Abstract
BACKGROUNG/OBJECTIVES
The study was performed to investigate the effects and mechanisms of action of high maysin corn silk extract on body weight and fat deposition in experimental animals.
MATERIALS/METHODS
A total of 30 male C57BL/6J mice, 4-weeks-old, were purchased and divided into three groups by weight using a randomized block design. The normal-fat (NF) group received 7% fat (diet weight basis), the high-fat (HF) group received 25% fat and 0.5% cholesterol, and the high-fat corn silk (HFCS) group received high-fat diet and high maysin corn silk extract at 100 mg/kg body weight through daily oral administration. Body weight and body fat were measured, and mRNA expression levels of proteins involved in adipocyte differentiation, fat accumulation, fat synthesis, lipolysis, and fat oxidation in adipose tissue and the liver were measured.
RESULTS
After experimental diet intake for 8 weeks, body weight was significantly lower in the HFCS group compared to the HF group (P < 0.05), and kidney fat and epididymal fat pad weights were significantly lower in the HFCS group compared to the HF group (P < 0.05). In the HFCS group, CCAAT/enhancer binding protein-β, peroxisome proliferator-activated receptor-γ1 (PPAR-γ1), and PPAR-γ2 mRNA expression levels were significantly reduced (P < 0.05) in the epididymal fat pad, whereas cluster of differentiation 36, lipoprotein lipase, acetyl-CoA carboxylase-1, sterol regulatory element binding protein-1c, pyruvate dehydrogenase kinase, isozyme-4, glucose-6-phosphate dehydrogenase, and stearoyl-CoA desaturase-1 mRNA expression levels were significantly decreased in liver and adipose tissues (P < 0.05). In the HFCS group, mRNA expression levels of AMP-activated protein kinase, hormone-sensitive lipase, and carnitine palmitoyltransferase-1 were elevated (P < 0.05).
CONCLUSIONS
It can be concluded that high maysin corn silk extract inhibits expression of genes involved in adipocyte differentiation, fat accumulation, and fat synthesis as well as promotes expression of genes involved in lipolysis and fat oxidation, further inhibiting body fat accumulation and body weight elevation in experimental animals.
Keywords: Corn silk, maysin, body weight, adipocyte differentiation, fat synthesis
INTRODUCTION
Corn silk, which is the stigma of maize, is a soft, silk thread-like waste material that is either green or yellow in color and abundantly distributed worldwide [1,2]. Corn silk has long been used for treatment of cystitis, edema, gout, kidney stones nephritis, prostatitis, and urinary infection in several countries [3,4,5]. In recent studies, corn silk aqueous extract was shown to reduce blood pressure [6], whereas corn silk polysaccharide was shown to elevate gastrointestinal movement by increasing the level of plasma cholecystokinin [7] and anti-tumor activity by increasing immune capability [8]. Corn silk extract has also been shown to have an anti-oxidative effect on lipid peroxidation [9], anti-fungal activity against Aspergillus flavus [10], and anti-inflammatory activity [11,12].
Corn silk contains various components, including protein, vitamins, carbohydrates, Ca, K, Mg, sitosterol, stigmasterol, alkaloids, saponins, tannins, and flavonoids (maysin, methoxymaysin, apimaysin, and luteolin derivatives) [13,14,15]. Among them, maysin contains luteolin attached to disaccharides and is known as a growth inhibitor of corn earworm [16]. Recently, maysin in corn silk has been reported to have physiological activities such as cytotoxicity and radical scavenging activity in tumor cell lines [15,17]. It has also been reported that maysin isolated from corn silk has a neuroprotective effect through anti-oxidative and anti-apoptotic activities [18] and acts as an immunomodulator in murine macrophage RAW 264.7 cells [19].
The WHO defines obesity as the over-accumulation of body fat and has reported that 39% of adults over 18 years are overweight worldwide with a 13% obesity rate in 2014 [20]. In Korea, according to KNHANES, the obesity rate in adults over 19 years was 26.0% in 1998 but increased to 31-32% and has maintained these rates for the past 7 years [21]. Obesity has become an important public health problem and is associated with health conditions such as diabetes, cardiovascular disease, hypertension, cancer, reduced life expectancy, and poor cognition and motor control [22,23,24]. It is now a very important goal in nutritional management to maintain a normal body weight and inhibit excessive fat accumulation.
To reduce accumulation of body fat, adipocyte differentiation in adipose tissue should be suppressed. Inhibition of lipogenesis and elevation of lipolysis in tissues other than adipose tissue are also necessary in order to reduce fat accumulation. Transcription factors related to adipocyte differentiation include CCAAT/enhancer binding protein-α, β (C/EBP-α, β) and peroxisome proliferator-activated receptor- γs(PPAR-γs) [25]. Cluster of differentiation-36 (CD-36) and activating protein-2 (AP-2) are key proteins involved in regulating uptake of fatty acid into adipose tissue [26], and acetyl-CoA carboxylase1 (ACC) and fatty acid synthase (FAS) are key enzymes in fatty acid synthesis [27]. Sterol regulatory element binding protein-1c (SREBP-1c) regulates the expression of enzymes such as FAS, ACC, ATP-citrate lyase (ACL), and malic enzyme (ME), which are involved with triglyceride synthesis [28]. AMP activated protein kinase (AMPK) is thought to inhibit triglyceride synthesis by inhibiting activities of lipogenesis enzymes like FAS as well as transcriptional regulation via SREBP-1c. It is also likely that AMPK promotes fat oxidation by activating enzymes like ACC and carnitine palmitoyltransferase 1(CPT1) [29].
Studies on the anti-obesity effects of corn silk extract have been carried out [30]. However, the effects of corn silk extract on mRNA expression levels of genes related with lipolysis, lipogenesis and triglyceride synthesis are not well known. Thus, this study was performed to investigate the anti-obesity effect and mechanism of high maysin corn silk extract in experimental animals fed high-fat diet.
MATERIALS AND METHODS
Animals and study design
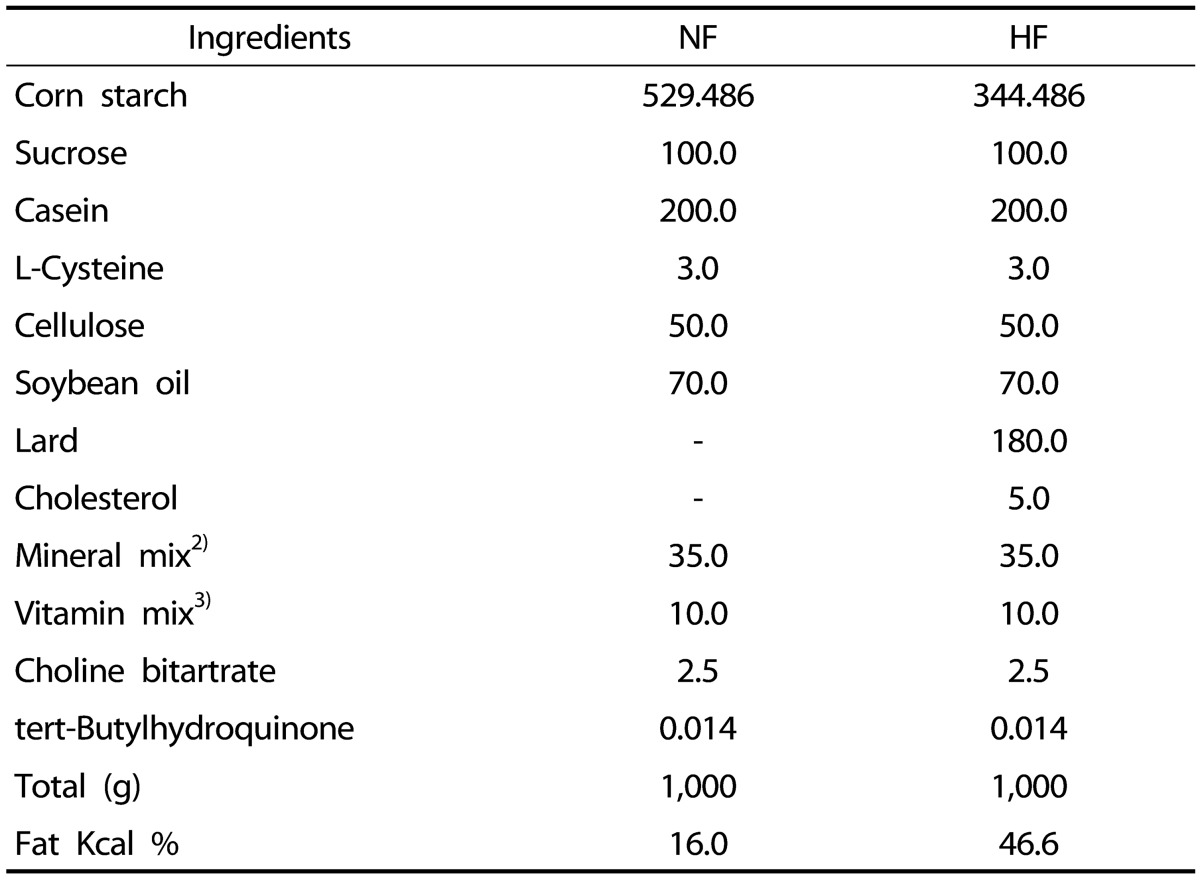
For experimental animals, 30 male C57BL/6J mice, 4-weeks-old, were purchased and divided into three groups by weight using a randomized block design: normal-fat group (NF group, n = 10), high-fat group (HF group, n = 10), and high-fat corn silk group (HFCS, n = 10). Experimental diet was prepared based on AIN-93G [31]; normal-fat diet contained 7% fat (diet weight basis) as soybean oil while high-fat diet contained 7% fat as soybean oil and 18% as lard, resulting in a total of 25% fat (diet weight basis) and 0.5% cholesterol (Table 1). The NF group received normal-fat diet, the HF group received high-fat diet, and the HFCS group received high-fat diet along with daily oral administration of high maysin corn silk extract at 100 mg/kg body weight. High maysin corn silk extract was provided by the Rural Development Administration, National Institute of Crop Science. In a previous study, consumption of ethanol extract of corn stigmas (4,000 mg/kg) improved diabetic conditions without toxicity [13]. In addition, rats fed 500 mg/kg of high maysin corn silk extract for 4 weeks showed no toxicity (data not shown). Thus, 100 mg/kg of corn silk extract was used in this study and can be considered as safe.
Table 1. Composition of the experimental diets.
1) NF, normal control group; HF, high-fat diet group
2) Mineral mixture (per kg): calcium carbonate anhydrous, 357 g; potassium phosphate monobasic, 196 g; potassium citrate tripotassium monohydrate, 70.78 g; sodium chloride, 74 g: magnesium oxide, 24 g; ferric citrate, 6.06 g; zinc carbonate, 1.65 g; sodium meta-silicate, 1.45 g; manganous carbonate, 0.63 g; cupric carbonate, 0.30 g; chromium potassium sulfate, 0.275 g; boric acid, 81.5 mg; sodium fluoride, 63.5 mg; nickel carbonate, 31.8 mg; lithium chloride, 17.4 mg; sodium selenate anhydrous, 10.25 mg; potassium iodate, 10.0 mg; ammonium paramolybdate, 6.66 mg; powdered sucrose, 221.026 g.
3) Vitamin mixture (per kg): nicotinic acid, 3.0 g; calcium pantothenate, 1.6 g; pyridoxine HCl 0.7 g; thiamine HCl, 0.6 g; riboflavin 0.6 g; folic acid, 0.2 g; biotin, 0.02 g; vitamin B12, 2.5 g; vitamin 15.0 g; vitamin A, 0.8 g; vitamin D3, 0.25 g; vitamin K-1, 0.075 g; powdered sucrose, 974.655 g.
The extraction procedure of high maysin corn silk was previously described [32]. In brief, unpollinated corn silk (Kwangpyeongok) were collected and extracted with prethanol A (C2H5OH, Duksan, Korea). The extracted samples were filtered and concentrated under reduced pressure. Chlorophyll, lipids, and saccharides of extracted samples were removed using methylene chloride (CH2Cl2), after which the active material from the extracts was eluted by addition of absolute ethanol. Extracts were completely dried in a vacuum concentrator. In order to separate high maysin containing corn silk extract, dried extracts were dissolved in ethanol and then injected into a preparative C18 chromatograph column (Büchi, Newcastle, DE).
In general, the maysin content of corn silk is 5.2-230.5 mg/100 g, depending on the species [33]. However, high maysin corn silk used in this study was 2.78 g/100 g. The NF and HF groups received the same amount of distilled water orally as the HFCS group. The experimental period was 8 weeks. Dietary intake was measured twice a week, and body weight was measured once a day. Four mice were housed in a plastic cage in conditioned rooms (24 ± 1℃, 12-h light/12-h dark cycle). All experimental procedures met the guidelines of the animal testing ethics committee of Dankook University (Approval Code: 15-025).
Sample preparations
After fasting for 12 hours, animals were anesthetized and the blood was drawn from the heart using a syringe. Liver, subcutaneous fat, intestinal fat, kidney fat, and epididymal fat pads were dissected and rinsed in 0.9% NaCl solution and weighed. The liver and epididymal fat pads were stored at -70℃ in a freezer before gene expression analysis.
Total RNA isolation, reverse transcription, and real-time PCR
The detailed methods for total RNA isolation, reverse transcription, and real-time PCR for measuring mRNA expression of genes related to adipocyte differentiation, triglyceride synthesis, and fat oxidation in liver or epididymal fat pad were performed as previously described [34]. Total liver or adipose tissue RNA was isolated using TRI-reagent (Sigma Aldrich, MO, USA) according to the manufacturer's protocol. Real-time PCR was performed using the modified method recently described [35]. Genes measured in the experiment were as follows: ACC-1, ACC-2 , acyl CoA oxidase (ACO), AMPK, AP-2, C/EBP-α, C/EBP-β, CD-36, CPT-1, FAS, glyceraldehyde 3-phosphate dehydrpgenase (GAPDH), glycerol-3-phosphate acyltransferase-1(GPAT-1), glucose-6-phosphate dehydrogenase (G6PDH), hormone-sensitive lipase (HSL), lipoprotein lipase (LPL), medium chain acyl-CoA dehydrogenase (MCAD), pyruvate dehydrogenase kinase, isozyme 4 (PDK4), PPAR-α , PPAR-γ1 , PPAR-γ2, stearoyl-CoA desaturase-1 (SCD-1), and SREBP-1c. Each forward/reverse primer used is shown in Table 2. Expression of mRNA was analyzed with an Applied Biosystems StepOne Plus RT-PCR system (Applied biosystems, CA, USA). Fold differences in gene expression were calculated using the 2-ΔΔCT method with the endogenous control gene.
Table 2. Primer sequences used for real-time polymerase chain reaction.
ACC1; acetyl-CoA carboxylase1, ACC2; acetyl-CoA carboxylase2, ACO; acyl CoA oxidase, AMPK; AMP-activated protein kinase, AP-2; activating protein -2, C/EBP-α; CCAAT/enhancer binding protein-α, C/EBP-β; CCAAT/enhancer binding protein-β, CD36; cluster of differentiation 36, CPT-1; Carnitine palmitoyltransferase-1, FAS; fatty acid synthase, GAPDH; glyceraldehyde 3-phosphate dehydrogenase, GPAT1; glycerol-3-phosphate acyltransferase-1, G6PDH; glucose-6-phosphate dehydrogenase, HSL; Hormone-sensitive lipase, LPL; lipoprotein lipase, MCAD; medium chain acyl-CoA dehydrogenase, PDK4; pyruvate dehydrogenase kinase, isozyme 4, PPAR-α; peroxisome proliferator-activated receptor, PPAR-γ1; peroxisome proliferator-activated receptor-γ1, PPAR-γ2; peroxisome proliferator-activated receptor-γ2, SCD1; stearoyl-CoA desaturase-1, SREBP-1c; sterol regulatory element binding protein-1c
Statistical analysis
Statistical analysis was performed using Statistical Analysis System software (SAS Institute, Cary, NC, USA). Data were expressed as means with standard deviation, and statistically significant differences among groups were evaluated using one way-ANOVA (analysis of variance). Statistically significant differences among means of groups were tested at α = 0.05 using Duncan's multiple range tests.
RESULTS
Effect of high maysin corn silk extract on body weight and fat accumulation
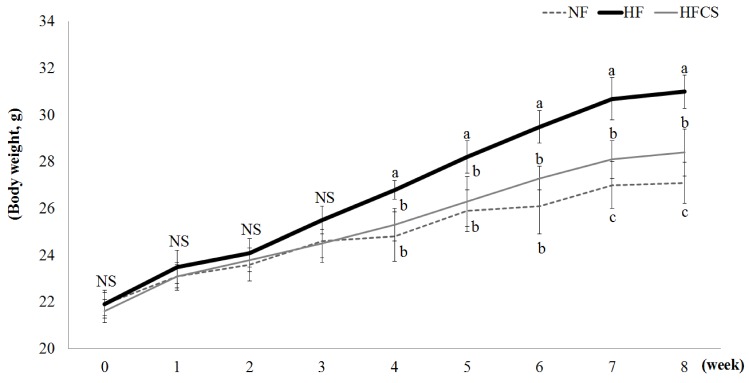
Changes in body weight during the experimental period are shown in Fig. 1. There were no significant differences in body weight among the groups until the 3rd week of experimental diets, whereas body weights of the HF and HFCS groups, both fed high-fat diet, significantly increased from the 4th week compared to the NF group fed low-fat diet. After 7 weeks, body weight was significantly reduced in the high-fat group compared to the high-fat only group (P < 0.05).
Fig. 1. Effect of corn silk extract on body weight.
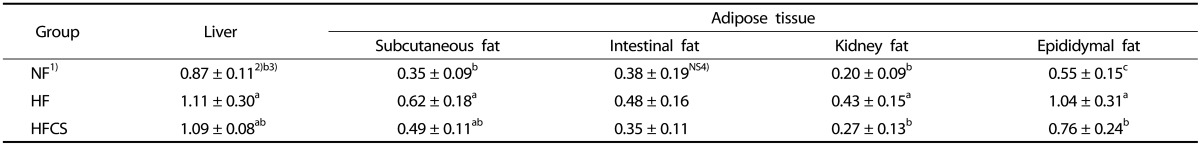
The liver and body adipose tissue weights were measured after completion of the experimental period. Liver weight was significantly higher in the HF group compared to the LF group (P < 0.05), whereas no difference in intake of high maysin corn silk extract was observed (Table 3). For body adipose tissue weight, subcutaneous fat and intestinal fat weights tended to be lower in the group with high maysin corn silk extract compared to the HF group, although no significant difference was observed. However, kidney fat and epididymal fat pad weights were significantly higher in the HF group compared to the other groups (P < 0.05) but significantly lower in the high-fat diet group with high maysin corn silk extract (Table 3).
Table 3. Liver and adipose tissue weights (g).
1) LF: Normal fat diet, HF: High-fat diet, HFCS; High-fat diet + Corn silk extract (100 mg/kg body weight)
2) Mean ± SD
3) Different letters indicate significant differences among groups at α = o.o5 as determined by Duncan's multiple range test
4) NS: Not significant
Effect of high maysin corn silk extract on adipocyte differentiation
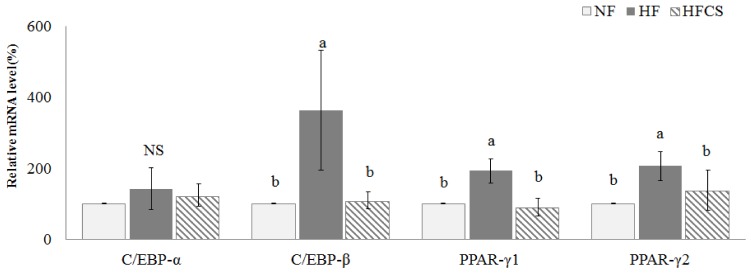
The mRNA expression levels of C/EBP-α, C/EBP-β, PPAR-γ1, and PPAR-γ2, which are related to adipocyte differentiation in epididymal fat tissue, were measured (Fig. 2). C/EBP-β, PPAR-γ1, and PPAR-γ2 mRNA expression levels were significantly elevated in the HF group compared to the NF group (P < 0.05) but were reduced to the same levels as in the NF group when high maysin corn silk extract was added.
Fig. 2. Effect of corn silk extract on mRNA expression of transcription factors related to adipocyte differentiation in adipose tissue of mice fed high-fat diet.
Total RNA was isolated using TRI-reagent, and cDNA was synthesized using 3 µg of total RNA with SuperScriptⅡ reverse transcriptase. Real-time PCR was performed using SYBR green and standard procedures to assess mRNA expression of primer in adipose tissue (epididymal fat pad) samples obtained from each group. Applied Biosystem StepOne software v2.1 was used. Each bar represents the mean ± SD, and different letters above each bar indicate significant differences among groups at α = o.o5 as determined by Duncan's multiple range test. C/EBP-α; CCAAT/enhancer binding protein-α, C/EBP-β; CCAAT/enhancer binding protein-β, PPAR-γ1; peroxisome proliferator-activated receptor-γ1, PPAR-γ2; peroxisome proliferator-activated receptor-γ2
Effect of high maysin corn silk extract on fat accumulation
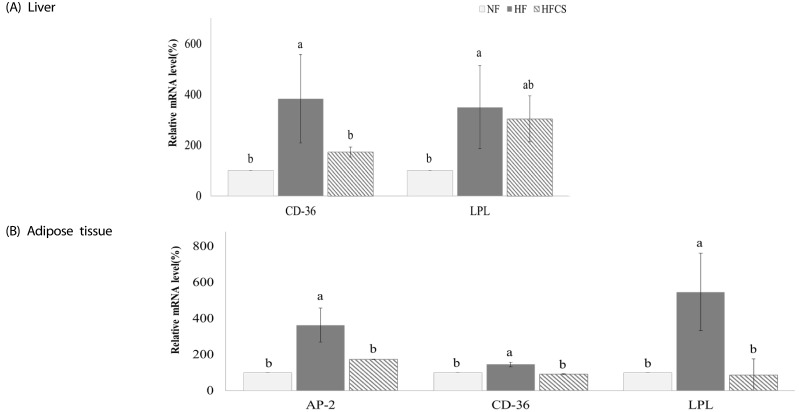
The mRNA expression levels of genes related to fat accumulation in the liver and adipose tissue were measured, as shown in Fig. 3. In the liver, mRNA expressions of CD-36 and LPL were significantly elevated in the HF group compared to the NF group (P < 0.05) but reduced in the group fed high-fat diet and high maysin corn silk extract. In adipose tissue, mRNA expressions of AP-2, CD-36, and LPL were also significantly elevated in the HF group compared to the NF group (P < 0.05) but reduced in the group fed high-fat diet and high maysin corn silk extract.
Fig. 3. Effect of corn silk extract on mRNA expression of transcription factors and enzymes related to fat deposition in the liver and adipose tissue of mice fed high-fat diet.
Total RNA was isolated using TRI-reagent, and cDNA was synthesized using 3 µg of total RNA with SuperScriptⅡ reverse transcriptase. Real-time PCR was performed using SYBR green and standard procedures to assess mRNA expression of primer in liver (A) and adipose tissue (B) (epididymal fat pad) samples obtained from each group. Applied Biosystem StepOne software v2.1 was used. Each bar represents the mean ± SD, and different letters above each bar indicate significant differences among groups at α = o.o5 as determined by Duncan's multiple range test. CD-36; cluster of differentiation 36, LPL; lipoprotein lipase, AP-2; activating protein-2
Effect of high maysin corn silk extract on fat synthesis
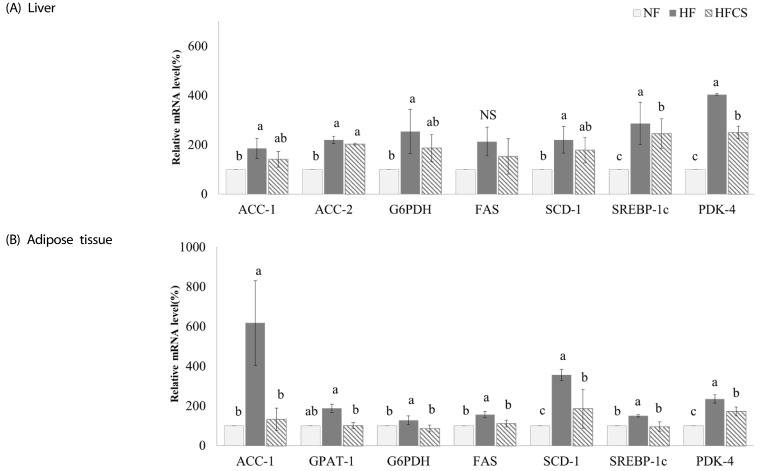
The expression levels of genes related to fat synthesis in the liver and adipose tissue were measured, as shown in Fig. 4. In the liver, all genes were significantly up-regulated in the HF group compared to the NF group, and mRNA expression levels of ACC-1, G6PDH, and SCD-1 were reduced upon intake of high maysin corn silk extract while levels of SREBP-1C and PDK-4 were significantly reduced (P < 0.05). In adipose tissue, expression levels of genes related to fat synthesis were significantly up-regulated in the HF group compared to the NF group, and intake of high maysin corn silk extract significantly reduced mRNA expression of all genes (P < 0.05).
Fig. 4. Effect of corn silk extract on mRNA expression of enzymes related to fat synthesis in the liver and adipose tissue of mice fed high-fat diet.
Total RNA was isolated using TRI-reagent, and cDNA was synthesized using 3 µg of total RNA with SuperScriptⅡ reverse transcriptase. Real-time PCR was performed using SYBR green and standard procedures to assess mRNA expression of primer in liver (A) and adipose tissue (B) (epididymal fat pad) samples obtained from each group. Applied Biosystem StepOne software v2.1 was used. Each bar represents the mean ± SD, and different letters above each bar indicate significant differences among groups at α = o.o5 as determined by Duncan's multiple range test. ACC1; acetyl-CoA carboxylase1, ACC2; acetyl-CoA carboxylase2, G6PDH; glucose-6-phosphate dehydrogenase FAS; fatty acid synthase, SCD1; stearoyl-CoA desaturase-1, SREBP-1c; sterol regulatory element binding protein1c, PDK-4; pyruvate dehydrogenase kinase, isozyme 4, GPAT-1; glycerol-3-phosphate acyltransferase-1.
Effect of high maysin corn silk extract on lipolysis and fat oxidation
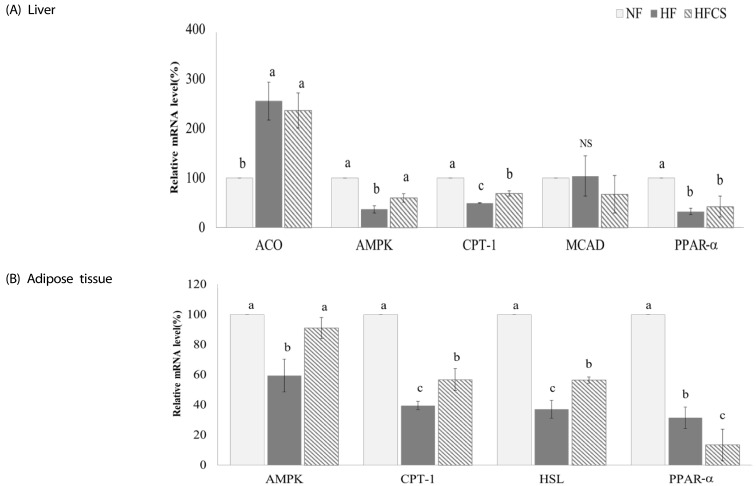
The mRNA expression levels of genes related to lipolysis and fat oxidation in the liver and adipose tissue were measured, as shown in Fig. 5. In the liver, intake of high maysin corn silk extract significantly increased mRNA expression levels of AMPK and CPT-1 (P < 0.05). In adipose tissue, mRNA expression levels of AMPK, CPT-1, and HSL were significantly up-regulated (P < 0.05) while expression of PPAR-α was reduced upon intake of high maysin corn silk extract.
Fig. 5. Effect of corn silk extract on mRNA expression of transcription factors and enzymes related to fat oxidation in the liver and adipose tissue of mice fed high-fat diet.
Total RNA was isolated using TRI-reagent, and cDNA was synthesized using 3 µg of total RNA with SuperScriptⅡ reverse transcriptase. Realtime PCR was performed using SYBR green and standard procedures to assess mRNA expression of primer in liver (A) and adipose tissue (B) (epididymal fat pad) samples obtained from each group. Applied Biosystem StepOne software v2.1 was used. Each bar represents the mean ± SD, and different letters above each bar indicate significant differences among groups at α = o.o5 as determined by Duncan's multiple range test. ACO; acyl CoA oxidase, AMPK; AMP-activated protein kinase, CPT-1; Carnitine palmitoyltransferase-1, MCAD: medium chain acyl-CoA dehydrogenase, PPAR-α; peroxisome proliferator-activated receptor, HSL; hormone-sensitive lipase.
DISCUSSION
Corn silk is a waste material produced in the corn cultivation process, and and the physiological activity of corn silk has been recently studied. However the anti-obesity effect and the related mechanism of corn silk extract has not been determined.
In this study, experimental animals that received high-fat diet along with 100 mg/kg of high maysin corn silk extract for 8 weeks showed significant reduction of body weight compared to the high-fat diet only group. Additionally, kidney fat and epididymal fat pad weights significant decreased, demonstrating high maysin corn silk extract had a weight-reducing effect by decreasing fat accumulation in the body. Min et al. [30] reported that administration of 100 mg/kg BW and 400 mg/kg BW of corn silk extract significantly lowered body weight after 2 weeks of intake in db/db mice, which is consistent with the weight-reducing effect in our study.
To elucidate the mechanism responsible for body weight and body fat reduction by high maysin corn silk extract, mRNA expression levels of proteins involved in adipocyte differentiation, fat accumulation, fat synthesis, lipolysis, and fat oxidation in adipose tissue and the liver were measured.
C/EBP-β and PPAR-γ are transcription factors that induce gene expression of glucose transporter 4 (GLUT4), FAS, SCD-1, and adiponectin to promote differentiation of fat cells [25]. In particular, PPAR-γ is a transcription factor mainly expressed in adipose tissue that increases fat accumulation by participating in the production of insulin-sensitive adipokines such as adiponectin [36,37,38]. As PPARγ induces transcription of AP-2 and LPL, decreased expression of PPAR-γ can reduce triglyceride synthesis in adipose tissue [39]. In a previous 3T3 L1 cell experiment, corn silk aqueous extract inhibited fat synthesis in fat cells and protein expression of PPAR-γ, a transcription factor playing an important role in adipocyte differentiation, in a concentration-dependent manner [30]. In our study, high intake of maysin corn silk extract reduced expression of PPAR-γ in adipose tissue, which is consistent with reduction of AP-2 in the liver and adipose tissue. Therefore, the results of this study suggest that corn silk extract suppresses lipogenesis in adipose tissue.
CD-36 is a protein that mediates transport of fatty acids into cells by interacting with AP-2 and fatty acid transport protein (FATP) [26], and expression of CD-36 was shown to be increased in the liver and adipose tissue of an obese experimental animal model [26,40]. Thus, high maysin corn silk extract inhibited expression of CD-36, thereby abrogating transport of fatty acids into adipose tissue and further inhibiting fat accumulation.
In this study, high maysin corn silk extract reduced mRNA expression of SREBP-1c. SREBP-1c regulates fat synthesis in adipose tissue and the liver and is known to regulate gene expression of enzymes related to triglyceride synthesis, including FAS, ACC, ACL, and ME [28]. As inhibition of SREBP-1c expression reduced fatty acid synthesis and consequently triglyceride accumulation in adipose tissue, high intake of maysin corn silk extract inhibited SREBP-1c expression, further reducing FAS and ACC expression and consequently decreasing fat synthesis and body fat weight.
AMPK reduces SREBP-1c expression to inhibit triglyceride synthesis and increase fatty acid oxidation in the liver, whereas CPT-1 is a protein that increases fatty acid oxidation by participating in the transport of fatty acids into mitochondria [41,42]. HSL is distributed in various organs, including muscles, testis, and the adrenal gland, but is mainly activated in adipose tissue and increases degradation of triglycerides stored in adipose tissue [43]. In this study, high maysin corn silk extract activated mRNA expression of AMPK and further suppressed triglyceride synthesis, possibly by inhibiting mRNA expression of transcription factors such as SREBP-1c and PPARγ. In addition, AMPK seemed to inhibit ACC and activate CPT1, resulting in increased beta- oxidation, decreased fat accumulation, and greater weight loss.
In conclusion, high maysin corn silk extract inhibits adipocyte differentiation through inhibition of C/EBP- β and PPAR-γ AMPK expression in adipose tissue, inhibits expression of CD-36, AP-2, and LPL related to fat accumulation in adipose tissue, inhibits expression of ACC-1, GPAT-1, G6PDH, FAS, SCD-1, SREBP-1c, and PDK-4 related to fat synthesis, and increases expression of AMPK, CPT-1, and HSL related to lipolysis and fatty acid oxidation to reduce body fat, consequently reducing body weight in experimental animals.
Footnotes
This work was carried out with the support of the "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ0113052015)" Rural Development Administration, Republic of Korea.
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Velazquez DV, Xavier HS, Batista JE, de Castro-Chaves C. Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine. 2005;12:363–369. doi: 10.1016/j.phymed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Maksimović Z, Malencić D, Kovacević N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol. 2005;96:873–877. doi: 10.1016/j.biortech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Hasanudin K, Hashim P, Mustafa S. Corn silk (Stigma maydis) in healthcare: a phytochemical and pharmacological review. Molecules. 2012;17:9697–9715. doi: 10.3390/molecules17089697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grases F, March JG, Ramis M, Costa-Bauzá A. The influence of Zea mays on urinary risk factors for kidney stones in rats. Phytother Res. 1993;7:146–149. [Google Scholar]
- 5.Barnes J, Anderson LA, Phillipson JD. Herbal Medicines: a Guide for Health-care Professionals. 2nd ed. London: Pharmaceutical Press; 1996. [Google Scholar]
- 6.George GO, Idu FK. Corn silk aqueous extracts and intraocular pressure of systemic and non-systemic hypertensive subjects. Clin Exp Optom. 2015;98:138–149. doi: 10.1111/cxo.12240. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Xu QT, Gao XH. Effects of stigma maydis polysaccharide on gastrointestinal movement. Zhongguo Zhong Yao Za Zhi. 2007;32:1203–1206. [PubMed] [Google Scholar]
- 8.Yang J, Li X, Xue Y, Wang N, Liu W. Anti-hepatoma activity and mechanism of corn silk polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2014;64:276–280. doi: 10.1016/j.ijbiomac.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Maksimović ZA, Kovacević N. Preliminary assay on the antioxidative activity of Maydis stigma extracts. Fitoterapia. 2003;74:144–147. doi: 10.1016/s0367-326x(02)00311-8. [DOI] [PubMed] [Google Scholar]
- 10.Zeringue HJ., Jr Identification and effects of maize silk volatiles on cultures of Aspergillus flavus. J Agric Food Chem. 2000;48:921–925. doi: 10.1021/jf990061k. [DOI] [PubMed] [Google Scholar]
- 11.Habtemariam S. Extract of corn silk (stigma of Zea mays) inhibits the tumour necrosis factor-alpha- and bacterial lipopolysaccharide-induced cell adhesion and ICAM-1 expression. Planta Med. 1998;64:314–318. doi: 10.1055/s-2006-957441. [DOI] [PubMed] [Google Scholar]
- 12.Wang GQ, Xu T, Bu XM, Liu BY. Anti-inflammation effects of corn silk in a rat model of carrageenin-induced pleurisy. Inflammation. 2012;35:822–827. doi: 10.1007/s10753-011-9382-9. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Liu T, Han L, Liu Y. The effects of corn silk on glycaemic metabolism. Nutr Metab (Lond) 2009;6:47. doi: 10.1186/1743-7075-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo BZ, Zhang ZJ, Butrón A, Widstrom NW, Snook ME, Lynch RE, Plaisted D. Lost P1 allele in sh2 sweet corn: quantitative effects of p1 and a1 genes on concentrations of maysin, apimaysin, methoxymaysin, and chlorogenic acid in maize silk. J Econ Entomol. 2004;97:2117–2126. doi: 10.1603/0022-0493-97.6.2117. [DOI] [PubMed] [Google Scholar]
- 15.Lee EA, Byrne PF, McMullen MD, Snook ME, Wiseman BR, Widstrom NW, Coe EH. Genetic mechanisms underlying apimaysin and maysin synthesis and corn earworm antibiosis in maize (Zea mays L.) Genetics. 1998;149:1997–2006. doi: 10.1093/genetics/149.4.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snook ME, Widstrom NW, Gueldner RC. Reversed-phase high-performance liquid chromatographic procedure for the determination of maysin in corn silks. J Chromatogr A. 1989;477:439–447. [Google Scholar]
- 17.Kim SL, Snook ME, Lee JO. Radical scavenging activity and cytotoxicity of maysin(C-glycosylflavone) isolated from silks of Zea mays L. Korean J Crop Sci. 2003;48:392–396. [Google Scholar]
- 18.Choi DJ, Kim SL, Choi JW, Park YI. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014;109:57–64. doi: 10.1016/j.lfs.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Kim SL, Lee S, Chung MJ, Park YI. Immunostimulating activity of maysin isolated from corn silk in murine RAW 264.7 macrophages. BMB Rep. 2014;47:382–387. doi: 10.5483/BMBRep.2014.47.7.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity and overweight [Internet] Geneva: World Health Organization; [cited 2016 April 20]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 21.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2014: Korea National Health and Nutrition Examination Survey (KNHANES VI-2) Sejong: Korea Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 22.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 23.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Chan JS, Ren L, Yan JH. Obesity reduces cognitive and motor functions across the lifespan. Neural Plast. 2016;2016:2473081. doi: 10.1155/2016/2473081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2012;109:14568–14573. doi: 10.1073/pnas.1211611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura J, Masaki T, Arakawa M, Seike M, Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J Nutr. 2010;140:496–500. doi: 10.3945/jn.109.108977. [DOI] [PubMed] [Google Scholar]
- 27.Thampy KG. Formation of malonyl coenzyme A in rat heart. Identification and purification of an isozyme of A carboxylase from rat heart. J Biol Chem. 1989;264:17631–17634. [PubMed] [Google Scholar]
- 28.Kim HJ, Miyazaki M, Man WC, Ntambi JM. Sterol regulatory element-binding proteins (SREBPs) as regulators of lipid metabolism: polyunsaturated fatty acids oppose cholesterol-mediated induction of SREBP-1 maturation. Ann N Y Acad Sci. 2002;967:34–42. doi: 10.1111/j.1749-6632.2002.tb04261.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min OJ, Sharma BR, Park CM, Rhyu DY. Effect of myadis stigma water extract on adipogenesis and blood glucose in 3T3-L1 adipocytes and db/db mice. Korean J Pharmacogn. 2011;42:201–208. [Google Scholar]
- 31.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 32.Kim SL, Kim MJ, Lee YY, Jung GH, Son BY, Lee JS, Kwon YU, Park YI. Isolation and identification of flavonoids from corn silk. Korean J Crop Sci. 2014;59:435–444. [Google Scholar]
- 33.Kim SL, Jung TW. Maysin and other flavonoid contents in corn silks. Korean J Breed. 2001;33:338–343. [Google Scholar]
- 34.Ha AW, Kim WK, Kim JH, Kang NE. The supplementation effects of peanut sprout on reduction of abdominal fat and health indices in overweight and obese women. Nutr Res Pract. 2015;9:249–255. doi: 10.4162/nrp.2015.9.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Qin G, Liu J, Mao L, Zhang Z, Shang J. Adipose tissue regulates hepatic cholesterol metabolism via adiponectin. Life Sci. 2014;118:27–33. doi: 10.1016/j.lfs.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 39.Floyd ZE, Wang ZQ, Kilroy G, Cefalu WT. Modulation of peroxisome proliferator-activated receptor gamma stability and transcriptional activity in adipocytes by resveratrol. Metabolism. 2008;57:S32–S38. doi: 10.1016/j.metabol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larter CZ, Yeh MM, Van Rooyen DM, Teoh NC, Brooling J, Hou JY, Williams J, Clyne M, Nolan CJ, Farrell GC. Roles of adipose restriction and metabolic factors in progression of steatosis to steatohepatitis in obese, diabetic mice. J Gastroenterol Hepatol. 2009;24:1658–1668. doi: 10.1111/j.1440-1746.2009.05996.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee MS, Kim IH, Kim CT, Kim Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J Nutr. 2011;141:1947–1953. doi: 10.3945/jn.111.146050. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol. 2013;366:135–151. doi: 10.1016/j.mce.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Berger JJ, Barnard RJ. Effect of diet on fat cell size and hormone-sensitive lipase activity. J Appl Physiol (1985) 1999;87:227–232. doi: 10.1152/jappl.1999.87.1.227. [DOI] [PubMed] [Google Scholar]