Abstract
Sunitinib is a chemotherapeutic agent that has been approved for renal cell carcinoma and gastrointestinal stromal tumors resistant to imatinib. It is usually well tolerated and severe gastrointestinal side effects are rare. There are very few reports of sunitinib causing severe esophagitis, and only one of them was previously reported as exfoliative esophagitis. We describe a case of severe sunitinib-induced exfoliative esophagitis that resulted in overt gastrointestinal bleed.
Introduction
Sunitinib is an orally available multireceptor tyrosine kinase inhibitor with antiangiogenic activity. It targets vascular endothelial growth factor (VEGF) receptors, platelet-derived growth factor receptors, and stem cell factor receptors.1 Generally well tolerated, sunitinib was approved in 2005 for metastatic renal cell carcinoma and GI stromal tumors resistant to imatinib.2,3 The standard dose of sunitinib is 50 mg per day, 4 weeks on and 2 weeks off.1,2
Exfoliative esophagitis is a rare condition caused by the dissection and exfoliation of esophageal mucosa from submucosal tissues.4,5 The usual etiologies of exfoliative esophagitis are chemical, thermal, or physical trauma from foods, from drugs like cyclooxygenase-2 (COX-2) inhibitors and nonsteroidal anti-inflammatory drugs, radiation, endoscopy,4,5 or from dermatological conditions like pemphigus vulgaris.4 To date, only one case of exfoliative esophagitis caused by sunitinib has been reported, thus making this a rare etiology, especially in its causation of gastrointestinal (GI) bleed.5
Case Report
A 66-year-old man presented to our hospital with a 1-day history of acute-onset solid- and liquid-phase dysphagia and odynophagia as well as an episode of melena. He had a known history of stage IV renal cell carcinoma treated with nephrectomy and sunitinib initiated 10 months prior to his presentation. His medical history was otherwise significant for atrial fibrillation managed with warfarin and gastroesophageal reflux disease managed with pantoprazole (40 mg daily) and sucralfate for many years. Admission hemoglobin was 10.6 g/dL, significantly lower than his baseline of 13.0 g/dL, and he had an international normalized ratio of 4.1.
An esophagogastroduodenoscopy (EGD) revealed extensive necrosis and ulcerated mucosa throughout the esophagus, with oozing blood and clots in the distal esophagus (Figure 1). Given the diffuse nature of the lesions, endoscopic intervention was not indicated. There were multiple openings in the wall of the esophagus that looked like fistulas (Figure 2). There was no narrowing noted in the esophagus (luminal diameter was estimated to be more than 20 mm), and the gastroesophageal junction was traversed easily. No discrete masses were noted in the esophagus. There was mild esophagitis proximal to the area of necrosis. Multiple biopsies were performed. The remainder of the examination showed a normal stomach and mild duodenitis in the bulb.
Figure 1.
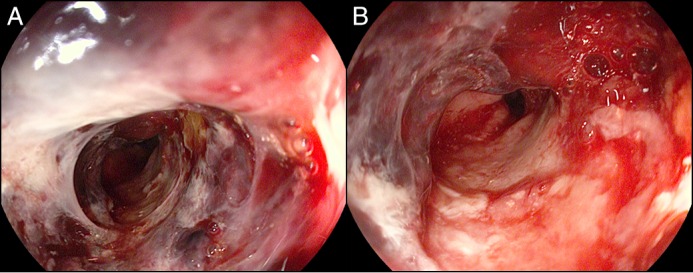
EGD revealed (A) extensive necrosis and ulcerated mucosa throughout the esophagus with (B) oozing blood and clots in the distal esophagus.
Figure 2.
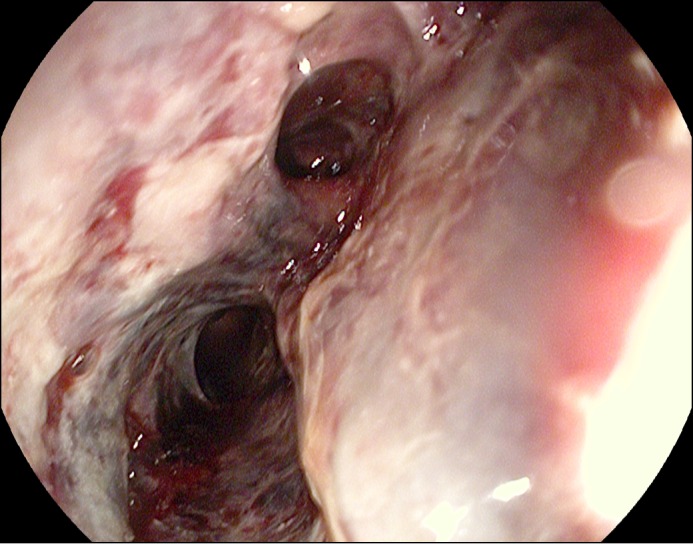
EGD showing a possible fistulous opening.
An omnipaque/barium esophagram showed multiple filling defects within the mid- to distal esophagus, as well as areas of mucosal irregularity possibly related to ulceration (Figure 3). The lack of contrast extravasation ruled out active fistulas. Pathologic examination of the biopsy specimens showed partially necrotic fragments of squamous mucosa, extensive acute inflammation, and fibrinous exudate. These morphologic findings were consistent with exfoliative esophagitis.
Figure 3.
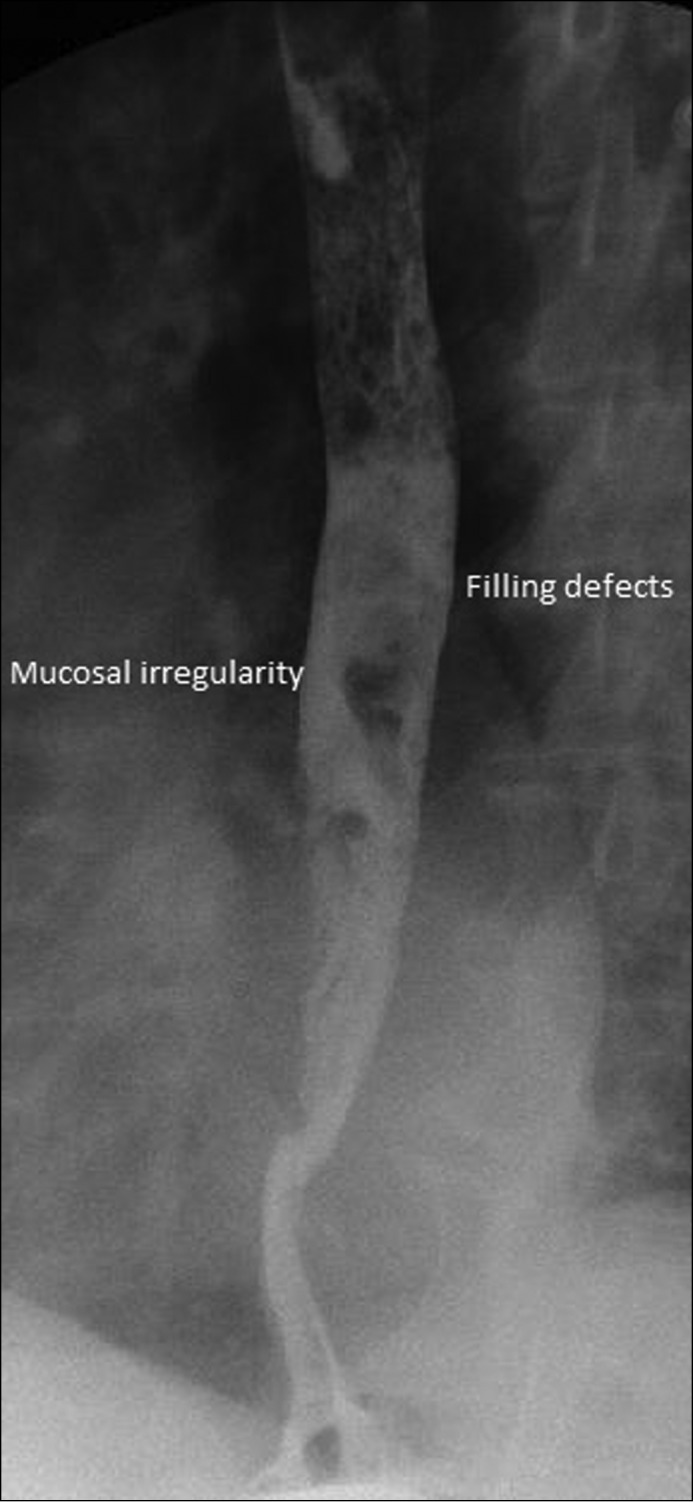
Omnipaque/barium esophagram showed multiple filling defects within the mid- to distal esophagus, as well as areas of mucosal irregularity possibly related to ulceration.
We suspected that sunitinib was responsible for these findings and promptly discontinued this medication. Pantoprazole was increased to 40 mg twice daily to aid in esophageal healing. The patient’s dysphagia improved over the next 3 days. He remained stable with no further GI bleeding and was discharged home. While on high-dose pantoprazole, the patient was restarted on a reduced dose of sunitinib 4 weeks later, which was well tolerated. Repeat EGD 7 weeks later demonstrated complete healing of the esophageal ulceration and necrosis (Figure 4). There were no strictures noted on this EGD.
Figure 4.
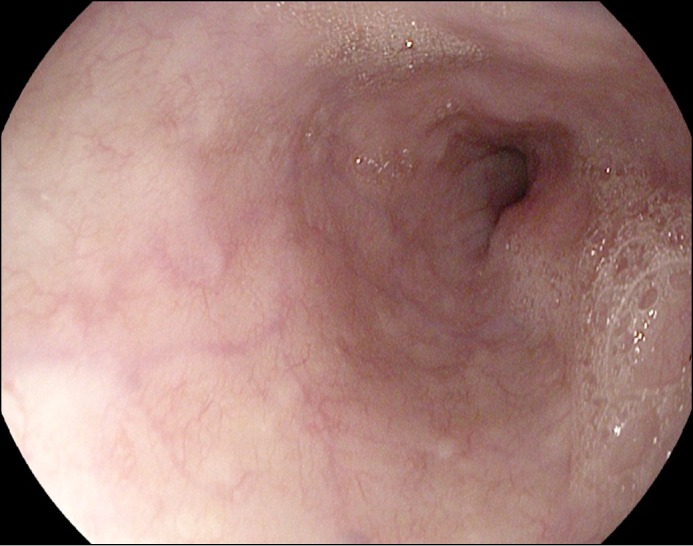
Repeat EGD 7 weeks after treatment demonstrated complete healing of the esophageal ulceration and necrosis.
Discussion
The efficacy and safety of sunitinib for patients with metastatic renal cell carcinoma or GI stromal tumors have been well documented.5 The non-GI side effects are usually mild and commonly include asthenia, stomatitis, hypertension, left heart failure, and skin manifestations, including hand-foot skin reaction.1,2,5,6 Gastrointestinal side effects include diarrhea, nausea, gastroesophageal reflux, mild esophagitis, and mild gastritis.1,5 Esophagitis/gastritis is thought to be due to mucosal irritation by peptic acid secretion.1 In most cases, either mild or no visible endoscopic findings can be seen.1,2,5 Patients may also report symptoms of dysphagia and odynophagia.1 Esophagitis with extensive exfoliation is a very rare manifestation of severe esophageal mucositis.5
The mechanism of the development of exfoliative esophagitis is not yet known. It is presumed that sunitinib promotes the development of gastroesophageal reflux, which then leads to severe esophagitis. It is believed to be related to VEGF and mitogen-activated protein kinase pathways that are involved in mucosal defense from gastric acid and repair of mucosal lesions.1,2 VEGF receptors are expressed in normal esophageal mucosa and are responsible for restoring connective tissue and angiogenesis in acutely and chronically injured mucosa.6,7,8 Acute and chronic injury can increase the expression of VEGF.5,8 VEGF inhibition results in impaired mucosal healing and increases the vulnerability of the mucosa.5 Additionally, as seen in rat models, COX-2 is an important inducer of VEGF in the esophageal mucosa and delays ulcer healing when exposed to nonsteroidal anti-inflammatory drugs.5,9
Our patient had no underlying conditions predisposing him to exfoliative esophagitis, and sunitinib was therefore considered as the cause. We encourage clinicians to consider medications, especially chemotherapeutic agents, as a cause for severe esophagitis and occult or overt GI bleeding.
Disclosures
Author contributions: Both authors contributed equally to literature review and to manuscript writing, revision, and review. S. Gayam is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandora’s vase: The growing problem of new toxicities from novel anti-cancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007; 7:127–34. [DOI] [PubMed] [Google Scholar]
- 2.Jeanniard-Malet O, Gravis G. Three cases of severe ulcerative esophagitis induced by SUTENT®. J Gastrointest Cancer. 2012; 43(1):128–30. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomezak P, et al. Sunitinib versus interferon alpha in metastatic renal-cell carcinoma. N Engl J Med. 2007; 356:115.. [DOI] [PubMed] [Google Scholar]
- 4.Fukuchi M, Otake S, Maitoh H, et al. A case of exfoliative esophagitis with pemphigus vulgaris. Dis Esophagus. 2011; 24(3):E23–5. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Wang T, Huang Y. Exfoliative esophagitis in a patient with metastatic renal cell carcinoma during sunitinib treatment. Medical Oncology. 2013; 30(1):436. [DOI] [PubMed] [Google Scholar]
- 6.Wood LS. Management of vascular endothelial growth factor and multikinase inhibitor side effects. Clin J Oncol Nurs. 2009; 13(suppl):13–8. [DOI] [PubMed] [Google Scholar]
- 7.Luo JC, Lin HY, Lu CL, et al. Growth factor expression in patients with erosive esophagitis. Transl Res. 2008; 152(2):81–7. [DOI] [PubMed] [Google Scholar]
- 8.Baatar D, Jones MK, Tsugawa K, et al. Esophageal ulceration triggers expression of hypoxia-inducible factor-1 alpha and activates vascular endothelial growth factor gene. Am J Pathol. 2002; 161:1449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baatar D, Jones MK, Pai R, et al. Selective cyclooxygenase-2 block delays healing of esophageal ulcers in rats and inhibits ulceration- induction and extracellular signal-regulated kinase 2 activation. Am J Pathol. 2002; 160:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


