Abstract
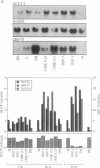
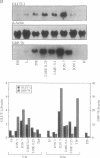
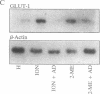
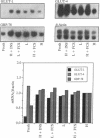
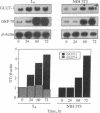
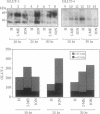
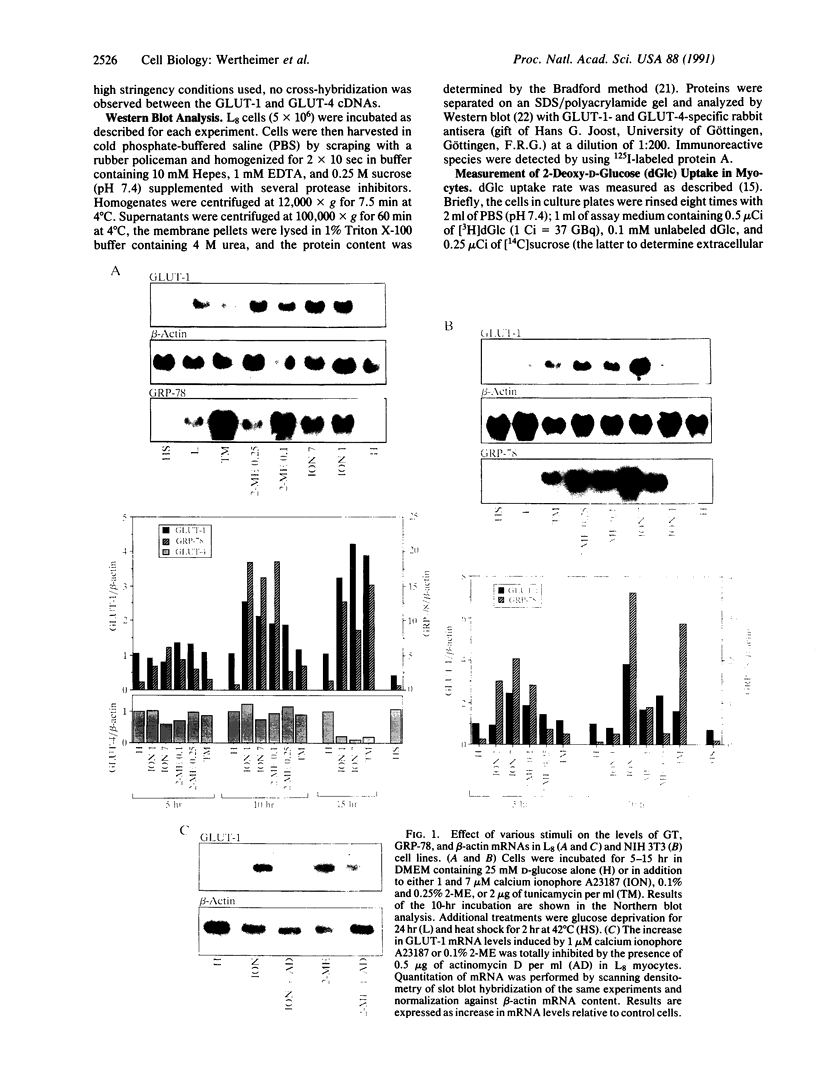
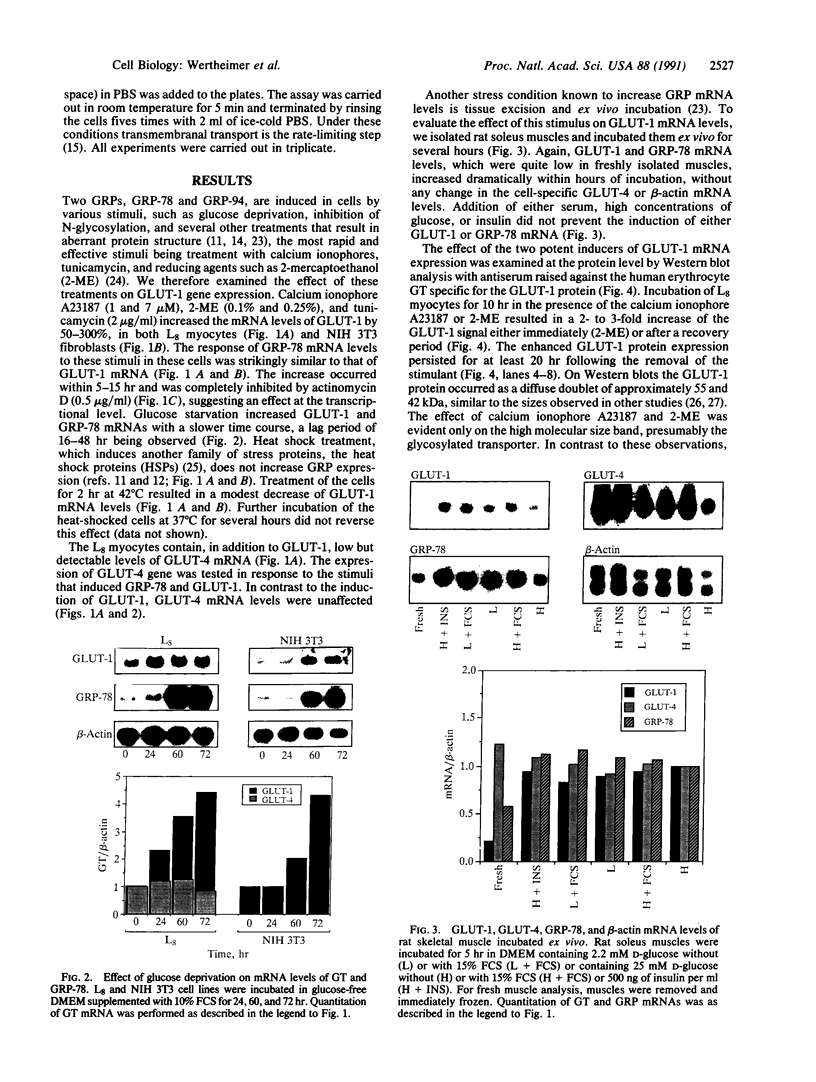
In mammals, glucose transport is mediated by five structurally related glucose transporters that show a characteristic cell-specific expression. However, the rat brain/HepG2/erythrocyte-type glucose transporter GLUT-1 is expressed at low levels in most cells. The reason for this coexpression is not clear. GLUT-1 is negatively regulated by glucose. Another family of proteins, glucose-regulated proteins (GRPs), is also ubiquitously expressed and stimulated by glucose deprivation and other cellular stresses. We therefore hypothesized that GLUT-1 may be a glucose-regulated stress protein. This was tested by subjecting L8 myocytes and NIH 3T3 fibroblasts to glucose starvation or exposure to the calcium ionophore A23187, 2-mercaptoethanol, or tunicamycin, all known to increase GRP levels. The mRNA for GLUT-1 was augmented by 50-300% in a time-dependent manner, similarly to the changes in GRP-78 mRNA. Ex vivo incubation of rat soleus muscles induced a marked and concomitant rise in the mRNA levels of GLUT-1 and GRP-78. Finally, calcium ionophore A23187 and 2-mercaptoethanol induced a 2- to 3-fold increase in the levels of the GLUT-1 protein and hexose uptake. In all instances in which GRP-78 and GLUT-1 responded to stress, the transcription of the cell-specific muscle/adipocyte-type insulin-responsive glucose transporter (GLUT-4) did not change. Thus, despite the lack of structural similarity, GLUT-1 and GRP-78 expression is regulated similarly, whereas the regulation of GLUT-4, which is structurally related to GLUT-1, is different. We propose that GLUT-1 belongs to the GRP family of stress proteins and that its ubiquitous expression may serve a specific purpose during cellular stress.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Birnbaum M. J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Hiraki Y., Garcia de Herreros A., Birnbaum M. J. Transformation stimulates glucose transporter gene expression in the absence of protein kinase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8252–8256. doi: 10.1073/pnas.86.21.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kim Y. K., Kim K. S., Lee A. S. Regulation of the glucose-regulated protein genes by beta-mercaptoethanol requires de novo protein synthesis and correlates with inhibition of protein glycosylation. J Cell Physiol. 1987 Dec;133(3):553–559. doi: 10.1002/jcp.1041330317. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Delegeane A. M., Baker V., Chow P. C. Transcriptional regulation of two genes specifically induced by glucose starvation in a hamster mutant fibroblast cell line. J Biol Chem. 1983 Jan 10;258(1):597–603. [PubMed] [Google Scholar]
- Lin A. Y., Chang S. C., Lee A. S. A calcium ionophore-inducible cellular promoter is highly active and has enhancerlike properties. Mol Cell Biol. 1986 Apr;6(4):1235–1243. doi: 10.1128/mcb.6.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes. 1990 Jan;39(1):6–11. doi: 10.2337/diacare.39.1.6. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Takano M., Gattoni-Celli S., Chen C. C., Isselbacher K. J. Evidence for expression of the facilitated glucose transporter in rat hepatocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9042–9046. doi: 10.1073/pnas.85.23.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson S., Cerasi E. Substrate regulation of the glucose transport system in rat skeletal muscle. Characterization and kinetic analysis in isolated soleus muscle and skeletal muscle cells in culture. J Biol Chem. 1986 Dec 25;261(36):16827–16833. [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y., Sugano S., Hampe A., Vashistha A., Pellman D., Hanafusa H. 78-kilodalton glucose-regulated protein is induced in Rous sarcoma virus-transformed cells independently of glucose deprivation. Mol Cell Biol. 1988 Jul;8(7):2675–2680. doi: 10.1128/mcb.8.7.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Charron M. J., Lodish H. F. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- Walker P. S., Donovan J. A., Van Ness B. G., Fellows R. E., Pessin J. E. Glucose-dependent regulation of glucose transport activity, protein, and mRNA in primary cultures of rat brain glial cells. J Biol Chem. 1988 Oct 25;263(30):15594–15601. [PubMed] [Google Scholar]
- Walker P. S., Ramlal T., Donovan J. A., Doering T. P., Sandra A., Klip A., Pessin J. E. Insulin and glucose-dependent regulation of the glucose transport system in the rat L6 skeletal muscle cell line. J Biol Chem. 1989 Apr 15;264(11):6587–6595. [PubMed] [Google Scholar]
- Walker P. S., Ramlal T., Sarabia V., Koivisto U. M., Bilan P. J., Pessin J. E., Klip A. Glucose transport activity in L6 muscle cells is regulated by the coordinate control of subcellular glucose transporter distribution, biosynthesis, and mRNA transcription. J Biol Chem. 1990 Jan 25;265(3):1516–1523. [PubMed] [Google Scholar]
- White M. K., Weber M. J. Transformation by the src oncogene alters glucose transport into rat and chicken cells by different mechanisms. Mol Cell Biol. 1988 Jan;8(1):138–144. doi: 10.1128/mcb.8.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala C. A., Salas-Prato M., Yan W. T., Banjo B., Perdue J. F. In cultured chick embryo fibroblasts the hexose transport components are not the 75 000 and 95 000 dalton polypeptides synthesized following glucose deprivation. Can J Biochem. 1980 Oct;58(10):1179–1188. doi: 10.1139/o80-158. [DOI] [PubMed] [Google Scholar]