Abstract
KCNQ1 gene mutation has a well-known association with long QT syndrome (LQTS). However, recent studies suggest that it may be implicated in intestinal neoplasia. We present a 27-year-old Hispanic man with a known history of LQTS secondary to KCNQ1 mutation, who presented with painless jaundice. Endoscopic retrograde pancreatic cholangiography revealed a prominent ampulla, with histology consistent with ampullary adenoma with high-grade dysplasia. Further endoscopic studies did not suggest familial adenomatous polyposis. To date, this is the index case of duodenal ampullary adenoma in the setting of KCNQ1 mutation.
Introduction
Duodenal ampullary adenoma has been classically described sporadically or in association with familial adenomatous polyposis (FAP).1 It is an important cause of morbidity and mortality in patients with FAP. Because it is a dysplastic condition, early identification is key. Recent research suggests that mutations in KCNQ1 may be implicated in the pathogenesis of intestinal neoplasia.2
Case Report
A 27-year-old Hispanic man with a past medical history significant for congenital LQTS due to KCNQ1 (G168R) mutation presented to the hospital with a 2-week history of deepening jaundice and pruritus. There was no concomitant fever, abdominal pain, or change in bowel habits. He did not have any sick contacts or recent travel. He did not report using any over-the-counter or prescribed medications. He was hemodynamically stable without any hepatosplenomegaly or stigmata of chronic liver disease on examination. Laboratory data were significant for total bilirubin 9.8 mg/dL (direct bilirubin 7.0 mg/dL) and alkaline phosphatase 214 U/L. Alanine aminotransferase, aspartate aminotransferase, serum albumin, and international normalized ratio were within normal limits.
Right upper quadrant ultrasound (US) revealed a prominent common bile duct measuring 1.2 cm without any evidence of gallstones or cholecystitis. A contrast-enhanced computed tomography (CT) scan showed obstruction at the level of the ampulla causing dilatation of the biliary tree and pancreatic duct. Subsequent endoscopic retrograde cholangiopancreatography revealed a prominent ampulla with 2 small (2 mm) stones retrieved after biliary sphincterotomy was done (Figure 1). Ampullary biopsy was significant for adenomatous change with high-grade dysplasia (Figure 2). Subsequent endoscopic US (EUS) revealed an isoechoic, homogeneous mass measuring 10 mm in the ampulla without invasion of the muscularis propria. Endoscopic ampullectomy using a snare and electrocautery was performed, and biopsy specimens from the resected tissue revealed no evidence of neoplastic tissue. The pancreatic duct was stented prophylactically to prevent pancreatitis. The patient’s jaundice and pruritus subsequently resolved with normalization of bilirubin and alkaline phosphatase levels.
Figure 1.
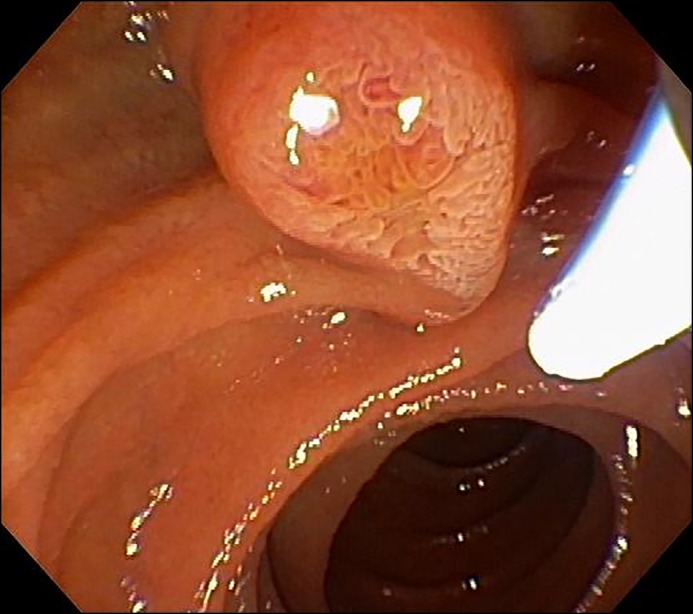
Endoscopic appearance of duodenal ampullary adenoma.
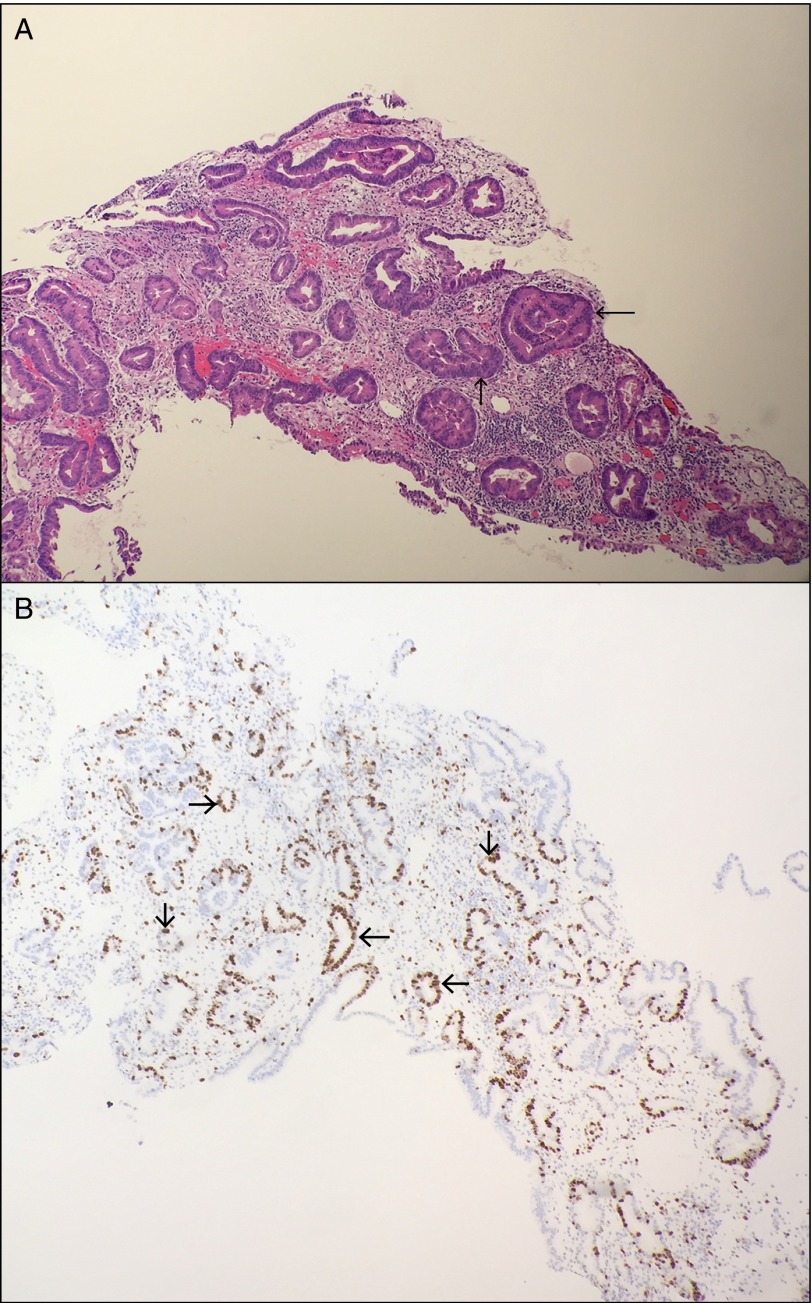
Figure 2.
(A) Hematoxylin and eosin staining of the specimen revealed adenomatous glands with high-grade dysplasia, as manifested by nuclear atypia (arrows). (B) Proliferation marker MIB1 showed strong nuclear staining in dysplastic cells (arrows). There is no evidence of dysplastic cells beyond the basement membrane.
A follow-up esophagogastroduodenoscopy 2 months later did not show any evidence of neoplastic recurrence. Screening colonoscopy to assess for FAP only revealed 3 hyperplastic polyps. The patient will undergo surveillance endoscopies every 3 to 6 months for 2 years to assess for recurrence.
Discussion
The KCNQ1 gene encodes the pore-forming alpha subunit of a voltage-gated potassium channel and is predominantly expressed in the myocardium, inner ear, stomach, intestine, and pancreas, where it is critical for ion homeostasis.3 Mutations in KCNQ1 have been studied in congenital LQTS. Variants of KCNQ1 are also associated with an increased risk of type 2 diabetes.4 Rice et al. demonstrated that patients with KCNQ1 mutation had increased level of gastrin, which increased the risk of carcinoid tumor.5
However, recent work in mice by Than et al. suggested that KCNQ1 gene mutations that have a well-known association with LQTS also increase the risk of intestinal tumors.2 A series of experiments were performed in mice with truncation mutation at codon 850 of the adenoma polyposis coli (APC) gene. These mice, designated APCmin (multiple intestinal neoplasia), demonstrated that both heterozygous and homozygous inactivation of the KCNQ1 gene resulted in adenomas in a significant proportion of APCmin mice. Loss in KCNQ1 promoted tumor progression and enhanced tumor multiplicity. The proposed mechanism of carcinogenesis stems from the interaction of KCNQ1 with cystic fibrosis (CF) transmembrane regulator (CFTR) and mucin 2 (MUC2). In human studies, low KCNQ1 protein expression showed a significantly lower overall survival in colorectal cancer metastasizing to the liver.2 These studies associate KCNQ1 mutations with tumor pathogenesis and progression. However, Girault et al. found that the expression of KvLQT1 (synonym for KCNQ1) was increased by 1.5- to 1.7-fold in adenocarcinomatous lung tissue compared to paired non-neoplastic tissue.6 Consideration for the molecular mechanisms involved, such as genetic imprinting or different gene mutations, may resolve this discrepancy and warrants further basic science research.
Ampullary adenomas are benign glandular lesions located in the duodenal papilla. They have been historically associated with FAP, in which they implicate a 124-fold increased risk of ampullary carcinoma.7 This risk is due to mutation in the APC gene that codes for a tumor suppressor protein, which functions as a “gatekeeper” in prevention of tumor development.8
Ampullary adenoma can present as painless jaundice (as in our patient), or it can lead to pancreatitis or cholangitis. Once it is identified on endoscopy and confirmed with a biopsy, EUS is superior to abdominal US, CT, and magnetic resonance imaging in providing information regarding the depth of the ampullary lesion as well as locoregional lymph node status.9 Most adenomas with no intraductal extension can be fully resected by endoscopic snare polypectomy.10 Treatment options are dictated by tumor size, firmness, friability, extent, and degree of dysplasia. Adenomas recur in about a third of cases, so surveillance endoscopy is recommended after endoscopic papillectomy. This is usually performed 1 to 6 months after the index procedure, with subsequent examinations every 3 to 12 months for at least 2 years.11
The KCNQ1 mutation is inherited in autosomal dominant fashion with high penetrance, so patients and their relatives should undergo genetic counseling. More research needs to be conducted in such patients and their families to develop appropriate guidelines for screening and surveillance.
Disclosures
Author contributions: FNU Asad-Ur-Rahman and L. Hughes wrote the article. MT Khan reviewed the literature. MK Hasan and I. Inayat reviewed the article. I. Inayat is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Groves CJ, Saunders BP, Spigelman AD, Phillips RKS. Duodenal cancer in patients with familial adenomatous polyposis (FAP): Results of a 10-year prospective study. Gut. 2002; 50:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Than BL, Goos JA, Sarver AL, et al. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene. 2014; 33(29):3861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peroz D, Rodriguez N, Choveau F, et al. Kv7. 1 (KCNQ1) properties and channelopathies. J Physiol. 2008; 586:1785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda K, Miyake K, Horikawa Y, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008; 40:1092–7. [DOI] [PubMed] [Google Scholar]
- 5.Rice KS, Dickson G, Lane M, et al. Elevated serum gastrin levels in Jervell and Lange-Nielsen syndrome: A marker of severe KCNQ1 dysfunction? Heart Rhythm. 2011; 8(4):551–4. [DOI] [PubMed] [Google Scholar]
- 6.Girault A, Prive A, Trinh NT, et al. Identification of KvLQT1 K+ channels as new regulators of non-small cell lung cancer cell proliferation and migration. Int J Oncol. 2014; 44(3):838–48. [DOI] [PubMed] [Google Scholar]
- 7.Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc. 2011; 3(12):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991; 66(3):589–600. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Tseng LJ, Yang CC, et al. The accuracy of endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, computed tomography, and transabdominal ultrasound in the detection and staging of primary ampullary tumors. Hepatogastroenterology. 2001; 48:1750–53. [PubMed] [Google Scholar]
- 10.Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004; 60:757–64. [DOI] [PubMed] [Google Scholar]
- 11.Chatadi, et al. American Society for Gastrointestinal Endoscopy. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc, 82.5. 2015;773–781. [DOI] [PubMed] [Google Scholar]