα1. Furthermore, we found that inhibition of IHH signaling by CPE suppressed Cbfα1 expression. This is consistent with findings by Shimoyama et al., who found that IHH regulates osteoblast differentiation of mesenchymal cells by upregulating Runx2 via Gli2 [24]. PPARγ2 is a critical regulator of the balance of differentiation between adipogenesis and osteogenesis [38]. We found that naringin-potentiated osteogenic differentiation of BMSCs is associated with marked downregulation of PPARγ2 in BMSCs, which is in accordance with a recent study by Fan et al., who demonstrated that naringin-potentiated osteogenic differentiation is associated with downregulation of PPARγ in BMSCs [33]. These data indicate that naringin-potentiated osteogenic differentiation is associated with changes in a myriad of osteogenesis-associated genes. Similar to our findings, FoxC2 activation has been associated with stimulating the expression of osteogenesis-associated genes and inhibiting the expression of PPARγ2 in BMSCs [18]. These results suggest that naringin may exert its effect on osteogenesis-associated genes by indirectly regulating FoxC2 expression.
The IHH signaling pathway is important for osteogenesis [24], and IHH inhibitor CPE significantly inhibits osteogenic differentiation of BMSCs [39]. In the present study, we show that CPE attenuates the naringin-induced increase in osteogenic differentiation-associated genes, including genes encoding OCN, BSP and Foxc2. This indicates that the effects of naringin on osteogenesis-associated genes could occur through activation of the IHH signaling pathway.
In conclusion, our study demonstrates that naringin promotes osteogenic differentiation of BMSCs in vitro by activating osteogenesis-associated genes, and is partly mediated by up-regulating Foxc2 expression via the IHH signaling pathway. Our findings shed light on the mechanisms whereby naringin potentiates osteogenesis of BMSCs. These findings indicate that naringin could be of value for treating osteogenic diseases such as osteoporosis.
Acknowledgements
The study is supported by the National Natural Science Foundation of China (No. 81341103), the Administration of Traditional Chinese Medicine of Guangdong Province, China (No. 20131248 and 20142084), the Natural Science Foundation of Guangdong Province, China (No. 2014A030313467) and the Department of Education, Guangdong Government under the Top-tier University Development Scheme for Research and Control of Infectious Diseases (2015092, 2016011). All research was completed at the Laboratory of Molecular Cardiology, First Affiliated Hospital of Shantou University Medical College. We gratefully acknowledge the support of Xin Zhang, Li-Biao Wu, and Bo-Zhi Cai for their helpful advice and collaboration. The authors declare that they have no conflict of interest.
Disclosure of conflict of interest
None.
References
- 1.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P, Busse B, Alliston T, Ager JW 3rd, Ritchie RO. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci U S A. 2011;108:14416–14421. doi: 10.1073/pnas.1107966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulder JE, Kolatkar NS, LeBoff MS. Drug insight: Existing and emerging therapies for osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:670–680. doi: 10.1038/ncpendmet0325. [DOI] [PubMed] [Google Scholar]
- 4.Deal C. Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol. 2009;5:20–27. doi: 10.1038/ncprheum0977. [DOI] [PubMed] [Google Scholar]
- 5.Sowers MR, Zheng H, Jannausch ML, McConnell D, Nan B, Harlow S, Randolph JF Jr. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010;95:2155–2162. doi: 10.1210/jc.2009-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng S, Zhang G, He Y, Wang X, Leung P, Leung K, Qin L. Epimedium-derived flavonoids promote osteoblastogenesis and suppress adipogenesis in bone marrow stromal cells while exerting an anabolic effect on osteoporotic bone. Bone. 2009;45:534–544. doi: 10.1016/j.bone.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 8.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh N, Viswanathan MB, Saraswathy A, Balakrishna K, Brindha P, Lakshmanaperumalsamy P. Phytochemical and antimicrobial studies on Drynaria quercifolia. Fitoterapia. 2001;72:934–936. doi: 10.1016/s0367-326x(01)00342-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, Dai KR, Yan SG, Yan WQ, Zhang C, Chen DQ, Xu B, Xu ZW. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 2009;607:1–5. doi: 10.1016/j.ejphar.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Wu JB, Fong YC, Tsai HY, Chen YF, Tsuzuki M, Tang CH. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur J Pharmacol. 2008;588:333–341. doi: 10.1016/j.ejphar.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 15.Nifuji A, Miura N, Kato N, Kellermann O, Noda M. Bone morphogenetic protein regulation of forkhead/winged helix transcription factor Foxc2 (Mfh1) in a murine mesodermal cell line C1 and in skeletal precursor cells. J Bone Miner Res. 2001;16:1765–1771. doi: 10.1359/jbmr.2001.16.10.1765. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Cho KW, Choi HS, Park SJ, Rhee Y, Jung HS, Lim SK. The forkhead transcription factor Foxc2 stimulates osteoblast differentiation. Biochem Biophys Res Commun. 2009;386:532–536. doi: 10.1016/j.bbrc.2009.06.071. [DOI] [PubMed] [Google Scholar]
- 17.Park SJ, Gadi J, Cho KW, Kim KJ, Kim SH, Jung HS, Lim SK. The forkhead transcription factor Foxc2 promotes osteoblastogenesis via up-regulation of integrin beta1 expression. Bone. 2011;49:428–438. doi: 10.1016/j.bone.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 18.You W, Fan L, Duan D, Tian L, Dang X, Wang C, Wang K. Foxc2 over-expression in bone marrow mesenchymal stem cells stimulates osteogenic differentiation and inhibits adipogenic differentiation. Mol Cell Biochem. 2014;386:125–134. doi: 10.1007/s11010-013-1851-z. [DOI] [PubMed] [Google Scholar]
- 19.Ingham PW. Transducing Hedgehog: the story so far. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 21.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilton MJ, Tu X, Cook J, Hu H, Long F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development. 2005;132:4339–4351. doi: 10.1242/dev.02025. [DOI] [PubMed] [Google Scholar]
- 23.Levi B, James AW, Nelson ER, Brugmann SA, Sorkin M, Manu A, Longaker MT. Role of Indian hedgehog signaling in palatal osteogenesis. Plast Reconstr Surg. 2011;127:1182–1190. doi: 10.1097/PRS.0b013e3182043a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoyama A, Wada M, Ikeda F, Hata K, Matsubara T, Nifuji A, Noda M, Amano K, Yamaguchi A, Nishimura R, Yoneda T. Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol Biol Cell. 2007;18:2411–2418. doi: 10.1091/mbc.E06-08-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lennon DP, Caplan AI. Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1606–1607. doi: 10.1016/j.exphem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Li SH, Guo DZ, Li B, Yin HB, Li JK, Xiang JM, Deng GZ. The stimulatory effect of insulin-like growth factor-1 on the proliferation, differentiation, and mineralisation of osteoblastic cells from Holstein cattle. Vet J. 2009;179:430–436. doi: 10.1016/j.tvjl.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Zou L, Zou X, Chen L, Li H, Mygind T, Kassem M, Bunger C. Effect of hyaluronan on osteogenic differentiation of porcine bone marrow stromal cells in vitro. J Orthop Res. 2008;26:713–720. doi: 10.1002/jor.20539. [DOI] [PubMed] [Google Scholar]
- 28.Allen JL, Cooke ME, Alliston T. ECM stiffness primes the TGFbeta pathway to promote chondrocyte differentiation. Mol Biol Cell. 2012;23:3731–3742. doi: 10.1091/mbc.E12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinert AF, Weissenberger M, Kunz M, Gilbert F, Ghivizzani SC, Gobel S, Jakob F, Noth U, Rudert M. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther. 2012;14:R168. doi: 10.1186/ar3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei M, Yang Z, Li P, Zhang Y, Sse WC. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med. 2007;35:663–667. doi: 10.1142/S0192415X07005156. [DOI] [PubMed] [Google Scholar]
- 31.Pang WY, Wang XL, Mok SK, Lai WP, Chow HK, Leung PC, Yao XS, Wong MS. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br J Pharmacol. 2010;159:1693–1703. doi: 10.1111/j.1476-5381.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Jiang Y, Wooley PH, Xu Z, Yang SY. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J Orthop Sci. 2013;18:478–485. doi: 10.1007/s00776-013-0362-9. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Li J, Fan Q. Naringin promotes differentiation of bone marrow stem cells into osteoblasts by upregulating the expression levels of microRNA-20a and downregulating the expression levels of PPARgamma. Mol Med Rep. 2015;12:4759–4765. doi: 10.3892/mmr.2015.3996. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Ma W, Wang F, Dong J, Wang D, Sun B, Wang B. Stimulation of Wnt/beta-Catenin Signaling to Improve Bone Development by Naringin via Interacting with AMPK and Akt. Cell Physiol Biochem. 2015;36:1563–1576. doi: 10.1159/000430319. [DOI] [PubMed] [Google Scholar]
- 35.Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068–3092. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, Park JH, El-Fiqi A, Kim JH, Yun YR, Jang JH, Han CM, Lee EJ, Kim HW. Biointerface control of electrospun fiber scaffolds for bone regeneration: engineered protein link to mineralized surface. Acta Biomater. 2014;10:2750–2761. doi: 10.1016/j.actbio.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Watt J, Schlezinger JJ. Structurally-diverse, PPARgamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology. 2015;331:66–77. doi: 10.1016/j.tox.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salem O, Wang HT, Alaseem AM, Ciobanu O, Hadjab I, Gawri R, Antoniou J, Mwale F. Naproxen affects osteogenesis of human mesenchymal stem cells via regulation of Indian hedgehog signaling molecules. Arthritis Res Ther. 2014;16:R152. doi: 10.1186/ar4614. [DOI] [PMC free article] [PubMed] [Google Scholar]


