Abstract
Background
Heart failure (HF) readmission results in substantial expenditure on HF management. This study aimed to evaluate the readmission rate, outcome, and predictors of HF readmission.
Methods
Patients with reduced left ventricular ejection fraction (LVEF < 40%) who were admitted for acute decompensation of de novo HF were enrolled to analyze readmission rate, mortality and predictors of readmission.
Results
A total of 433 de novo HF patients with LVEF < 40% were enrolled during the period August 2013 to December 2014. The in-hospital and 6-month mortality rates were 3.9% and 15.2%, respectively. In those patients surviving the index HF hospitalization, the 30-day and 6-month readmission rates were 10.9% and 27%, respectively. At the end of the 6-month follow-up, the readmission group had higher mortality than the non-readmission group (27.66% vs. 10.36%; p = 0.001). The survivors of the 30-day readmission had similar mortality rates at 6 months, regardless of the cause of readmission (cardiovascular vs. non-cardiovascular: 25% vs. 30.43%, p = 0.677). Among all the parameters, prescription of beta blockers independently reduced the risk of 30-day readmission (odds ratio 0.15; 95% confidence interval 0.02-0.99; p = 0.049).
Conclusions
Those HF patients who suffered from 30-day readmission had worse prognosis at the 6-month follow-up. Regardless of the readmission causes, the patients surviving the 30-day readmission had similar mortality rates at 6-month follow-up. These results underscored the importance of reducing readmission as a means to improve HF outcome.
Keywords: Heart failure, Prognosis, Readmission
INTRODUCTION
Heart failure (HF), a growing epidemic worldwide, affects 1-2% of the adult population in developed countries, and the prevalence rises to more than 10% in people aged 70 years or older.1 The prevalence of HF is estimated to increase to 3.5% in the United States by 2030, and the medical costs of HF are projected to increase from $24.7 billion in 2010 to $77.7 billion in 2030.2 Hospital admission accounts for more than 50% of healthcare costs associated with HF treatment both in the United States and Europe.3,4 In a medicare database study, HF is the most frequent reason for readmission within 30 days of discharge in both medical and surgery populations.5 Despite the advances in management and improved prognosis of chronic HF in the past two decades, patients with acute decompensated HF continue to have a high mortality rate, varying from 5-15% at 60 to 90 days post-discharge.6 The readmission rate remains high, however, wherein approximately 24% at 30 days7-9 and 30% at 60 to 90 days post-discharge10 and ≥ 50% within 6 months.11,12 This all leads to the large burden of disease and heavy expenditure on HF management, implicating the desperate need to improve prediction and intervention strategies of HF readmissions.
Epidemiologic data of HF in Asia are limited. A community-based cohort study has revealed a HF prevalence of approximately 5.5% in an ethnic Chinese population in northern Taiwan, with the 5- and 10-year mortality rates being 14.1% and 24.4%, respectively, in patients with HF with preserved left ventricular ejection fraction (LVEF), and 29.2% and 48.2%, respectively, in those with HF with reduced ejection fraction (EF).13 The major etiology of HF in this study is hypertension, consistent with the result of another study in an Asian-Chinese population, in which the prevalence of coronary heart disease has been proposed to be less than in western countries.14 Nevertheless, given the high prevalence of cigarette smoking and westernization of dietary patterns, myocardial infarction (MI) has been supposed to be an increasingly common cause of HF in Taiwan.15 As the population ages and survival of various cardiovascular (CV) diseases improves, challenges have emerged with respect to the escalating burden of HF, a situation paralleling that seen in western societies. Owing to the comprehensive coverage of the National Health Insurance, the health care system in Taiwan has been recognized for its good accessibility, relatively low costs and short waiting times.16 Despite the efficiency of the Taiwanese healthcare facilities, prior studies have reported high in-hospital and annual mortality rate, 23.5% and 40-50%, respectively, in patients with New York Heart Association (NYHA) functional class III to IV waiting for cardiac transplantation,17,18 appearing to be inferior to those in the western countries.
Apart from the similarities and differences regarding HF epidemiology between the eastern and the western countries, data on the pattern of HF readmission and related outcomes in Asia population is scarce. Readmission would increase health care costs and may be associated with higher morbidity and mortality compared with the index HF admission. The present study aimed to evaluate the readmission rate, subsequent outcome and predictors of readmission in de novo HF patients with reduced EF (HFrEF) after the index admission for acute decompensated heart failure (ADHF).
METHODS
Study population and protocol
This retrospective study enrolled de novo HFrEF, defined as LVEF < 40%,19 who were admitted for ADHF to the Department of Cardiology of the Linkou Chang Gung Memorial Hospital in Taiwan from August 2013 to December 2014. ADHF is defined as gradual or rapid change in HF signs and symptoms resulting in a need for urgent therapy, usually resulting in unplanned office visits, emergency room visits, or hospitalization.20-22 Patients with a history of HF or LFEF < 40% on previous echocardiography were excluded from this study. Patients with in-hospital mortality or lost to follow-up and not further contacted by telephone during the follow-up period of 6 months were excluded from the readmission analysis. The remaining patients were then grouped into the readmission and the non-readmission groups. Readmission was defined as any rehospitalization or emergency department visit longer than 24 hours (Figure 1).
Figure 1.
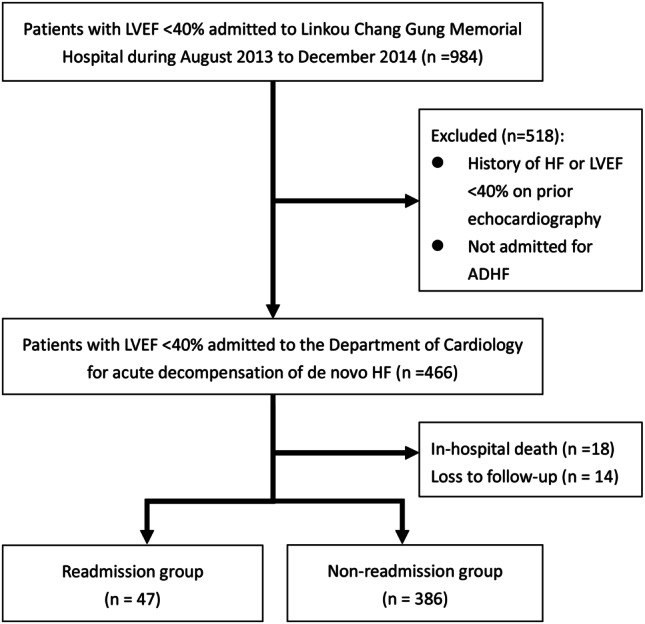
Patient enrollment. ADHF, acute decompensated heart failure; HF, heart failure; LVEF, left ventricular ejection fraction.
Data collection
Patients’ baseline characteristics were recorded in a standard case report form. The demographic data included age, sex, occupation, marital status, and educational background. The clinical variables included CV risk factors and comorbidities such as hypertension, diabetes, coronary artery disease (CAD), MI, dyslipidemia, peripheral artery disease, valvular heart disease, atrial fibrillation, smoking, family history, stroke, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and baseline medications. Past intervention history included percutaneous coronary intervention (PCI), cardiac implantable electronic devices, and coronary artery bypass graft (CABG) and valve surgeries. Other information included body mass index (BMI), blood pressure and heart rate on admission date, precipitating factors of HF admission, and NYHA functional class. Other data collected were daily input/output records, body weight (BW), dosage and duration of diuretics and inotropic agents, use of intra-aortic balloon pump (IABP), extracorporeal membrane oxygenation (ECMO) and mechanical ventilation. Laboratory data included complete blood count and leukocyte differential count, biochemistry and urinalysis. Additionally, 12-lead electrocardiography and chest X-ray findings were recorded, M-mode, 2-dimensional, and Doppler echocardiography were performed according to standard criteria.23 In-hospital outcomes included in-hospital mortality, length of hospital stay, and development of acute kidney injury (AKI). AKI was defined according to the Acute Kidney Injury Network as an absolute increase in serum creatinine of at least 0.3 mg/dL, or a minimum increment of 50% within 48 hours, or oliguria of < 0.5 mL/kg per hour for more than six hours.24
Follow-up evaluation
Patients enrolled in this study were followed for a period of 6 months. After discharge from the index admission, the first follow-up clinic visit was scheduled within the first two weeks post-discharge. Readmission information was obtained mainly from the primary care physicians in the outpatient department, electronic medical records, and telephone contact with the patients or their relatives by the HF case managers. Information collected during the follow-up period included symptoms and signs of HF, medication compliance and adverse effects, dietary pattern, nutritional status, BW changes, and rehabilitation program. Information on readmission included the frequency and reasons for readmission, and the date of readmission and discharge. The CV causes of readmission included HF exacerbation, angina, acute coronary syndrome, ventricular tachyarrhythmia, atrial tachyarrhythmia, and bradycardia. The non-CV causes of readmission included but were not limited to pneumonia, urinary tract infection, other infection, gastrointestinal tract bleeding, renal function deterioration, and COPD with acute exacerbation.
Statistical analysis
The patients were divided into the readmission and the non-readmission groups. Categorical variables were expressed as percentages, and continuous variables were expressed as mean ± standard deviation. The chi-square test with Fisher’s exact test was used to compare categorical variables between the two groups. Continuous variables were compared utilizing the student’s t-test. Variables with a p value < 0.05 were considered to be statistically different between the two groups, and then were incorporated into the multivariate logistic regression to determine the independent predictors of readmission. Kaplan-Meier survival curves and the log-rank test were used to compare survival of the readmission vs. the non-readmission groups and the CV vs. the non-CV causes of readmissions. Data were analyzed with the SPSS version 19.0 (SPSS, Chicago, Illinois, USA).
RESULTS
In-hospital and 6-month mortality and readmission rates
A total of 984 patients with reduced LVEF were admitted to Linkou Chang Memorial Hospital during the study period. Among them, 466 were diagnosed as de novo HF with acute decompensation. The in-hospital mortality of the index HF admission was 3.9%, and the 6-month mortality was 15.2%. Fourteen patients (3%) were excluded from the readmission analysis because of loss to follow-up and lack of telephone contact. The 30-day readmission rate was 10.9%. One hundred seventeen patients (27%) were readmitted at least once at the end of the 6-month follow-up. Twenty-six of the 117 patients were admitted twice, 8 patients three times, 6 patients four times, and one patient was admitted 5 times. Overall, the CV and the non-CV causes comprised 56.7% and 43.3% of the readmissions, respectively (Figure 2).
Figure 2.
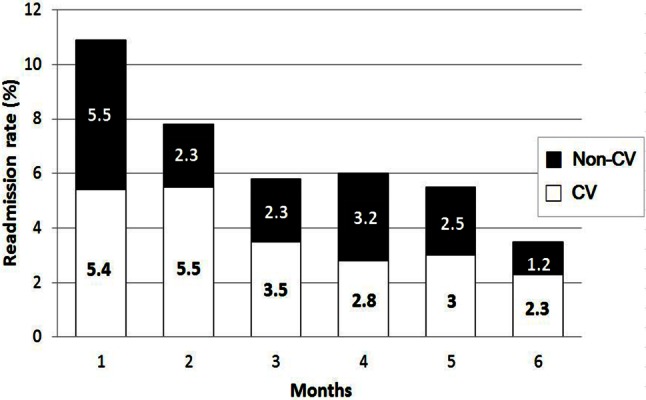
Cardiovascular and non-cardiovascular readmissions during 6-month follow-up. CV, cardiovascular.
Clinical characteristics associated with readmission
Table 1 demonstrated the baseline characteristics of the study population. The cohort of all patients had an average age of 65.4 years, with 68.6% men. Patients in the readmission group were older (69.4 vs. 64.93; p = 0.025) and more frequently diagnosed with hypertension (85.11% vs. 61.92%; p = 0.002) than those in the non-readmission group. The non-readmission group tended to be male gender (69.9% vs. 57.4%, p = 0.081) and had higher BMI (25.3 kg/m2 vs. 25.3 kg/m2, p = 0.097) than the readmission group. There was no significant difference between the two study groups in terms of percentage of NYHA function class III or IV, admission blood pressure and heart rate, and comorbidities such as CAD, MI, diabetes, CKD, atrial fibrillation, COPD and malignancy. Regarding the laboratory data, the readmitted patients had relatively higher levels of blood urea nitrogen (45 mg/dL vs. 29.86 mg/dL; p = 0.06) and creatinine on admission (2.8 mg/dL vs. 1.95 mg/dL; p = 0.06; Table 2). The readmitted patients also had lower levels of eGFR (31.33 mL/min vs. 54.03 mL/min; p = 0.034), sodium (137.37 mg/dL vs. 138.98 mg/dL, p = 0.016), and hemoglobin (11.78 mg/dL vs. 12.87 mg/dL; p = 0.006). In spite of the lack of statistical difference, more patients in the readmission group had moderate to severe mitral (42.55% vs. 29.79%; p = 0.075) and tricuspid regurgitation (29.79% vs. 15.54%; p = 0.094).
Table 1. Baseline characteristics of heart failure patients.
| All patients (n = 433) | Readmission (n = 47) | Non-readmission (n = 386) | p value | |
| Age (years) | 65.42 ± 15.67 | 69.4 ± 12.17 | 64.93 ± 15.99 | 0.025 |
| Male gender (%) | 68.6 | 57.4 | 69.9 | 0.081 |
| Body mass index (kg/m2) | 25.23 ± 4.62 | 24.4 ± 4.23 | 25.33 ± 4.66 | 0.097 |
| NYHA functional class III or IV (%) | 82.91 | 82.98 | 82.9 | 0.989 |
| Systolic blood pressure (mm Hg) | 138.73 ± 32.99 | 137.51 ± 31.72 | 138.88 ± 33.18 | 0.8 |
| Diastolic blood pressure (mm Hg) | 84.25 ± 21.55 | 81.34 ± 19.35 | 84.6 ± 21.8 | 0.328 |
| Heart rate (beasts/min) | 101.03 ± 26.06 | 101.66 ± 20.58 | 100.95 ± 26.67 | 0.861 |
| Cardiovascular risk factors/comorbidities (%) | ||||
| Coronary artery disease | 52.19 | 55.32 | 51.81 | 0.168 |
| Myocardial infarction | 18.24 | 14.89 | 13.73 | 0.366 |
| Diabetes mellitus | 41.57 | 48.94 | 40.67 | 0.278 |
| Hypertension | 64.43 | 85.11 | 61.92 | 0.002 |
| Dyslipidemia | 57.04 | 63.83 | 56.22 | 0.32 |
| Smoking | 44.8 | 34.04 | 46.11 | 0.266 |
| CKD stage ≥ 3 | 41.28 | 51.35 | 40 | 0.187 |
| Dialysis | 10.17 | 12.77 | 9.84 | 0.531 |
| Stroke | 16.4 | 23.4 | 15.54 | 0.169 |
| Atrial fibrillation | 27.02 | 25.53 | 27.2 | 0.945 |
| COPD | 11.78 | 14.89 | 11.4 | 0.483 |
| Malignancy | 3.75 | 6.52 | 3.41 | 0.417 |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association.
Table 2. Laboratory tests and cardiac examinations on admission.
| All patients (n = 433) | Readmission (n = 47) | Non-readmission (n = 386) | p value | |
| Laboratory tests | ||||
| Blood urea nitrogen (mg/dL) | 31.55 ± 21.5 | 45 ± 32.85 | 29.86 ± 19.04 | 0.06 |
| Creatinine (mg/dL) | 2.05 ± 2.22 | 2.8 ± 2.88 | 1.95 ± 2.11 | 0.06 |
| eGFR (ml/min) | 52.54 ± 29.37 | 31.33 ± 20.93 | 54.03 ± 29.37 | 0.034 |
| Sodium (mg/dL) | 138.81 ± 4.28 | 137.37 ± 5 | 138.98 ± 4.15 | 0.016 |
| Potassium (mg/dL) | 4.07 ± 0.56 | 4.02 ± 0.66 | 4.07 ± 0.55 | 0.546 |
| AST (mg/dL) | 67.33 ± 239.88 | 38.49 ± 27.35 | 70.79 ± 253.5 | 0.452 |
| ALT (mg/dL) | 52.86 ± 137.65 | 42.12 ± 47.97 | 54.15 ± 144.67 | 0.598 |
| Total bilirubin (mg/dL) | 0.91 ± 1.13 | 0.88 ± 0.82 | 0.91 ± 1.16 | 0.87 |
| Fasting glucose (mg/dL) | 124.08 ± 61.68 | 117.86 ± 38.83 | 124.97 ± 64.32 | 0.623 |
| HbA1C (%) | 6.71 ± 1.51 | 6.66 ± 1.08 | 6.71 ± 1.55 | 0.858 |
| BNP (pg/mL) | 1574.39 ± 1331.19 | 1509.21 ± 1405.97 | 1583.13 ± 1323.43 | 0.758 |
| Troponin I (ng/mL) | 3.44 ± 13.09 | 4.89 ± 16.91 | 3.26 ± 12.57 | 0.475 |
| Uric acid (mg/dL) | 8.19 ± 3.19 | 7.64 ± 3.46 | 8.27 ± 3.16 | 0.323 |
| Hemoglobin (mg/dL) | 12.76 ± 2.53 | 11.78 ± 2.69 | 12.87 ± 2.48 | 0.006 |
| Proteinuria ≥ 2+ | 29.6 | 41.94 | 28.05 | 0.11 |
| Electrocardiography (%) | ||||
| Rhythm (%) | 0.789 | |||
| Sinus | 72.66 | 73.91 | 72.51 | |
| Atrial fibrillation/flutter | 24.77 | 23.91 | 24.87 | |
| Pacemaker | 1.4 | 0 | 1.57 | |
| Others | 1.17 | 2.17 | 1.05 | |
| LBBB (%) | 6.06 | 4.35 | 6.27 | 0.606 |
| LV hypertrophy (%) | 17.95 | 15.22 | 18.28 | 0.609 |
| Pathologic Q wave (%) | 0.47 | 0 | 0.52 | 0.623 |
| QRS duration (ms) | 103.51 ± 25.58 | 103.65 ± 27.5 | 103.49 ± 25.38 | 0.967 |
| Corrected QT interval (ms) | 476.4 ± 43.11 | 481.09 ± 42.71 | 475.84 ± 43.18 | 0.436 |
| Echocardiography | ||||
| LVEF (%) | 30.45 ± 7.34 | 31.78 ± 6.73 | 30.29 ± 7.41 | 0.19 |
| Left atrial diameter (mm) | 44.39 ± 8.24 | 43.15 ± 7.54 | 44.54 ± 8.32 | 0.248 |
| LVEDD (mm) | 58.19 ± 8.69 | 56.52 ± 9.73 | 58.39 ± 8.54 | 0.165 |
| Left ventricular mass (g) | 284.25 ± 105.13 | 265.25 ± 98.65 | 286.12 ± 105.76 | 0.365 |
| E/A ratio | 1.39 ± .87 | 1.46 ± 0.83 | 1.38 ± 0.88 | 0.666 |
| E/E’ | 23.1 ± 11.8 | 23.08 ± 7.08 | 23.11 ± 12.15 | 0.63 |
| Moderate or severe MR (%) | 31.18 | 42.55 | 29.79 | 0.075 |
| Moderate or severe TR (%) | 17.09 | 29.79 | 15.54 | 0.094 |
| Moderate or severe AR (%) | 6.46 | 4.26 | 6.74 | 0.878 |
ALT, alanine aminotransferase; AR, aortic regurgitation; AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; EF, ejection fraction; eGFR, estimated glomerular filtration rate; LBBB, left bundle branch block; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; MR, mitral regurgitation; TR, tricuspid regurgitation.
Comparison of management during the index admission
The average length of hospital stay of all study patients was 16.2 days, with 20.4% requiring inotropic agents and 29.2% developing AKI (Table 3). The readmission group had a numerically longer length of stay (21.11 days vs. 15.62 days; p = 0.091) and a higher risk of AKI (45% vs. 27.84%; p = 0.09). There was no significant difference in revascularization procedures, valve surgery, implantation of electronic devices, or the use of inotropic agents, IABP and ECMO between the two groups. After treatment, there was an average weight reduction of 3.3 kg in all patients upon discharge. However, the readmission group had a higher average heart rate (83.74 beats/min vs. 77.73 beats/min; p = 0.015) upon discharge and a higher percentage of patients discharged with NYHA function class 3 or 4 (47.83% vs. 22.87%; p < 0.001).
Table 3. Comparison of management during the index admission and post-discharge mortality at 6 months.
| All patients (n = 433) | Readmission (n = 47) | Non-readmission (n = 386) | p value | |
| Length of hospital stay (days) | 16.22 ± 17.86 | 21.11 ± 20.98 | 15.62 ± 17.38 | 0.091 |
| Inotropic agents (%) | 20.37 | 21.74 | 20.21 | 0.807 |
| IV diuretics use (%) | 64.35 | 69.57 | 63.73 | 0.435 |
| Development of AKI (%) | 29.16 | 45 | 27.84 | 0.09 |
| Interventional therapy (%) | ||||
| CIED | 0.468 | |||
| Pacemaker | 1.85 | 2.13 | 1.81 | |
| ICD | 0.69 | 2.13 | 0.52 | |
| CRT | 0.46 | 0 | 0.52 | |
| PCI | 16.01 | 19.15 | 15.63 | 0.524 |
| CABG | 4.39 | 6.38 | 4.15 | 0.479 |
| Valve surgery | 1.85 | 2.13 | 1.81 | 0.88 |
| IABP | 3.94 | 0 | 4.4 | 0.146 |
| ECMO | 1.39 | 0 | 1.55 | 0.394 |
| Cardiac rehabilitation | 20.09 | 23.91 | 19.63 | 0.494 |
| Discharge status | ||||
| Systolic blood pressure (mm Hg) | 121.54 ± 22.58 | 122.51 ± 20.91 | 121.49 ± 22.75 | 0.771 |
| Diastolic blood pressure (mm Hg) | 73.20 ± 14.03 | 72.68 ± 14.92 | 73.20 ± 13.95 | 0.811 |
| Heart rate (beasts/min) | 78.56 ± 15.90 | 83.74 ± 16.91 | 77.73 ± 15.76 | 0.015 |
| Weight changes (kg) | -3.33 ± 4.68 | -3.28 ± 4.27 | -3.33 ± 4.73 | 0.928 |
| NYHA functional class III or IV on discharge (%) | 25.59 | 47.83 | 22.87 | < 0.001 |
| Discharge medications (%) | ||||
| ACEI | 24.04 | 25.53 | 23.85 | 0.799 |
| ARB | 59.62 | 40.43 | 53.93 | 0.081 |
| Beta blocker | 75.06 | 65.96 | 76.17 | 0.032 |
| Aldosterone antagonist | 34.86 | 19.14 | 36.85 | 0.016 |
| CCB | 9.86 | 14.89 | 9.21 | 0.219 |
| Thiazide or loop diuretics | 54.04 | 46.81 | 54.92 | 0.292 |
| Antiplatelet agent | 60.74 | 57.44 | 61.14 | 0.23 |
| Anticoagulation agent | 20.67 | 17.02 | 21.14 | 0.512 |
| Antiarrhythmic agent | 4.33 | 2.13 | 4.61 | 0.431 |
| Nitrate | 38.22 | 29.79 | 39.3 | 0.206 |
| Digoxin | 17.79 | 14.89 | 18.16 | 0.582 |
| 6-month mortality (%) | 0.001 | |||
| All-cause mortality | 12.24 | 27.66 | 10.36 | |
| Cardiovascular | 7.85 | 14.89 | 6.99 | |
| Non-cardiovascular | 4.39 | 12.77 | 3.37 |
ACEI, angiotensin-converting-enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CCB, calcium channel blocker; CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ECMO, extracorporeal membrane oxygenation; IABP, intraaortic balloon pumping; ICD, implantable cardioverter defibrillator; ICU, intensive care unit; IV, intravenous; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Predictors of readmission
Table 4 revealed the result of the multivariate logistic regression analysis. Among all the parameters, prescription of beta blockers independently reduced the risk of readmissionin HF patients (odds ratio 0.15; 95% confidence interval 0.02-0.99; p = 0.049).
Table 4. Multivariate analysis of predictors of 30-day readmission.
| Odds ratio | 95% confidence interval | p value | ||
| Low | Upper | |||
| Age | 1.04 | 0.97 | 0.90 | 0.335 |
| Hypertension | 52.21 | 4.66 | 0.42 | 0.212 |
| eGFR | 1.03 | 1.00 | 0.96 | 0.894 |
| Hemoglobin | 1.10 | 0.74 | 0.50 | 0.141 |
| Heart rate on discharge | 1.13 | 1.05 | 0.99 | 0.126 |
| NYHA functional class III or IV on discharge | 45.10 | 5.26 | 0.61 | 0.130 |
| Beta blocker | 0.99 | 0.15 | 0.02 | 0.049 |
| Aldosterone antagonist | - | - | - | 0.997 |
eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association.
Association of 30-day readmission and 6-month mortality
In patients surviving the index HF admission, the post-discharge 6-month mortality rate was 12.2%. The post-discharge 6-month mortality was significantly higher in the readmission group than in the non-readmission group (27.66% vs. 10.36%; p = 0.001; Table 3). We also evaluated the influence of the causes of 30-day readmission on outcome. There was no significant difference in 6-month mortality between the patients readmitted for CV and non-CV causes (25% vs. 30.43%, p = 0.677). Figure 3 illustrates the Kaplan-Meier survival analysis of readmission vs. non-readmission groups (A) and CV vs. non-CV causes of readmission (B).
Figure 3.
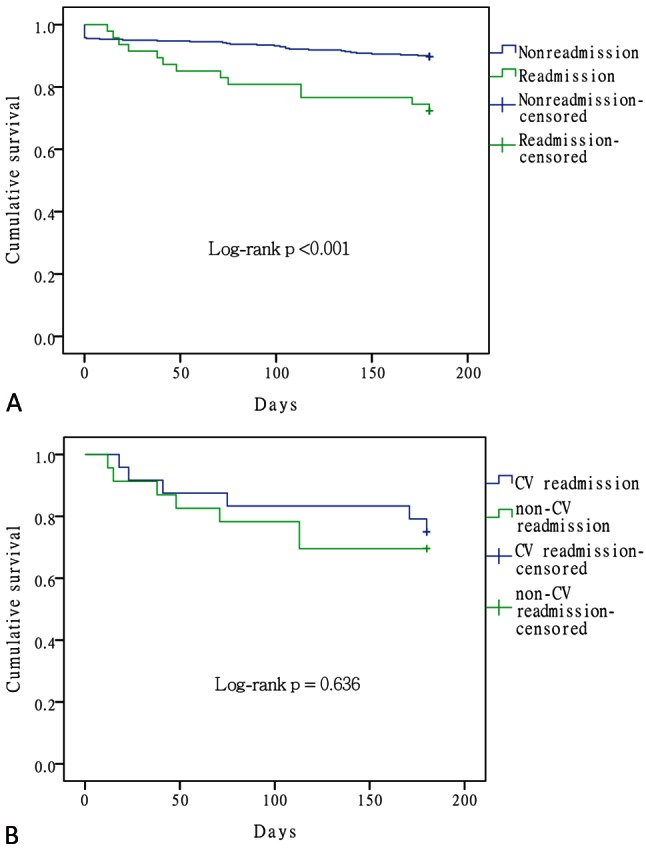
Kaplan-Meier survival analysis of readmission vs. non-readmission groups (A) and cardiovascular (CV) vs. non-CV causes of readmission (B).
DISCUSSION
This retrospective study analyzed readmission rate, subsequent outcome, and predictors of readmission in patients with LVEF < 40% who were admitted for de novo HF with decompensation. The 30-day and 6-month readmission rates were 10.9% and 27%, respectively. The post-discharge mortality at 6 months was significantly higher in the readmission group than in the non-readmission group. The patients surviving the 30-day readmission had similar mortality rate at the end of follow-up, regardless of the causes of the initial readmission.
The common comorbidities of HF patients in this study included hypertension (64.4%), CAD (52.2%), and diabetes (41.3%), a pattern similar to the ADHF registries the EHFS II study in Europe (hypertension 62.5%, 53.6%, and diabetes 32.8%)25 and the ADHERE study in the United States (hypertension 73%, CAD 57%, and diabetes 44%).26 In contrast to the prior belief of low prevalence of CAD among Taiwan’s HF patients (2.5% in a community cohort study13 and 31.5% in a more recent claims database study27), it is worth noting that the prevalence of CAD in this study was comparable to that in the western societies.25,26 Although there was potential selection bias of higher comorbidity burden in patients admitted to this tertiary referral center, increasing prevalence of western dietary pattern may also in part account for the changes in epidemiology of cardiovascular disease in Taiwan. Furthermore, there was a trend that BMI was lower in the readmission group than that in the non-readmission group, a finding that may be attributed to the “obesity paradox” — clinical outcomes of CV diseases, including HF, are better in obese patients than in their leaner counterparts.28-30 However, data on the association between BMI and HF readmission are scarce. In a retrospective study by Zapatero et al., obesity in patients admitted for ADHF substantially reduces in-hospital mortality and the possibility of 30-day readmission, whereas malnutrition was associated with increases in in-hospital mortality and the risk 30-day readmission.31 Further large, multicenter studies are warranted to establish the association between BMI and HF readmission.
The 30-day and 6-month readmission rates in this study were much lower than the 24%7-9 and ≥ 50% observed in western societies, respectively.11,12 It has been estimated that worsening chronic HF, de novo HF and advanced or end-stage HF comprised 80%, 15% and 5% of hospitalization for HF, respectively.32 The present study only analyzed patients with de novo HF and therefore the readmission rates may be underestimated. In addition, the average length of stay was 16.22 days, much longer than that of the United States (6.4 ± 85.2 days).32 The longer hospital stay in this study may be due to comprehensive assessment of de novo HF patients as well as cardiac rehabilitation during hospitalization. Whether longer hospital stay leads to reduction of 30-day readmission is yet to be determined. Furthermore, we could not exclude the possibility that some patients were admitted to other hospitals, an inherent limitation of this retrospective analysis that may lead to underestimation of the readmission rates.
Although rehospitalizations of HF patients are frequently due to congestion, a significant number of readmissions are associated with cardiac and noncardiac comorbidities.33,34 It is noteworthy that the non-CV causes comprised 43.3% of all readmissions during the 6-month follow-up in this study. Importantly, we found that the 6-month mortality of the patients who were readmitted for non-CV causes within 30 days of discharge was similar to those rehospitalized for CV causes. This suggested that any causes of 30-day readmission in patients with HFrEF were relevant for HF prognosis and should be given equal attention to prevent adverse outcomes. A recent analysis of the CHARM studies (CHARM-Added, CHARM-Preserved, and CHARM-Alternative) also reported a similar risk of subsequent mortality in HF patients across the spectrum of EF, regardless of whether they were first hospitalized for CV or non-CV causes.33
Analysis of readmission in one institution may help improve its efficiency and quality of care and facilitate risk stratification based on patient characteristics. In this study, the lack of beta blocker prescription on discharge was an independent predictor of HF readmission. However, we should also note that patients in the readmission group were discharged with a higher average heart rate, and a higher percentage of them had NYHA functional class 3 or 4 upon discharge, indicating that many of the readmitted patients had been discharged from the index hospitalization without adequate decongestion. So far, a robust and well-validated statistical model for prediction of HF readmission or risk stratification is not yet available.35,36 It has been demonstrated that physiologic indicators of HF severity and serum makers of neurohormonal activation foretell higher readmission rates.37-40 Associated non-CV comorbidities, including diabetes,41-43 renal dysfunction,44,45 anemia46 and pulmonary disease,11 also increased HF or non-HF related complications, including readmission. The inconsistencies in data on readmission prediction may result from a diverse patient spectrum and other non-measurable life events that dominate the clinical variables from the large administrative database. Nevertheless, given the high prevalence of HF comorbidities and their association with readmission and outcome, addressing the non-CV comorbidities is still an important adjunct to the multidisciplinary approach of HF management.
Limitations
This retrospective study had several inherent limitations. First, it was conducted in a tertiary referral center in Taiwan and only de novo HF patients with LVEF < 40% were analyzed. These results could not be generalized to the whole spectrum of HF patients because of potential selection bias. A larger and multicenter registry is needed to represent the epidemiology of HF readmission in Taiwan. Second, despite the extensive effort to identify all readmissions, some readmissions to other hospitals may not be recorded. Third, psychosocial and/or social economic factors were not analyzed in this study. Furthermore, the patient number was relatively small, and only one independent predictor of readmission was derived from the multivariate analysis. This result may be a chance finding and could not allow for the development of a risk prediction model.
CONCLUSIONS
The readmission rates in de novo HF patients with LV EF < 40% in this study were 10.9% and 27% at 30 and 6 months, respectively. The readmitted patients had a higher mortality rate than the non-readmitted patients upon 6-month follow-up. The patients surviving the CV or the non-CV readmissions within 30 days of discharge had a similar mortality rateat the end of follow-up. These results underscored the importance of reducing readmission as a means to improve HF outcome. The high burden of HF comorbidities and the association with readmission rate and mortality also underscored the idea that addressing the non-CV comorbidities should serve as an adjunct to the multidisciplinary approach of HF management.
DISCLOSURES
The authors have no conflicts of interest to declare in this study. This work is supported by Chang Gung Memorial Hospital, CIRPG3E0011 and CMRPG3F1631.
REFERENCES
- 1.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: apolicy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart S, MacIntyre K, Hole DJ, et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. New Engl J of Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2009;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 9.Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309:587–593. doi: 10.1001/jama.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure a report from the OPTIMIZE-HF registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 11.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 12.Butler J, Kalogeropoulos A. Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat! J Am Coll Cardiol. 2008;52:435–437. doi: 10.1016/j.jacc.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Huang CH, Chien KL, Chen WJ, et al. Impact of heart failure and left ventricular function on long-term survival - report of a community-based cohort study in Taiwan. Eur J Heart Fail. 2007;9:587–593. doi: 10.1016/j.ejheart.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls MG, Richards AM. Is hypertension a leading cause of heart failure in Chinese? Clin Exp Pharmacol Physiol. 2002;29:850–851. doi: 10.1046/j.1440-1681.2002.03735.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Chen M, Tang WW. Improving care for patients with heart failure: what can Taiwan accomplish? Acta Cardiol Sin. 2007;23:211. [Google Scholar]
- 16.Wu TY, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. Lond J Prim Care. 2010;3:115–119. doi: 10.1080/17571472.2010.11493315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MC, Chang HW, Cheng CI, et al. Risk stratification of in-hospital mortality in patients hospitalized for chronic congestive heart failure secondary to non-ischemic cardiomyopathy. Cardiology. 2003;100:136–142. doi: 10.1159/000073931. [DOI] [PubMed] [Google Scholar]
- 18.Huang CM, Young MS, Wei J. Predictors of short-term outcome in Chinese patients with ambulatory heart failure for heart transplantation with ejection fraction < 25%. Jpn Heart J. 2000;41:349–369. doi: 10.1536/jhj.41.349. [DOI] [PubMed] [Google Scholar]
- 19.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Joseph SM, Cedars AM, Ewald GA, et al. Acute decompensated heart failure:contemporary medical management. Tex Heart I J. 2009;36:510. [PMC free article] [PubMed] [Google Scholar]
- 23.Henry WL, DeMaria A, Gramiak R, et al. Report of the American Society of Echocardiography Committee on nomenclature and standards in two-dimensional echocardiography. Circulation. 1980;62:212–215. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 24.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieminen MS, Brutsaert D, Dickstein K, et al. Euro Heart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients:description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 26.Adams KF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Tseng CH. Clinical features of heart failure hospitalization in younger and elderly patients in Taiwan. Eur J Clin Invest. 2011;41:597–604. doi: 10.1111/j.1365-2362.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 28.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 31.Zapatero A, Barba R, Gonzalez N, et al. Influence of obesity and malnutrition on acute heart failure. Rev Esp Cardiol. 2012;65:421–426. doi: 10.1016/j.recesp.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure. J Am Coll Cardiol. 2013;61:391–403. doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Desai AS, Claggett B, Pfeffer MA, et al. Influence of hospitalization for cardiovascular versus noncardiovascular reasons on subsequent mortality in patients with chronic heart failure across the spectrum of ejection fraction. Circ Heart Fail. 2014;7:895–902. doi: 10.1161/CIRCHEARTFAILURE.114.001567. [DOI] [PubMed] [Google Scholar]
- 34.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–433. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 36.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Linked to Medicare Claims. Circ Heart Fail. 2011;4:628–636. doi: 10.1161/CIRCHEARTFAILURE.111.962290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure data from 2 large randomized clinical trials. Circulation. 2012;125:280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 39.Allen LA, Gheorghiade M, Reid KJ, et al. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4:389–398. doi: 10.1161/CIRCOUTCOMES.110.958009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunlay SM, Gheorghiade M, Reid KJ, et al. Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12:367–374. doi: 10.1093/eurjhf/hfq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 42.Krumholz HM, Chen YT, Wang Y, et al. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139:72–77. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2007;154:277. e1-8. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Butler J, Chirovsky D, Phatak H, et al. Renal function, health outcomes, and resource utilization in acute heart failure a systematic review. Circ Heart Fail. 2010;3:726–745. doi: 10.1161/CIRCHEARTFAILURE.109.920298. [DOI] [PubMed] [Google Scholar]
- 45.Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 46.Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]


