Abstract
Background
To assess the influence of combined intracoronary application of high-dose adenosine and tirofiban in primary percutaneous coronary intervention (PCI) on clinical events and cardiac function.
Methods
Our study evaluated consecutive patients with acute ST-segment elevation myocardial infarction undergoing primary PCI, who were randomly divided into adenosine group (n = 130) and control group (n = 128). Combined with thrombus aspiration and then intracoronary tirofiban, the adenosine group received intracoronary adenosine (2 mg) through the aspiration catheter 2 times. After thrombus aspiration and stenting of the infarct- related artery, the control group received placebo. The primary endpoint of our investigation was major adverse cardiac events (MACE) at the 1-year and 3-year marks. The secondary endpoint comprised left ventricular remodeling (LVR) at 6 months, myocardial blush grade (MBG), thrombolysis in myocardial infarction (TIMI) flow grade and corrected TIMI frame count (CTFC) after PCI.
Results
Our study found that TIMI flow grade post-PCI did not differ significantly between the 2 groups, while CTFC favored the adenosine-treated patients (21.6 ± 6.5 vs. 25.1 ± 7.8, p = 0.001). Although the adenosine group achieved a higher rate of MBG 3 (45.1% vs. 32.0%, p = 0.035) and MBG 2-3 (76.2% vs. 62.3%, p = 0.018) than the control group, the incidences of MACE at 1 year (20.0% vs. 25.0%, p = 0.373) and 3 years (26.9% vs. 32.0%, p = 0.413) were comparable. LVR occurred in 23.1% (27/117) of adenosine-treated patients and in 29.8% (43/114) of the controls (p = 0.296).
Conclusions
Intracoronary administration of high-dose adenosine combined with intracoronary tirofiban and thrombus aspiration may further improve myocardial perfusion after primary PCI.
Keywords: Adenosine, Angioplasty, Myocardial infarction, Remodeling
INTRODUCTION
In the clinical setting of acute ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI), no-reflow phenomenon affects more than 20% of patients.1-3 This “angiographic no-reflow” was associated with reduced myocardial salvage, larger infarct size, poor left ventricular functional recovery and increased risk of short-term mortality.4-6 It furthermore was an independent predictor of long-term cardiac death and cardiac events in STEMI patients treated with primary PCI.7,8
Thus, efforts have been undertaken to reduce the incidence of no-reflow, such as thrombus aspiration9 and distal protection device.10 On the other hand, some agents who administered intravenously or intracoronarily during primary PCI have been demonstrated to be effective. It has been reported that intravenous or intracoronary application of platelet glycoprotein (GP) IIb/IIIa receptor antagonist11,12 or adenosine,13,14 could raise the myocardial perfusion level, reduce the incidence of no-reflow, and improve clinical outcomes in patients who underwent primary PCI.
Although a number of studies had evaluated the effects of intravenous or intracoronary application of adenosine in primary PCI, some were limited by their non-controlled design or low sample size, and few had assessed the influence of adenosine on cardiac function and long-term clinical outcomes. In the previous study, it was demonstrated that combined intracoronary administration of high-dose adenosine and tirofiban during primary, and PCI could further reduce the risk of no-reflow.15 Whether this beneficial effect could translate into advantageous influence on clinical outcomes was worth clarifying. This study with a 3-year follow-up was intended to indentify the influence of combined intracoronary application of high-dose adenosine and tirofiban during primary PCI on clinical events and cardiac function.
PATIENTS AND METHODS
Patients
This was a single-center, open-label, prospective, randomized controlled study. Its eligible participants consisted of consecutive patients over 18 years of age, presenting to the Capital Medical University Beijing Chao-yang Hospital with suspected acute STEMI, as well as candidates for primary PCI who were eligible for participation. Inclusion criteria were: symptoms of chest pain suggestive of myocardial ischemia for at least 30 minutes, less than 12 hours from onset of symptoms to hospital admission, and an electrocardiography (ECG) showing ST-segment elevation of > 0.1 mV in 2 or more leads, or new left bundle branch block. Exclusion criteria were: 1) contraindications for anticoagulant, antiplatelet therapy; 2) left main coronary artery lesion or cardiogenic shock; 3) previous history of coronary artery bypass grafting; 4) any past thrombolytic therapy; 5) second-degree or higher atrioventricular blockage with no cardiac pacing protection; 6) concomitant asthma or chronic obstructive pulmonary disease or history of theophylline, dipyridamole, or glyburide treatment; and 7) life expectancy < 6 months. This study was conducted from January 2007-September 2010. The study protocol was approved by our hospital ethics committee, and all randomized patients gave written informed consent before enrollment.
Randomization and treatment
After an initial coronary angiography was performed, the operator determined if the patient qualified for randomization. The eligible patients were randomized (1:1) to 2 high-dose bolus injections of intracoronary adenosine (2 × 2 mg in 20 mL 0.9% NaCl) or placebo (2 × 20 mL 0.9% NaCl). This occurred after thrombus aspiration and intracoronary injection of tirofiban, and after stenting, by hand distal to the culprit lesion over 1 minute. Sealed sequentially numbered opaque allocation envelopes were used for randomization. Additionally, the allocation schedule was based on computer-generated random numbers (block size 20).
Ultimately, the procedure was performed through the radial (preferred) or femoral artery at the operator’s discretion utilizing standard techniques. However, only the culprit lesion was treated. After a guide wire crossing the culprit lesion, manual thrombus aspiration were performed at least 2 times using thrombus aspiration catheter (ZEEK, Zeon Medical, Tokyo, Japan). If the aspiration catheter could not cross the lesion, a predilation could be conducted. Then, through the aspiration catheter, a bolus of 10 μg/kg tirofiban was injected by hand distal to the culprit lesion over 3 minutes, followed by continuous intravenous administration at 0.15 μg·kg-1·min-1 for 24 h. Intracoronary nitroglycerin (200 ug) administered through the guiding catheter was recommended after the 2 times bolus of intracoronary adenosine or placebo. Other medications, such as sodium nitroprusside, verapamil, and diltiazem, were not routinely recommended but were applied when necessary.
Before the procedure was conducted, all patients received 300 mg of aspirin and 600 mg of clopidogrel. Unfractionated heparin was administered, with a bolus of 70 IU/Kg given via the arterial sheath, maintaining an activated clotting time of 250 seconds or longer. Aspirin (75 to 100 mg/day) was prescribed indefinitely and clopidogrel (75 mg/day) for at least 12 months after procedure. Patients were treated with beta blocking agents, statins, and angiotensin-converting enzyme inhibitors or angiotensin II blockers according to the judgment of the patients’ physician.
Angiographic and electrocardiographic analysis
Coronary angiograms obtained before and after primary PCI were analyzed by two experienced observers blinded to treatment allocation and clinical data. On the initial angiogram and on the angiogram after stenting, thrombolysis in myocardial infarction (TIMI) flow grade16 was assessed. In addition, corrected TIMI frame count (CTFC) and myocardial blush grade (MBG) were assessed after stenting, as previously described.17,18 The 12-lead ECG obtained upon presentation and post-intervention were used to measure ST-segment elevation at 20 ms after the QRS complex. For anterior myocardial infarction, leads I, aVL, and V1-V6 were measured; for non-anterior myocardial infarction, leads II, III, aVF, and V5-V6 were measured. The sum of ST-segment resolution (sum STR) was calculated, and categorized as complete resolution (> 70%), partial resolution (30-70%), and no resolution (< 30%).19
Myocardial biomarkers
Serum creatine kinase (CK), myocardial band of CK (CK-MB), and troponin I (Tn-I) were measured in all patients on admission, just before and after primary PCI, and at 8, 16, 24 and 48 hours after the procedure. The maximal value was defined as the peak value.
Echocardiographic study
All subjects underwent two 2-dimensional echocardiographic examinations, at discharge and 6 months after the procedure, with the commercially available ultrasound scanner iE33 (Philips; Andover, MA, USA). LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV ejection fraction (LVEF) were calculated by the modified biplane Simpson’s rule algorithm.20 Left ventricular remodeling (LVR) was defined as a ≥ 20% increase in the LVEDV at 6-month follow-up assessed as compared with that at the time of discharge.21
Follow-up and endpoints
Clinical follow-up was performed at 30 days, 3 months, 6 months, and then every 6 months for a total of 3 years after the procedure. The primary end point of the study was major adverse cardiac events (MACE), defined as the composite of cardiac death, recurrent infarction, target vessel revascularization (TVR), and heart failure (defined as cardiac function ≥ NYHA class II combined with LVEF < 50%) at the 1-year and 3-year marks. The secondary endpoints comprised LVR at 6 months, and TIMI flow grade, CTFC, and MBG after revascularization. Stent thrombosis (ST) was classified as definite, probable, and possible according to the Academic Research Consortium definition. The clinical events committee, whose members were blinded to the assigned groups, reviewed and adjudicated all serious clinical events.
Statistical analysis
According to the medical records, the incidence of no-reflow in the control group was approximately 45%,1-3,13,14 and adenosine application reduced the incidence of no-reflow by approximately 15%.13,14 Based on a sample size calculation formula with α set at 0.05, statistical significance was set at a sample size of 120 per group, with a total required sample size of 240. Continuous data are expressed as mean ± standard deviation (SD) or as median (interquartile range), and dichotomous data are presented as numbers and percentages. All continuous variables were compared using the Student’s t-test or, in the case of a non-Gaussian distribution, with a nonparametric test. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test as appropriate. Three-year event curves were generated by the Kaplan-Meier method, and incidence between groups was compared utilizing the log-rank test. Hazard ratios with 95% confidence intervals were calculated by Cox proportional hazards regression model. A 2-sided p value < 0.05 was considered significant for all tests. All analyses were conducted using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
A total of 392 STEMI patients who received primary PCI were screened, after which 264 patients were enrolled in the present study. Among these patients, 3 in the adenosine group ceased intravenous tirofiban due to bleeding and were excluded (one case of hematochezia, one of stress ulcer combined with upper gastrointestinal bleeding, and one for bleeding gums). Additionally, 2 patients in the control group stopped administration of tirofiban due to bleeding (one case of stress ulcer combined with upper gastrointestinal bleeding and one for epistaxis), and were excluded. One case in the control group ceased application of tirofiban due to a substantial decrease in platelet count (< 50 × 109/L) and was excluded. Ultimately, data of 130 patients in the adenosine group and 128 patients in the control group were analyzed. The two groups did not differ significantly in basic clinical information, coronary angiogram, or PCI data (Table 1).
Table 1. Baseline characteristics.
| Adenosine group (n = 130) | Control group (n = 128) | p value | |
| Age (years) | 61.5 ± 11.7 | 60.2 ± 12.5 | 0.595 |
| Male | 102 (78.5) | 95 (74.2) | 0.465 |
| Heart rate (bpm) | 76.5 ± 17.8 | 75.0 ± 18.2 | 0.451 |
| Systolic blood pressure (mmHg) | 129.3 ± 25.8 | 131.7 ± 23.8 | 0.659 |
| Diastolic blood pressure (mmHg) | 76.4 ± 14.2 | 75.1 ± 14.4 | 0.528 |
| Current smoker | 80 (61.5) | 69 (53.9) | 0.257 |
| History | |||
| Hypertension | 55 (42.3) | 58 (45.3) | 0.707 |
| Diabetes | 22 (16.9) | 27 (21.1) | 0.43 |
| Hyperlipidemia | 46 (35.3) | 41 (32.0) | 0.6 |
| Myocardial infarction | 4 (3.0) | 3 (2.3) | 1.000 |
| PCI | 7 (5.4) | 5 (3.9) | 0.769 |
| Family history | 38 (29.2) | 30 (23.4) | 0.324 |
| Ischemic time (min) | 214 ± 147 | 225 ± 152 | 0.772 |
| Killip class | 0.891 | ||
| I | 97 (74.6) | 94 (73.4) | |
| II | 27 (20.8) | 29 (22.7) | |
| III | 6 (4.6) | 5 (3.9) | |
| Angiographic | |||
| Infarct-related vessel | 0.872 | ||
| LAD | 62 (47.7) | 57 (44.5) | |
| LCX | 18 (13.8) | 18 (14.1) | |
| RCA | 50 (38.5) | 53 (41.4) | |
| Multivessel disease | 75 (57.7) | 70 (54.7) | 0.707 |
| TIMI flow pre-PCI | 0.652 | ||
| Grade 0, 1 | 85 (65.4) | 81 (63.3) | |
| Grade 2 | 19 (14.6) | 24 (18.8) | |
| Grade 3 | 26 (20.0) | 23 (17.9) | |
| Primary PCI | |||
| Stent diameter (mm) | 3.3 ± 0.47 | 3.3 ± 0.49 | 0.695 |
| Stent length (mm) | 25.1 ± 12.5 | 24.2 ± 13.6 | 0.429 |
| Direct stenting | 22 (16.9) | 26 (20.3) | 0.525 |
| Post-stent dilation | 102 (78.5) | 94 (73.4) | 0.383 |
| DES use | 126 (96.9) | 125 (97.7) | 1.000 |
| IABP use | 4 (3.1) | 4 (3.1) | 1.000 |
Data are presented as mean (SD) or n (%), except where noted.
DES, drug eluting stent; IABP, intra-aortic balloon counterpulsation; LAD, left artery descendent; LCX, circumflex artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
Angiographic results
There was no significant difference in the TIMI flow grade after procedure between the adenosine group and the control group, with 123 (94.6%) and 118 (92.2%) patients achieving TIMI grade 3 flow, respectively (p = 0.625). The CTFC of the adenosine group was significantly more favorable than that of the control group (21.6 ± 6.5 frames vs. 25.1 ± 7.8 frames, p = 0.001) (Table 2). MBG evaluation revealed that the rate of MBG 3 [45.1% (55/122) vs. 32.0% (39/122), p = 0.035] and of MBG 2-3 [76.2% (93/122) vs. 62.3% (76/122), p = 0.018] were significantly higher in the adenosine group than that in the control group (Table 2).
Table 2. Myocardial reperfusion after primary percutaneous coronary intervention (n, %).
| Adenosine group | Control group | p value | |
| TIMI flow grade | n = 130 | n = 128 | |
| 0 or 1 | 1 (0.8) | 2 (1.5) | 0.552 |
| 2 | 6 (4.6) | 8 (6.3) | 0.562 |
| 3 | 123 (94.6) | 118 (92.2) | 0.432 |
| CTFC | n = 119 | n = 116 | |
| Frames, x ± s | 21.6 ± 6.5 | 25.1 ± 7.8 | 0.001 |
| MBG | n = 122 | n = 122 | |
| 0 | 7 (5.7) | 14 (11.5) | 0.11 |
| 1 | 22 (18.0) | 32 (26.2) | 0.123 |
| 2 | 38 (31.2) | 37 (30.3) | 0.89 |
| 3 | 55 (45.1) | 39 (32.0) | 0.035 |
| Sum STR | n = 125 | n = 124 | |
| < 30% | 20 (16.0) | 29 (23.4) | 0.143 |
| 30%-70% | 38 (30.4) | 43 (34.7) | 0.471 |
| > 70% | 67 (53.6) | 52 (41.9) | 0.065 |
CTFC, corrected TIMI frame count; MBG, myocardial blush grade; STR, ST-segment resolution; TIMI, thrombolysis in myocardial infarction.
ST-segment resolution
The ECG results of 125 patients in the adenosine group and 124 patients in the control group were reviewed for sum STR analysis. The ECGs were recorded at 29 min (20-42 min) in the adenosine group and 32 min (18-45 min) in the control group after PCI. The rates of complete ST-segment resolution were comparable between the two groups [53.6% (67/125) vs. 41.9% (52/ 124), p = 0.065] (Table 2).
Biomarkers
In the adenosine group, the peak values of CK (1738 ± 772 U/L vs. 1975 ± 897 U/L, p = 0.023), CK-MB (124 ± 51 U/L vs. 141 ± 57 U/L, p = 0.009) and Tn-I (75 ± 26 ng/ml vs. 84 ± 29 ng/ml, p = 0.011) were all significantly lower than those in the control group.
Echocardiographic results
Data of echocardiographic examinations achieved at discharge and at 6 months after the procedure of 117 adenosine patients and 114 controls had been analyzed. The adenosine group and the control group showed similar LVEDV (121 ± 31 mL vs. 122 ± 32 mL, respectively; p = 0.681), LVESV (70 ± 31 mL vs. 74 ± 29 mL, respectively; p = 0.277), and LVEF (42 ± 14% vs. 40 ± 12%, respectively; p = 0.519) at discharge and at 6 months (LVEDV 125 ± 38 mL vs. 130 ± 42 mL, respectively, p = 0.417; LVESV 64 ± 29 mL vs. 69 ± 30 mL, respectively, p = 0.374; LVEF 49 ± 15% vs. 47 ± 12%, respectively, p = 0.319). There were 27 patients in the adenosine group and 34 patients in the control group exhibiting LVR (p = 0.296).
Clinical outcomes
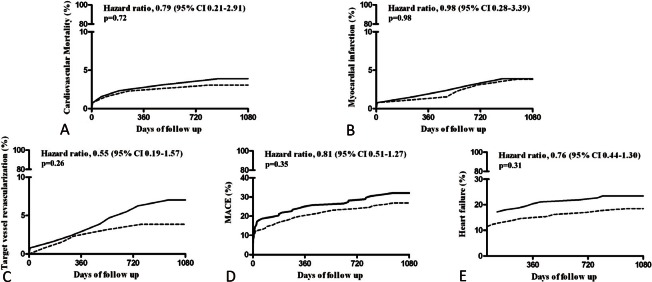
All patients in both groups fulfilled their follow-up at 30 days and 1-year. However, two cases in the adenosine group and one case in the control group were lost to follow-up at 3-year. The two groups did not differ significantly in the incidence of MACE or heart failure at 30 days (Table 4). Additionally, the two groups received similar medical treatments (Table 3), and showed comparable incidence rates of cardiac death, re-infarction, TVR, heart failure, or MACE at 1-year and 3-year follow-up (Table 4, Figure 1). Cumulative ST event rates up to 3 years after the procedure were similar between the 2 groups (Table 4). The 3-year cumulative incidence of heart failure was 18.5% and 23.4% in the adenosine group and the control group (p = 0.31; HR 0.76, 95%CI 0.44-1.30), respectively, and the rate of MACE was 26.9% and 32.0% in the 2 groups (p = 0.35; HR 0.81, 95%CI 0.51-1.27) (Figure 1).
Table 4. Clinical outcomes (n, %).
| Adenosine group | Control group | p value | |
| At 30 days | n = 130 | n = 128 | |
| Death | 1 (0.8) | 2 (1.6) | 1.000 |
| Cardiac death | 1 (0.8) | 1 (0.8) | 1.000 |
| Recurrent infarction | 1 (0.8) | 1 (0.8) | 1.000 |
| TVR | 0 | 1 (0.8) | 1.000 |
| Heart failure | 14 (10.8) | 20 (15.6) | 0.274 |
| MACE | 16 (12.3) | 22 (17.2) | 0.295 |
| At 1 year | n = 130 | n = 128 | |
| Death | 4 (3.1) | 4 (3.1) | 1.000 |
| Cardiac death | 3 (2.3) | 3 (2.3) | 1.000 |
| Recurrent infarction | 1 (0.8) | 2 (1.6) | 1.000 |
| TVR | 3 (2.3) | 4 (3.1) | 1.000 |
| Heart failure | 19 (14.6) | 26 (21.1) | 0.253 |
| MACE | 26 (20.0) | 32 (25.0) | 0.373 |
| At 3 years | n = 128 | n = 127 | |
| Death | 6 (4.7) | 7 (5.5) | 0.785 |
| Cardiac death | 4 (3.1) | 5 (3.9) | 0.749 |
| Recurrent infarction | 5 (3.9) | 5 (3.9) | 1.000 |
| TVR | 7 (5.5) | 9 (7.1) | 0.617 |
| Heart failure | 24 (18.5) | 30 (23.4) | 0.360 |
| MACE | 35 (26.9) | 41 (32.0) | 0.413 |
| Stent thrombosis | 4 (3.1) | 4 (3.1) | 1.000 |
| Definite | 1 (0.8) | 2 (1.6) | 0.622 |
| Probable | 2 (1.6) | 1 (0.8) | 1.000 |
| Possible | 1 (0.8) | 1 (0.8) | 1.000 |
MACE, major adverse cardiac events; TVR, target vessel revascularization.
Table 3. Medication status.
| Adenosine group | Control group | p value | |
| At 1 year | n = 130 | n = 128 | |
| Aspirin | 128 (98.5) | 126 (98.4) | 1.000 |
| Clopidogrel | 129 (99.2) | 128 (100) | 1.000 |
| Betablocking agents | 96 (73.8) | 91 (71.1) | 0.677 |
| ACEI or ARB | 95 (73.1) | 98 (76.6) | 0.567 |
| Statin | 122 (93.8) | 122 (95.3) | 0.785 |
| At 3 years | n = 128 | n = 127 | |
| Aspirin | 122 (95.3) | 120 (94.5) | 0.785 |
| Clopidogrel | 20 (15.6) | 23 (18.1) | 0.620 |
| Betablocking agents | 96 (75.0) | 92 (72.4) | 0.671 |
| ACEI or ARB | 85 (66.4) | 90 (70.9) | 0.500 |
| Statin | 118 (92.2) | 120 (94.5) | 0.617 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II blockers.
Figure 1.
Clinical outcomes with three-year follow-up.
DISCUSSION
The present study suggests that, for STEMI patients undergoing primary PCI, on the basis of thrombus aspiration and intracoronary tirofiban infusion, intracoronary bolus of high-dose adenosine (2 mg, 2 times) through aspiration catheter can further improve myocardial perfusion and reduce the incidence of no-reflow, with a trend of promoting ST-segment resolution. However this benefit would not likely improve clinical cardiovascular events and cardiac function.
Many methods and criteria have been applied to evaluate and diagnose no-reflow in PCI. Due to the difference in subjects, methods of evaluation, and diagnostic criteria among different studies, the incidence of no-reflow reported covered a wide range, from 5-50%.22 As coronary angiographic criteria, no-reflow is defined as TIMI grade < 3 or TIMI grade 3 but MBG grade 0-1. When using ST-segment resolution for evaluation of myocardial perfusion, only about 35% of patients could achieve sufficient myocardial tissue perfusion (defined as STR > 70%) after primary PCI.22 In the present study, more patients in the adenosine group achieved MBG 2-3 after primary revascularization, namely reducing the incidence of angiographic no-reflow.
Many factors contribute to no-reflow, including distal embolization, coronary spasm, ischemia-related and reperfusion-related injury. Coronary microvascular dysfunction and ischemia-reperfusion injury are important mechanisms. A variety of medications [e.g., verapamil, diltiazem, sodium nitroprusside, adenosine, and glycoprotein IIb/IIIa inhibitors (GPI)] and measures (e.g., thrombus aspiration and distal protection devices) targeting different pathological aspects of no-reflow have been explored to reduce the risk of no-reflow. The efficacy of thrombus aspiration9,23 and application of GPI11,12 in reducing no-reflow during primary PCI has been demonstrated. Medication bolus injected through the thrombus aspiration catheter into the infarct-related coronary artery is a simple and effective method. During primary PCI, combined with thrombus aspiration intracoronary administration of GPI, adenosine, and other medications through the aspiration catheter may be an effective means to further improve myocardial perfusion.
The mechanisms of action of adenosine are multifaceted and not yet entirely clear. In circumstances involving ischemia, myocardial cells produce endogenous adenosine. Experimental studies have shown that adenosine has a strong effect on vasodilation, and the capacity to inhibit platelet aggregation, inflammatory cell activation, oxygen free radical production, and intracellular calcium influx. In this manner, adenosine can reduce the severity of reperfusion injury and improve myocardial perfusion.24,25 Acute Myocardial Infarction STudy of ADenosine (AMISTAD)-I trial26 and the AMISTAD-II27 trial have shown that intravenous use of high doses of adenosine (70 μg/kg/min) could reduce infarction area. Intracoronary use of adenosine can offer a higher local concentration and less side effects. Intracoronary administration of high doses of adenosine during primary PCI have been shown to be safe and improve myocardial perfusion, reduce the incidence of no-reflow, promote ST-segment resolution, and narrow the infarction size.13,14,28,29 However, these studies included a limited number of samples, and some of them were not randomized controlled trials. In the subsequent two randomized controlled studies, the efficacy of intracoronary application of adenosine in primary PCI was not confirmed.30,31 Using cardiac magnetic resonance imaging (MRI), Desmet et al.30 found that intracoronary injection of adenosine (4 mg) during primary PCI could not improve MBG after and salvage more myocardium. In another study recruiting 448 STEMI patients, intracoronary bolus of adenosine (120 μg, twice) in primary PCI also failed to improve myocardial perfusion.31
Unfortunately, improvement of myocardial perfusion failed to benefit clinical outcomes at 1-year and 3-year, and cardiac function and LVR at 6 months in this study. To date, studies on intravenous or intracoronary use of adenosine during primary PCI had not demonstrated any advantageous influence on long-term clinical events. The potential causation of such lack of correlation may include the following: 1) the sample size may not being large enough; 2) even though intravenous and intracoronary administration of adenosine can reduce myocardial necrosis, the extent may not being enough to lead to a decrease of clinical events; 3) the use of β-blockers, ACEI or ARB and statins improving ventricular remodeling after myocardial infarction, reducing the incidence of clinical events, and thus desalinating the possible clinical benefits of adenosine.
Although it remains controversial to apply a prophylactic application of adenosine to all STEMI patients undergoing primary PCI, prophylactic use of adenosine targeting patients at high risk of no-reflow or therapeutic intracoronary administration of adenosine in patients with poor myocardial perfusion after intervention is a worthwhile undertaking.29 The efficacy of intracoronary administration of adenosine targeting these patients awaits further exploration.
CONCLUSIONS
In conclusion, this study confirmed that intracoronary administration of high-dose adenosine could further improve myocardial perfusion during primary PCI. However, whether this effect could benefit clinical outcomes and cardiac function needs further evaluation
CONFLICT OF INTEREST
There is no conflict of interest in this manuscript.
SOURCES OF SUPPORT
This project was supported by the Beijing Science Foundation of China (7142062) and Clinical-Basic Foundation of Capital Medical University (14JL40).
REFERENCES
- 1.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72:950–957. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 2.Roe MT, Ohman EM, Maas AC, et al. Shifting the open-artery hypothesis downstream: the quest for optimal reperfusion. J Am Coll Cardiol. 2001;37:9–18. doi: 10.1016/s0735-1097(00)01101-3. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe R, Charron T, Puley G, et al. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152–3156. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 4.Morishima I, Sone T, Mokuno S, et al. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J. 1995;130:239–243. doi: 10.1016/0002-8703(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 5.Brosh D, Assali AR, Mager A, et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction in six-month mortality. Am J Cardiol. 2007;99:442–445. doi: 10.1016/j.amjcard.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Ndrepepa G, Tiroch K, Keta D, et al. Predictive factors and impact of no-reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Intervent. 2010;3:27–33. doi: 10.1161/CIRCINTERVENTIONS.109.896225. [DOI] [PubMed] [Google Scholar]
- 7.Morishima I, Sone T, Okumura K, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 8.Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55:2383–2389. doi: 10.1016/j.jacc.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 10.Dangas G, Stone GW, Weinberg MD, et al. Contemporary outcomes of rescue percutaneous coronary intervention for acute myocardial infarction: comparison with primary angioplasty and the role of distal protection devices (EMERALD trial). Am Heart J. 2008;155:1090–1096. doi: 10.1016/j.ahj.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Ottani F, La Vecchia L, De Vita M, et al. Comparison by meta-analysis of eptifibatide and tirofiban to abciximab in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2010;106:167–174. doi: 10.1016/j.amjcard.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Thiele H, Schindler K, Friedenberger J, et al. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation. 2008;118:49–57. doi: 10.1161/CIRCULATIONAHA.107.747642. [DOI] [PubMed] [Google Scholar]
- 13.Claeys MJ, Bosmans J, De Ceuninck M, et al. Effect of intracoronary adenosine infusion during coronary intervention on myocardial reperfusion injury in patients with acute myocardial infarction. Am J Cardiol. 2004;94:9–13. doi: 10.1016/j.amjcard.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Stoel MG, Marques KM, de Cock CC, et al. High dose adenosine for suboptimal myocardial reperfusion after primary PCI: a randomized placebo-controlled pilot study. Cath Cardiovasc Interv. 2008;71:283–289. doi: 10.1002/ccd.21334. [DOI] [PubMed] [Google Scholar]
- 15.Tong ZC, Li Q, Chen M, et al. Efficacy comparison of combined intracoronary administration of high-dose adenosine and tirofiban versus intracoronary tirofiban during primary percutaneous coronary intervention in patients with acute myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41:839–844. [PubMed] [Google Scholar]
- 16.TIMI Study Group. The Thrombolysis and Myocardial Infarction (TIMI) Trial: phase I findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 17.Gibson CM, Cannon CP, Daley WL, et al. Frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 18.van’t Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 19.van’t Hof AW, Liem A, de Boer MJ, Zijlstra F. Clinical value of 12-lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Zwolle Myocardial Infarction Study Group. Lancet. 1997;350:615–619. doi: 10.1016/s0140-6736(96)07120-6. [DOI] [PubMed] [Google Scholar]
- 20.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 21.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 22.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 24.Forman MB, Stone GW, Jackson EK. Role of adenosine as adjunctive therapy in acute myocardial infarction. Cardiovasc Drug Rev. 2006;24:116–147. doi: 10.1111/j.1527-3466.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction and cardioprotection. The ‘Dark Side’ of reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- 26.Mahaffey KW, Puma JA, Barbagelata A, et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction. Results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 27.Ross AM, Gibbons RJ, Stone GW, et al. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 28.Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101:2154–2159. doi: 10.1161/01.cir.101.18.2154. [DOI] [PubMed] [Google Scholar]
- 29.Grygier M, Araszkiewicz A, Lesiak M, et al. New method of intracoronary adenosine injection to prevent microvascular reperfusion injury in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am J Cardiol. 2011;107:1131–1135. doi: 10.1016/j.amjcard.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Desmet W, Bogaert J, Dubois C, et al. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J. 2011;32:867–877. doi: 10.1093/eurheartj/ehq492. [DOI] [PubMed] [Google Scholar]
- 31.Fokkema ML, Vlaar PJ, Vogelzang M, et al. Effect of high-dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: a randomized controlled trial. Circ Cardiovasc Interv. 2009;2:323–329. doi: 10.1161/CIRCINTERVENTIONS.109.858977.109.858977. [DOI] [PubMed] [Google Scholar]