Abstract
The objective of the present study was to systematically assess the association between dioxin/2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and cancer incidence and mortality. Systematic literature searches were conducted until July 2015 in Pubmed, Embase and Cochrane library to identify relevant studies. A random-effects model was applied to estimate the pooled odds ratio (OR), risk ratio (RR), standard incidence ratio (SIR) or standard mortality ratio (SMR) for cancer incidence or mortality. In addition, dose-response, meta-regression, subgroup, and publication bias analyses were conducted. Thirty-one studies involving 29,605 cancer cases and 3,478,748 participants were included. Higher external exposure level of TCDD was significantly associated with all cancer mortality (pooled SMR = 1.09, 95% CI: 1.01–1.19, p = 0.04), but not all cancer incidence (pooled RR = 1.01, 95% CI: 0.97–1.06, p = 0.49). Higher blood level of TCDD was both significantly associated with all cancer incidence (pooled RR = 1.57, 95% CI: 1.21–2.04, p = 0.001) and all cancer mortality (pooled SMR = 1.45, 95% CI: 1.25–1.69, p < 0.001). Subgroup analysis suggested that higher external exposure and blood level of TCDD were both significantly associated with the mortality caused by non-Hodgkin’s lymphoma. In conclusion, external exposure and blood level of TCDD were both significantly associated with all cancer mortality, especially for non-Hodgkin’s lymphoma.
Cancer constitutes an enormous burden on society in more and less economically developed countries. An estimated 14.1 million new cancer cases and 8.2 million cancer deaths occurred in 2012 worldwide1. As one of the important established risk factors for cancer, environmental carcinogen like dioxin might contribute to its increasing prevalence2,3. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD or dioxin) is the most toxic halogenated aromatic hydrocarbon4, which is a widespread the environmental contaminant released by various sources of combustion, incineration, and chemical manufacturing5,6. This compound is extremely stable and thus accumulates in the food chain with a half-life of 7–9 years in humans7,8. In 1997, the International Agency for Research on Cancer (IARC) has classified it as a known human carcinogen (group 1) on the basis of animal studies and mechanistic information, but the epidemiology data was limited2. In 2012, the IARC illustrated the associations between TCDD and human cancers according to many observational studies3, but these issues were not systematically reviewed and quantified by a meta-analysis. Molecular studies has proven that TCDD is a potent a carcinogen which could disrupt multiple endocrine pathways via aryl-hydrocarbon receptors (AhR) widely present in animals and humans2,8,9.
As mentioned above, many epidemiological cohort studies and case-control studies have evaluated the association between TCDD/dioxin and cancer incidence and mortality10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40, but the results remained inconsistent. In addition, two previous meta-analyses reported the association between TCDD exposure and prostate cancer41 and lung cancer42, while another43 reported the dose-response relationship for blood level of TCDD and cancer mortality based on 3 cohort studies. However, to date, no study has systematically analyzed the association between external exposure or blood level of TCDD and all cancer incidence and mortality. Thus, the aim of this study was to provide a systematically quantitative assessment of the association from an epidemiological point of view, and fill in gaps in the IARC deficiencies on this issue.
Materials and Methods
Data sources, search strategy and selection criteria
Systematic literature searches were conducted in PUBMED, EMBASE and Cochrane library (up to July 2015) to identify eligible studies. The following terms were used in the search procedure: (“dioxin” or “TCDD” or “Tetrachlorodibenzodioxin” or “2,3,7,8-Tetrachlorodibenzo-p-dioxin” or “Tetrachlorodibenzo-p-dioxin”) AND (“cancer” or “tumor” or “tumour” or “carcinoma” or “neoplasm” or “sarcoma” or “melanoma” or “malignancy” or “leukemia” or “leukeamia” or “myeloma” or “lymphoma” or “adenoma”). Reports cited the references identified in this systematic review and relevant reviews were also searched to include potentially missed studies. Titles and abstracts were first scanned, and then full articles of potential eligible studies were reviewed. The retrieved studies were carefully examined to exclude potential duplicates or overlapping data. For duplicate reports, the ones with larger sample size, longer follow-up time and/or more detailed information were selected. This meta-analysis was designed, conducted and reported according to PRISMA and MOOSE statements44,45.
Studies were eligible for inclusion if all the following criteria were fulfilled: (1) prospective or retrospective cohort studies and case-control studies evaluated the association between dioxin/TCDD and cancer incidence and mortality; (2) the odds ratio (OR), risk ratio (RR), standard incidence ratio (SIR) or standard mortality ratio (SMR) estimates and their 95% confidence intervals (95% CI) were given or sufficient data were available for evaluation; (3) articles as full papers in English were evaluated for eligibility. Studies reported the association between Agent Orange/herbicides and cancer incidence and mortality were excluded because the limitation of precise data on TCDD. For studies conducted in the same population, the criteria priority was established according to (1) whether the detailed information of different cancer subtypes and dioxin exposure level was provided or studies with a larger sample size and (2) the publication time. Reviews, meeting abstracts, notes, comments, editorials, and case reports were excluded because of the limited data.
Data extraction and quality assessment
Data extraction was carried out independently by two investigators (Drs. Xu JM and Ye Y). Discrepancies were resolved by a third investigator. The endpoints of this analysis were all cancer incidence and mortality as most of the included studies adopted, as well as site/type-specific cancers. The following information was extracted from each study: authors, year of publication, country of each study, study period, population characteristics (sample size, gender and age), and cancer subtypes. ORs (RRs, SIRs or SMRs) reflected the greatest degree of control for potential confounders were adopted in this meta-analysis. The quality of each study was assessed according to NEWCASTLE-OTTAWA quality assessment46. The total score ranges from 0 to 9, and a higher score indicates higher quality. Sensitivity analyses are further conducted according to the quality assessment results to explore the source of heterogeneity.
Data synthesis and statistical analysis
The primary meta-analyses were conducted to assess the association between external exposure and blood level of TCDD and all cancer incidence and mortality. Heterogeneity between individual studies was assessed by the chi-square test and I2 test; P ≤ 0.10 and/or I2 > 50% indicates significant heterogeneity47. Summary ORs (RRs, SIRs or SMRs) and 95% CI were calculated using a random-effects model. The significance of the pooled ORs (RRs, SIRs or SMRs) were determined by Z test (p < 0.05 was considered to be significant). Studies that reported results of a specific type of cancer but no data on all cancer were not pooled for all cancer analysis. Subgroup analyses were applied to explore source of heterogeneity and to evaluate potential effect of modification of variables including cancer subtype, exposure way and TCDD exposure reference category. In order to avoid bias and make the analysis more accurate, subgroup results were shown in pooled form if there were three or more studies for one subtype, otherwise, it was listed in an original form. Funnel plots were constructed and Begg’s and Egger’s tests were performed to assess the publication bias (p ≤ 0.10 was considered to be significant).
We analyzed the dose-response relationship using first-order, and second-order, and three-order fractional polynomial regression of the inverse variance-weighted data to estimate a curve of best fit. Best-fit curves were selected using decreased deviance compared with the reference model48. Comparisons of curves to determine best fit were done using a chi-square distribution. The average values within the blood TCDD categories were specified as the midpoint for bounded ranges, and 0.75 times the higher bound for the lowest (unbounded) range, and 1.25 times the lower bound for the highest (unbounded) range. RRs or SMRs (the ratio of observed to expected cancer deaths multiplied by 100) was the response measure used in these studies. All analyses were conducted using Stata software (version 12.0; StatCorp, College Station, TX, USA).
Results
Study characteristics and data quality
After searching PUBMED, EMBASE and Cochrane library, 6446 articles were identified. 4437 articles were assessed after removing 2009 duplicate papers. Review of titles and abstracts resulted in exclusion of 4206 articles. For the remaining 231 articles, 163 were excluded for the following reasons: insufficient data (n = 60), foreign languages (n = 17), not on the right topic or targeted population (the outcomes of these studies were not cancer incidence or mortality, or the study interests were not dioxin) (n = 56), review articles (n = 14), meeting abstracts (n = 6), letters or comments (n = 10). 68 studies were included for further consideration and then 37 duplicate reports49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85 from the same population were excluded. The detailed study selection methods for the same population are shown in Supplementary Table 1. Finally, a total of 31 studies10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 were included for the meta-analysis, including 22 cohort studies and 9 case-control studies. There were different TCDD exposure ways as follow: occupational exposure, non-occupational exposure, industrial accidents, and soldiers exposed to herbicides used in Vietnam War. The reference categories also varied among different studies, some adopted the non-exposed population to calculate SIRs or SMRs (external reference), and others adopted the lowest exposure categories (internal reference). We pooled the RRs or SMRs of high-exposed versus non-exposed categories for external reference, and highest versus lowest categories for the internal reference. Of note, all the included case-control studies only provided data on specific cancer types but no combined data on all cancer, and these studies were only pooled for the subgroup analysis but not for the all cancer analysis in order to ensure the accuracy of the results. The selection process is shown in Fig. 1, and the characteristics of the included studies are shown in Table 1. The exposure level and adjustment for confounders of included studies are shown in Supplementary Table 2.
Figure 1. Flow diagram of study selection process.
Table 1. Characteristics of included studies.
| No. | Study | Country/cohort | Time period | Exposure way | Exposure assessment | Reference category | Cancer types | Gender | No. of cancer cases/cohort or controls | Study quality | Age (years) | Duplicated reports |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort studies | ||||||||||||
| Exposure incidence | ||||||||||||
| 1 | Kogevinas13 | part of IARC* | 1955–1988 | occupational | job records, company records and detailed company exposure questionnaires | External: SIR and SMR | all cancer, breast cancer | F | 29/701 | 6 | N/A | |
| 2 | Read20 | New Zealand | 1970–2001 | non-occupational | individual’s recorded Territorial Authority for usual place of residence at death or cancer registration | External: New Plymouth population | all cancer, lymphocytic leukemia, Hodgkin’s disease, Non-Hodgkin’s lymphoma, soft tissue sarcoma | F/M | 8013/375583 | 8 | N/A | |
| 3 | Viel22 | French# | 1990–1999 | non-occupational | modelled ground-level concentrations | External: Isere population | non-Hodgkin’s lymphoma | F/M | 3974/2487274 | 8 | mean 61.49 ± 16.21 | |
| 4 | Pesatori25 | Italy, Seveso | 1977–1996 | industrial accident | measurements of TCDD soil levels | External: surrounding non-contaminated territory including 11 municipalities | All cancer, Esophagus, stomach, colon, rectum, liver, biliary tract, pancreas, lung, pleura, soft tissue sarcoma, melanoma, skin, breast, genito-urinary tract, ovary, prostate, testis, bladder, kidney, brain, thyroid, Hodgkin’s disease, non-Hodgkin’s lymphoma, leukemia | F/M | 2122/218761 | 8 | 0–74 | Pesatori72, Bertazzi52, Pesatori73 |
| 5 | Danjou31 | French, E3N cohort | 1993–2008 | non-occupational | diet history questionnaire | Internal: the lowest category | breast cancer | F | 3465/63830 | 9 | mean 52.73 ± 6.58 | |
| Exposure mortality | ||||||||||||
| 1 | Michalek10 | USA, vietnam veterans-AFSH | 1982–1987 | Vietnam war | physical Examination, Ranch Hands veterans | External: the comparison veterans | all cancer | M | 12/2294 | 6 | 48.5 | |
| 2 | Zober11 | Germany-BASF Aktiengesellschaft | 1953–1987 | industrial accident | company records | External: national mortality rate | all cancer, buccal cavity and pharynx, esophagus, stomach, colon, rectum, larynx, lung, bone, skin, prostate, bladder, leukemia | F/M | 23/247 | 8 | mean 63.4 | |
| 3 | Collins12 | USA, West Virginia, Monsanto company | 1949–1987 | industrial accident | work records and Internal Revenue Service Form | External: local population mortality rate | all cancer, stomach, colorectal, liver and biliary, respiratory system, bone, skin, prostate, bladder, lymphatic and hematopoietic, soft-tissue sarcoma | M | 102/754 | 7 | N/A | |
| 4 | Kogevinas15 | IARC, 36cohorts$ | 1939–1992 | occupational | job records, company records and detailed company exposure questionnaires | External: SIR and SMR | all cancer, buccal cavity and pharynx, esophagus, stomach, colon, rectum, liver and biliary, pancreas, peritoneum, nose and nasal sinuses, larynx, lung, bone, skin, prostate, kidney, testis, bladder, breast, cervix, endometrium and uterus, leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, myeloma, brain, soft tissue sarcoma, thyroid | F/M | 710/21863 | 7 | N/A | Saracci76, Kogevinas67, Bueno de Mesquita58, Kogevinas13, Vena79, Kogevinas85 |
| 5 | Steenland16 | USA, NIOSH | 1942–1993 | occupational | job records, job-exposure matrix and blood sample test | External (US non-exposed people) and Internal (the lowest category) | all cancer, esophagus, stomach, colon, rectum, liver and biliary, pancreas, peritoneum, larynx, lung, prostate, kidney, bladder, lymphatic and hematopoietic, leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, myeloma, brain and nervous system, connective tissue and soft tissue | M | 377/5172 | 7 | N/A | Fingerhut61, Steenland78, Salvan75 |
| 6 | Revich17 | Russia | 1983–1997 | non-occupational | food and soil concentration test | External: death rate in Samara Region | all cancer, intestine, stomach, colon, rectum, larynx, lung, bone, soft-tissue, breast, cervix, urinary organs, leukemia, lymphomas | F/M | 803/- | 8 | N/A | |
| 7 | Bodner18 | USA-Michigan, Dow chemical company | 1940–1994 | occupational | job records and exposure score | External: other area workers with background exposure to dioxin | all cancer, lung, soft-tissue sarcoma, non-Hodgkin’s lymphoma | M | 168/2187 | 7 | N/A | Cook60, Ott70, Bond57, Ramlow74 |
| 8 | Read20 | New Zealand | 1970–2001 | non-occupational | individual’s recorded Territorial Authority for usual place of residence at death or cancer registration | External: New Plymouth population | all cancer, lymphocytic leukemia, Hodgkin’s disease, Non-Hodgkin’s lymphoma, soft tissue sarcoma | F/M | 4235/375583 | 8 | N/A | |
| 9 | Consonni21 | Italy, Seveso | 1976–2001 | industrial accident | measurements of TCDD soil levels | External: surrounding non-contaminated territory including 11 municipalities | all cancer, stomach, colon, rectum, liver, biliary tract, pancreas, lung, soft tissue sarcoma, melanoma, breast, genito-urinary tract, ovary, prostate, bladder, kidney, brain, Hodgkin’s disease, non-Hodgkin’s lymphoma, leukemia | F/M | 2278/278108 | 8 | 0–74 | Bertazzi56, Bertazzi55, Bertazzi54, Bertazzi53, Baccarelli50 |
| 10 | Manuwald29 | Germany, Hamburg, Boehringer Ingelheim | 1952–2007 | occupational | company records and blood or fat tissue samples | External: Hamburg population | all cancer, hypo pharynx, digestive organs, esophagus, stomach, colon, rectum, pancreas, larynx, lung, pleura, breast, prostate, kidney, bladder, hematopoietic system, non-Hodgkin’s lymphoma | F/M | 291/1589 | 7 | N/A | Manz68 |
| 11 | Wang30 | China | 1980–2005 | occupational | air sample concentration test | External: Chinese national mortality rates | all cancer, lung, liver, gastric | F/M | 121/3529 | 7 | N/A | |
| Blood incidence | ||||||||||||
| 1 | Ott14 | Germany, Ludwigshafen | 1959–1992 | occupational | questionnaire and blood sample | External: West Germany population | all cancer, buccal cavity, digestive organs, stomach, colorectal, liver, gall bladder or bile duct, respiratory system, lung, prostate, bladder or kidney, lymphatic or hematopoietic tissue, skin | M | 47/243 | 7 | N/A | |
| 2 | Pavuk19 | USA, vietnam veterans | 1982–2003 | Vietnam war | physical examination and blood sample | Internal: the lowest category | all cancer, all SEER sites, digestive system, respiratory system, melanoma, basal or squamous cell, prostate | M | 402/1482 | 8 | mean 63.7 | Ketchum66, Akhtar49, Pavuk71, Michalek69 |
| 3 | Warner26 | Italy, Seveso, SWHS cohort | I:1976–1996, II:1997–2009 | industrial accident | interview, physical examination and blood sample | Internal: the lowest category | all cancer, breast cancer | F | 66/981 | 9 | 0–40 | Warner80 |
| Blood mortality | ||||||||||||
| 1 | Ott14 | Germany, Ludwigshafen | 1959–1992 | occupational | questionnaire and blood sample | External: West Germany population | all cancer, digestive organs, respiratory system, prostate, bladder or kidney, lymphatic or hematopoietic tissue | M | 31/243 | 7 | N/A | Zober11 |
| 2 | Steenland16 | USA, NIOSH | 1942–1993 | occupational | job records, job-exposure matrix and blood sample test | External (US non-exposed people) and Internal (the lowest category) | all cancer, lung cancer | M | 256/5172 | 8 | N/A | Steenland77, Cheng59 |
| 3 | Collins23 | USA, Michigan | 1937–1980 | occupational | job records and blood sample test | External (US population) and Internal (the lowest category) | all cancer, lung, prostate, kidney, non-Hodgkin’s lymphomas | M | 94/773 | 8 | mean 31.1 | |
| 4 | McBride24 | New Zealand | 1969–2004 | occupational | job records and blood sample test | External (New Zealand population) and internal (the lowest category) | all cancer, digestive organs, lung, soft-tissue sarcoma, lymphatic and hematopoietic tissue, non-Hodgkin’s lymphoma | F/M | 61/1599 | 8 | mean 52.9 | |
| 5 | Boers27 | Netherlands, Dutch cohort | 1955–2006 | occupational | blood sample test and predictive model | Internal (background exposure level as reference) | all cancer, digestive organs, stomach, pancreas, respiratory system, lung, skin, genital and urinary cancer, prostate, bladder, kidney, lymphatic and hematopoietic cancer, non-Hodgkin’s lymphoma, leukemia | M | 192/2056 | 8 | N/A | Heederik64, Hooiveld65 |
| 6 | Lin28 | USA, NHANES | 1999–2006 | non-occupational | blood sample test | Internal (the lowest category) | all cancer | F/M | 72/2361 | 8 | >40 | |
| 7 | Manuwald29 | Germany, Hamburg | 1952–2007 | occupational | company records and blood or fat tissue samples | External: Hamburg population | all cancer, digestive organs, respiratory system, breast cancer | F/M | 291/1589 | 7 | N/A | Flesch-Janys62, Bencher/51, Flesch-Janys63 |
| Case-control studies | ||||||||||||
| Exposure incidence | ||||||||||||
| 1 | Hardell32 | Sweden | 1970–1986 | non-occupational | structured questionnaire and work history | Internal (unexposed) | soft-tissue sarcoma | M | 434/948 | 6 | 25–80 | Hardell81, Eriksson82, Hardell83, Eriksson84 |
| 2 | Floret34 | France, Besançon | 1980–1995 | non-occupational | modeled ground-level according to meteorological conditions | Internal (the lowest category) | non-Hodgkin’s lymphoma | F/M | 222/2220 | 6 | median 66 | |
| 3 | Zambon38 | Italy, Venice | 1990–1996 | non-occupational | survey of the incinerators and industrial sources of airborne dioxin | Internal (the lowest category) | sarcoma | F/M | 172/405 | 6 | N/A | |
| 4 | Viel39 | France, Besançon | 1996–2002 | non-occupational | modeled ground-level according to meteorological conditions | Internal (the lowest category) | breast cancer | F | 434/2170 | 6 | >20 | |
| 5 | Villeneuve40 | Eight European countries‖ | 1995–1997 | occupational | structured questionnaire and work history | Internal (the lowest category) | male breast cancer | M | 104/1901 | 6 | 35–70 | |
| Blood and adipose tissue incidence | ||||||||||||
| 1 | Hardell33 | Sweden | 1994–1997 | non-occupational | adipose tissue sample test | Internal (the lowest category) | non-Hodgkin’s lymphoma | NA | 33/39 | 7 | NA | |
| 2 | Tuomisto35 | Finland | 1997–1999 | non-occupational | fat sample test and questionnaire | Internal (the lowest category) | soft-tissue sarcoma | F/M | 110/227 | 7 | 15.0–91.1 | |
| 3 | De Roos36 | US, NCI; SEER, the parent study | 1998–2000 | non-occupational | blood sample test | Internal (the lowest category) | non-Hodgkin’s lymphoma | F/M | 100/100 | 5 | 20–74 | |
| 4 | Reynolds37 | US | mid-1990s | non-occupational | adipose tissue sample test and questionnaire | Internal (the lowest category) | breast cancer | F | 79/52 | 6 | mainly 40–59 | |
IARC: The International Agency for Research on Cancer.
E3N: Etude Epidémiolog ique auprès de femmes de la Mutuelle Générale de l’Education Nationale.
AFSH: air force health study.
NIOSH: National Institute for Occupational Safety and Health.
SWHS: the Seveso Women’s Health Study.
NHANES: National Health and Nutrition Examination Survey.
F: female, M: male, N/A: not available.
Study quality was judged on the basis of the Newcastle-Ottawa Scale (1–9 stars).
*Austria, Denmark, Finland, Italy, Netherlands, New Zealand, and Sweden.
#Four administrative departments, Isère, Bas-Rhin, Haut-Rhin and Tarn.
$Australia, Austria, Canada, Denmark, Finland, Italy, the Netherlands, New Zealand, Sweden, UK, Germany, USA.
‖Denmark, France, Germany, Italy, Sweden, Latvia, Portugal and Spain.
Among the included studies, ten13,20,22,25,31,32,34,38,39,40 assessed the association between external exposure level of TCDD and cancer incidence. Eleven10,11,12,15,16,17,18,20,21,29,30 evaluated the association between external exposure level of TCDD and cancer mortality. For blood and adipose tissue level of TCDD, seven14,19,26,33,35,36,37 assessed cancer incidence and seven14,16,23,24,27,28,29 evaluated cancer mortality. Ott et al.14 reported the association between blood level of TCDD and both cancer incidence and mortality. Read et al.20 reported the association between external exposure of TCDD and both cancer incidence and mortality. Steenland et al.16 and Manuwald et al.29 reported the association between both external exposure and blood level of TCDD and cancer mortality. The results of quality assessment were shown in the Supplementary Table 3. The scores of most studies ranged from seven to nine (except for two studies got six points), which indicated the high quality of included studies and enhanced the reliability of the analysis. The PRISM checklist and flow diagram were shown in Supplementary Tables 4 and 5, respectively.
External exposure of TCDD and cancer incidence and mortality
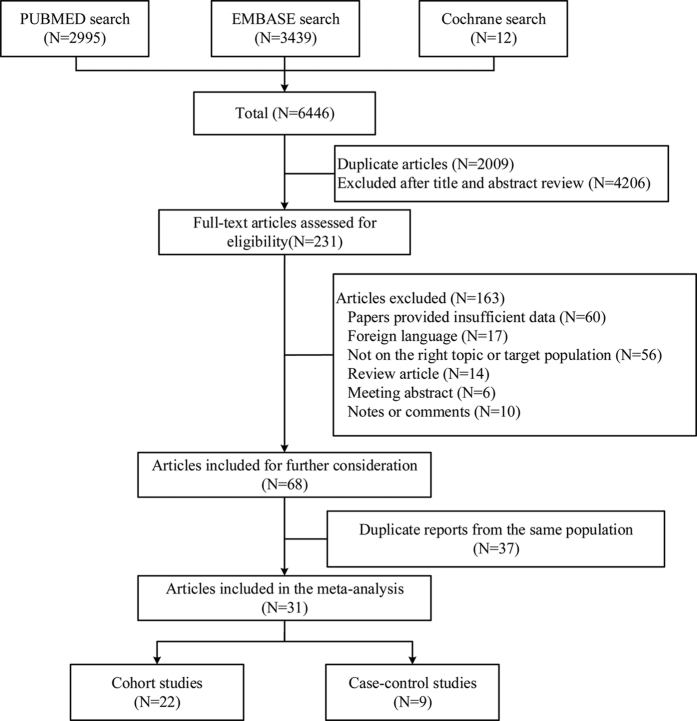
Ten studies involving 18,969 cancer cases and 3,155,159 participants assessed the association between external exposure of TCDD and cancer incidence, including five cohort studies and five case-control studies. The pooled RR of all cancer incidence of TCDD exposure level was 1.01 (95% CI: 0.97–1.06), indicating no significant association (Fig. 2a). There was significant heterogeneity across the included studies (I2 = 73.5%, p < 0.001), as shown in Fig. 2a. Subgroup analysis was conducted according to cancer subtype, as shown in Table 2. The pooled RRs of different cancer types were all not significant, including breast cancer, Hodgkin’s lymphoma, lymphatic leukemia, non-Hodgkin’s lymphoma, and soft-tissue sarcoma. The results of subgroup analysis suggested the heterogeneity may be caused by special cancer types. Sensitivity analysis was also conducted to further explain the source of heterogeneity according to quality assessment results. After exclusion of the study13 of the lowest score (six points), the pooled RR was 1.01(95% CI: 0.97–1.05), while the heterogeneity was not significantly changed (from I2 = 73.5% to I2 = 72.7%).
Figure 2.
Meta-analysis of the association between external exposure level of TCDD and (a) all cancer incidence and (b) all cancer mortality.
Table 2. Subgroup analyses of the association between TCDD and cancer incidence and mortality.
| Categories | Classification | Study number | No. of cases | RR or SMR (95% CI) | Heterogeneity |
Study | |
|---|---|---|---|---|---|---|---|
| I2 | p | ||||||
| Exposure incidence | |||||||
| cancer type | breast cancer | 3 | 3768 | 0.99(0.93–1.06) | 9.30% | 0.356 | |
| Hodgkin’s lymphoma | 2 | 49 | 1.13(0.83–1.54) | — | — | Read20 | |
| 26 | 1.42(0.93–2.18) | — | — | Pesatori25 | |||
| lymphatic leukemia | 2 | 104 | 1.35(0.93–1.97) | — | — | Read20 | |
| 13 | 0.83(0.46–1.48) | — | — | Pesatori25 | |||
| non-Hodgkin’s lymphoma | 4 | 4263 | 1.09(0.92–1.30) | 65.80% | 0.001 | ||
| soft-tissue sarcoma | 4 | 105 | 1.37(0.97–1.93) | 48.70% | 0.041 | ||
| Exposure mortality | |||||||
| cancer type | buccal cavity and pharynx | 2 | 22 | 1.30(0.82–1.97) | — | — | Kogevinas15 |
| 11 | 2.17(1.08–3.87)* | — | — | Manuwald29 | |||
| esophagus | 3 | 44 | 1.52(1.09–2.13)* | 9.10% | 0.333 | ||
| stomach | 7 | 433 | 1.02(0.82–1.27) | 68.10% | 0.001 | ||
| colorectal | 7 | 453 | 1.05(0.94–1.19) | 20.10% | 0.214 | ||
| colon | 5 | 298 | 0.97(0.86–1.09) | 0.00% | 0.532 | ||
| rectum | 5 | 154 | 1.18(0.97–1.44) | 25.10% | 0.238 | ||
| liver and biliary | 5 | 212 | 1.01(0.79–1.30) | 0.00% | 0.046 | ||
| pancreas | 4 | 139 | 0.93(0.78–1.11) | 0.00% | 0.719 | ||
| peritoneum | 2 | 5 | 2.19(0.45–6.41) | — | — | Steenland16 | |
| 3 | 1.23(0.40–2.80) | — | — | Kogevinas15 | |||
| larynx | 4 | 45 | 2.20(1.61–3.02)* | 0.00% | 0.563 | ||
| trachea/lung | 8 | 1190 | 1.21(0.89–1.65) | 95.20% | <0.001 | ||
| prostate | 5 | 172 | 1.14(0.97–1.34) | 0.00% | 0.830 | ||
| kidney | 4 | 90 | 1.39(1.08–1.78)* | 16.60% | 0.309 | ||
| bladder | 5 | 117 | 1.73(0.95–3.18) | 89.00% | <0.001 | ||
| Hodgkin’s disease | 4 | 43 | 1.35(0.97–1.88) | 0.00% | 0.895 | ||
| non-Hodgkin’s lymphoma | 6 | 239 | 1.18(1.01–1.37)* | 20.10% | 0.235 | ||
| myeloma | 3 | 50 | 1.49(1.03–2.15)* | 24.80% | 0.256 | ||
| leukemia | 5 | 156 | 1.14(0.96–1.35) | 0.00% | 0.464 | ||
| skin | 2 | 9 | 0.89(0.36–2.18) | — | — | Kogevinas15 | |
| 3 | 0.85(0.49–1.48) | — | — | Consonni21 | |||
| brain nervous system | 3 | 57 | 0.91(0.69–1.20) | 0.00% | 0.418 | ||
| bone | 2 | 2 | 5.00(0.60–18.1) | — | — | Collins12 | |
| 3 | 1.08(0.22–3.14) | — | — | Kogevinas15 | |||
| soft-tissue sarcoma | 6 | 46 | 1.60(1.15–2.23)* | 0.00% | 0.550 | ||
| breast | 4 | 234 | 1.27(0.78–2.06) | 87.80% | <0.001 | ||
| endometrium and uterus | 2 | 3 | 3.41(0.70–9.96) | — | — | Kogevinas15 | |
| 43 | 0.99(0.44–2.24) | — | — | Consonni21 | |||
| exposure way | non-occupational | 2 | 803 | 1.28(0.65–2.52) | — | — | Revich17 |
| 4235 | 1.00(0.97–1.04) | — | — | Read20 | |||
| occupational | 5 | 1667 | 1.25(1.07–1.46)* | 78.30% | 0.001 | ||
| industrial accident | 3 | 2405 | 1.02(0.91–1.14) | 44.80% | 0.093 | ||
| Vietnam war | 1 | 12 | 0.70(0.30–1.10) | — | — | Michalek10 | |
| Serum mortality | |||||||
| cancer type | digestive organs | 4 | 82 | 1.22(0.88–1.69) | 44.00% | 0.147 | |
| respiratory system | 3 | 82 | 1.25(0.86–1.81) | 57.50% | 0.095 | ||
| lung | 4 | 74 | 0.99(0.86–1.15) | 0.00% | 0.450 | ||
| prostate | 2 | 4 | 1.40(0.40–3.60) | — | — | Collins23 | |
| 14 | 1.08(0.79–1.49) | — | — | Boers27 | |||
| non-Hodgkin’s lymphoma | 2 | 4 | 4.50(1.20–11.50)* | — | — | Collins23 | |
| 7 | 1.36(1.06–1.74)* | — | — | Boers27 | |||
| exposure way | non-occupational | 1 | 72 | 2.34(1.08–5.08)* | — | — | Lin28 |
| occupational | 4 | 925 | 1.43(1.23–1.66)* | 0.00% | 0.442 | ||
| reference category | external | 5 | 733 | 1.39(1.18–1.63)* | 0.00% | 0.458 | |
| internal | 2 | 192 | 1.80(1.16–2.82)* | — | — | Boers27 | |
| 72 | 2.34(1.08–5.08)* | — | — | Lin28 | |||
— Could not be calculated.
*Significant association was indicated, statistical z test: p < 0.05.
Eleven studies involving 9,122 cancer deaths and 691,326 participants assessed the association between external exposure of TCDD and cancer mortality. The pooled SMR of all cancer mortality of TCDD exposure level was 1.09 (95% CI: 1.01–1.19), indicating a significant positive association (Fig. 2b). There was significant heterogeneity across the included studies (I2 = 90.8%, p < 0.001), as shown in Fig. 2b. Subgroup analyses for the association between external exposure of TCDD and cancer mortality were conducted according to cancer types and TCDD exposure ways, as shown in Table 2. The pooled SMRs of cancer mortality were significant in esophagus cancer (pooled SMR = 1.52, 95% CI: 1.09–2.13), larynx cancer (pooled SMR = 2.2, 95% CI: 1.61–3.02), kidney cancer (pooled SMR = 1.39, 95% CI: 1.08–1.78), non-Hodgkin’s lymphoma (pooled SMR = 1.18, 95% CI: 1.01–1.37), myeloma (pooled SMR = 1.49, 95% CI: 1.03–2.15), soft-tissue sarcoma (pooled SMR = 1.60, 95% CI: 1.15–2.23), and occupational exposed population (pooled SMR = 1.25, 95% CI: 1.07–1.46). Subgroup analyses suggested that heterogeneity was partly influenced by cancer type and TCDD exposure way (Table 2). To further explore the potential impact of within-study heterogeneity, we also conducted sensitivity analyses according to the quality assessment results. After excluded the study10 of the lowest score (six points), the pooled SMR was 1.10 (95% CI: 1.01–1.20), while the heterogeneity was not significantly changed (from I2 = 90.8% to I2 = 91.2%). The efficiency of the current sensitivity analysis was not able to provide evidence to further explain the source of heterogeneity.
Blood level of TCDD and cancer incidence and mortality
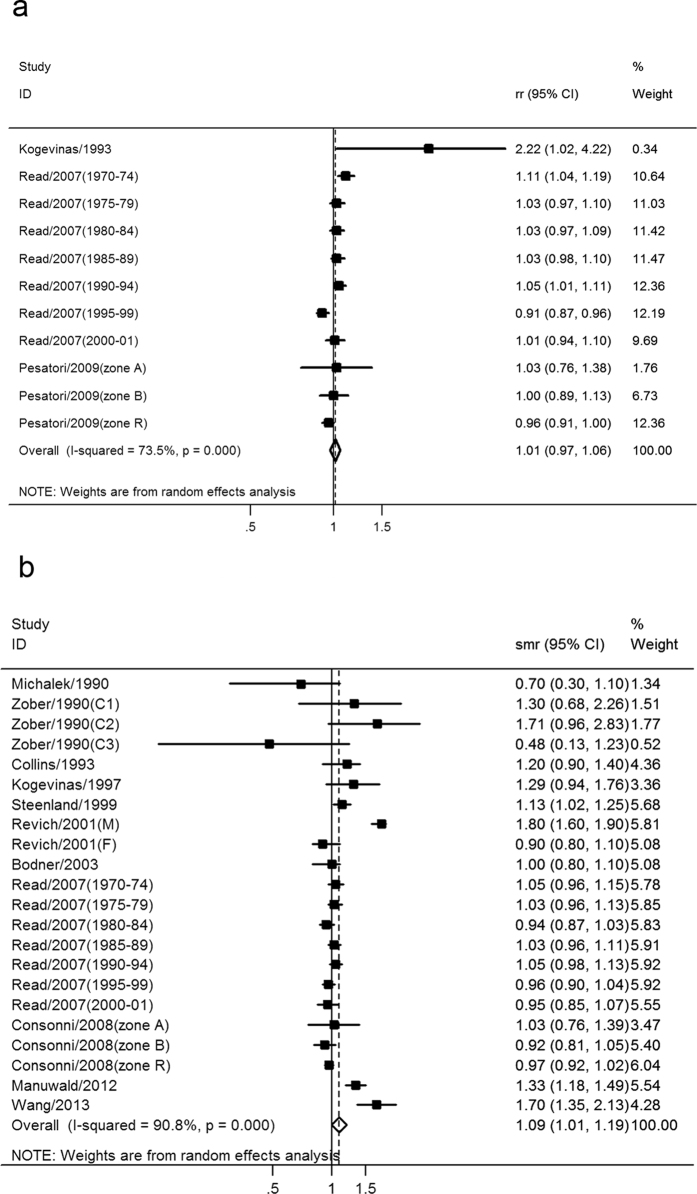
Seven studies comprising 837 cancer cases and 3,446 participants evaluated the association between blood of TCDD and cancer incidence, including three cohort studies and four case-control studies. The pooled RR of all cancer incidence for the highest versus lowest categories of TCDD exposure level was 1.57 (95% CI: 1.21–2.04), indicating a positive significant association (Fig. 3a). The I2 and p value for heterogeneity across the included studies were 7.0% and 0.341 respectively, as shown in Fig. 3a. Subgroup analysis was not conducted due to the limited data.
Figure 3.
Meta-analysis of the association between blood level of TCDD and (a) all cancer incidence and (b) all cancer mortality.
Seven studies involving 997 cancer deaths and 13,793 participants assessed the association between blood level of TCDD and cancer mortality. The pooled SMR of all cancer mortality for the highest versus lowest categories of TCDD exposure level was 1.45 (95% CI: 1.25–1.69), indicating a significant positive association (Fig. 3b). There was no significant heterogeneity across the included studies (I2 = 4.7%, p = 0.394), as shown in Fig. 3b. Subgroup analysis was conducted according to cancer type, exposure way and reference category. Two studies assessed the association between blood level of TCDD and non-Hodgkin’s lymphoma, and the SMRs (95% CI) were 4.50 (1.20–11.50) and 1.36 (1.06–1.74), respectively. The results suggested a significant positive association, which was consistent with the results of higher exposure level of TCDD. However, the results should be treated cautiously considering the relatively small sample size (n = 11), and more studies were needed to validate it. The subgroup analyses also indicated that it was all significant for occupational exposed and non-occupational exposed population, and for external and internal reference category, which further verified the stability of the results.
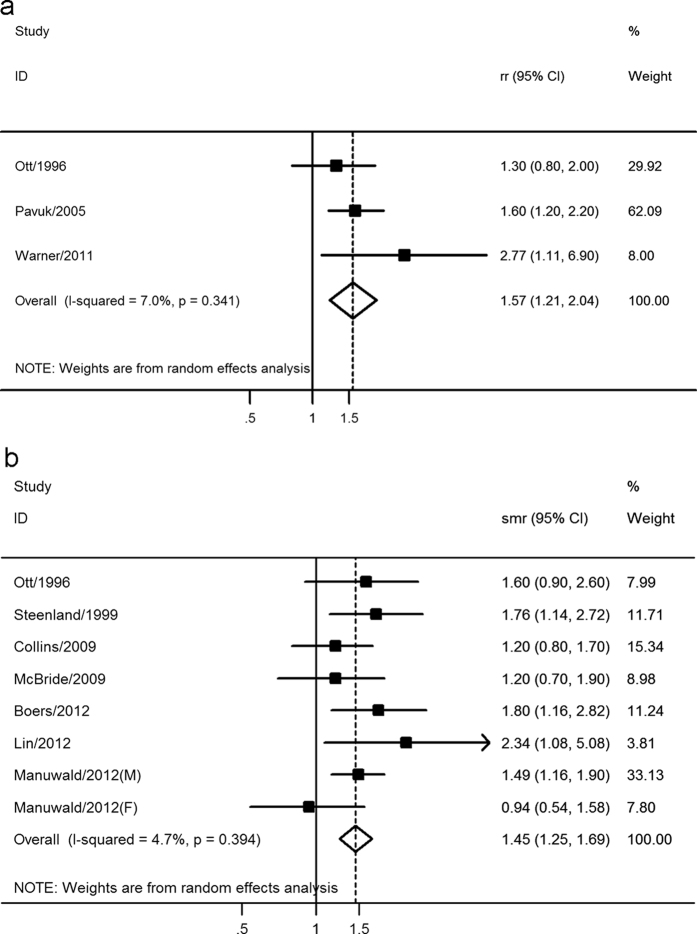
Dose-response analysis was conducted based on five studies14,16,23,24,29 according to the model of two-order fractional polynomial regression. RRs or SMRs using the low exposure group as the reference group were not appropriate for the dose-response analysis, which needs the RRs or SMRs relative to the normal background uncontaminated by occupational dioxin exposure43. Crump et al.43 conducted a dose-response analysis in 2003 with only three studies. The raw data of Ott et al.14 and Steenland et al.16,77 was obtained by personal communication by the authors43, thus we used these data extracted from Crump et al.43 to improve the validity of our analysis. We adopted Manuwald et al.’s study29 rather than Flesch-Janys et al.’s63 for the Hamburg cohort since the former had a longer follow-up time. Cumulative serum lipid concentration (CSLC, ppt-years) was selected as the exposure metric to relate to risk, and the second-order fractional polynomial regression plot indicated a positive correlation between blood TCDD level and all cancer SMR, as shown in Fig. 4a. After log transformation of TCDD dose, the curve showed a non-linear increasing trend (Fig. 4b). The size of the circles in Fig. 4 represented the study sample size. The SMRs remained below 114.02 for serum TEQ dose from 316.23 ppt-years to 5141.62 ppt-years. For the TEQ dose of 1000, 10000, 100000 ppt-years, the SMRs with 95% CIs were 110.67(99.09–122.26), 119.82(105.79–133.23) and 167.68(141.77–194.21), respectively. With SMRs increased from 114.02 to 124.02, the TEQ dose increased form 5141.62 ppt-years to 14883.33 ppt-years.
Figure 4. Dose-response analysis of the association between blood level of TCDD and all cancer mortality.
(a) Dose relationship between blood TCDD level and all cancer SMR. (b) Log dose relationship between blood TCDD level and all cancer SMR. The solid line represents SMRs and the dotted line represents 95% confidence intervals.
Publication bias
Begg’s funnel plots and Egger’s linear regression test indicated no evidence of publication bias in the present study (TCDD external exposure and cancer incidence PBegg = 0.755 and PEgger = 0.245, and mortality PBegg = 0.150 and PEgger = 0.521; blood level of TCDD and cancer incidence PBegg = 1.000 and PEgger = 0.620, and mortality PBegg = 0.711 and PEgger = 0.834). The funnel plots were shown in Supplementary Figures 1 to 4.
Discussion
The current meta-analysis summarized the results of twenty-two cohort studies and nine case-control studies, including ten on external exposure level of TCDD and cancer incidence, eleven on external exposure level and cancer mortality, seven on blood level of TCDD and cancer incidence, and seven on blood level of TCDD and cancer mortality. The results indicated that higher external exposure level of TCDD was significantly associated with all cancer mortality but not all cancer incidence. For external exposure studies, the dioxin exposure ways, exposure quantification methods, reference categories, exposure level and adjustment for potential confounders differed greatly among included studies, which could cause heterogeneity and these results should be taken cautiously. Besides, there was a significantly positive association between higher blood level of TCDD and both all cancer incidence and mortality. The subgroup analysis for TCDD exposure mortality reported significant results for esophagus cancer, larynx cancer, kidney cancer, non-Hodgkin’s lymphoma, myeloma, soft-tissue sarcoma and occupational exposed population. However, the IARC’s review suggested that the evidence for specific cancers was strongest for lung cancer, soft-tissue sarcoma and non-Hodgkin’s lymphoma3. The IARC’s review listed the related publications, while they didn’t distinguish the duplicated studies based on the same population and didn’t provided quantitatively pooled results. Thus, the results of the current study may provide relatively more detailed indications on specific cancer types. Interestingly, the subgroup analysis also suggested consistence for increased mortality ratio of non-Hodgkin’s lymphoma in both higher external exposure and blood level of TCDD, which may provide evidence on the precise carcinogenic potency of TCDD from an epidemiological point of view. The dose-response analysis showed an increasing trend of SMR with higher blood TEQ dose. For the TEQ dose of 1000, 10000, 100000 ppt-year, the SMRs were 110.67, 119.82 and 167.68, respectively.
The present meta-analysis provided epidemiological evidence for the carcinogenic potency of TCDD and the subgroup analysis showed specific cancer sites. Importantly, the consistent results for non-Hodgkin’s lymphoma mortality of both external exposure and blood level of TCDD may indicate its specific effect on hematopoietic system. Although the sample size was relative small in the blood level of TCDD and non-Hodgkin’s lymphoma mortality subgroup analysis, the results of the included two studies were both significant, independently. The SMRs and sample size of non-Hodgkin’s lymphoma by Collins et al.23 and Boers et al.27 were 4.50 (1.2–11.5, n = 4) and 1.36 (1.06–1.74, n = 7), respectively, which suggested possibility that the association may be especially significant for non-Hodgkin’s lymphoma. It has been reported by Hardell et al.86 that exposure to phenoxy acids, chlorophenols and organic solvents may be a causative factor in malignant lymphoma as early as 1981. And based on decades of research, it has been realized that, exposure to dioxins, in particular TCDD could induce chloracne87, and WHO has also classified it as a human carcinogen3. In consideration of the extensive sources, widespread trend and the strong toxicity of TCDD, the present results have considerable epidemiological and public health importance for humans. However its carcinogenic potential to humans and the mechanisms are not clearly demonstrated. It’s commonly believed that AhR activation accounted for most biological properties of dioxins, including various physiological and developmental processes, tumor promotion, thymic involution, craniofacial anomalies, skin disorders and alterations in the endocrine, immunological and reproductive systems50,88. Furthermore, TCDD may also up-regulate drug-metabolizing enzymes, thus increasing the presence of highly reactive intermediates that form during metabolic activation and/or transformation of several key hormones3. Animal experiment also suggested that intraperitoneal injection of TCDD could cause increased incidence of lymphomas in male and female mice89.
Determining the sources of heterogeneity is an important goal of meta-analysis. The heterogeneity of our study mainly existed in external exposure level of TCDD and all cancer incidence (I2 = 73.5%, p < 0.001) and mortality (I2 = 90.8%, p < 0.001). Subgroup analyses suggested that cancer subtype and dioxin exposure way can partially explain heterogeneity across the studies. Sensitivity analysis was also conducted according to the quality assessment results, while the efficiency was not able to provide evidence to further explain the source of heterogeneity. However, the heterogeneity caused by different TCDD exposure ways, quantification methods, reference categories (internal or external), lag time, background exposure levels and adjustment for confounders couldn’t be fully quantified due to the limitation of individual participant data. The future research should pay more attention to the unity of survey methods and the standardization of the exposure reference category to control heterogeneity.
Our study has several strengths. First, we adopted the external exposure and blood level of TCDD to thoroughly assess the association between TCDD and cancer incidence and mortality. Second, subgroup analyses and dose-response analyses were applied, which further strengthened the conclusions and emphasized the TCDD effects on some specific cancer sites. Although the 2012 IARC monographs3 evaluated the evidence in humans for the carcinogenicity of TCDD and made a list of cohort studies, these issues were not systematically reviewed and quantified by a meta-analysis. Thus, the current meta-analysis fill in gaps in the IARC deficiencies on this issue and it’s of considerable interest and public health importance. In addition, no publication bias was observed, indicating that the pooled results should be unbiased.
However, the current analysis is restricted by several limitations. First, the number of studies involved in blood level of TCDD and all cancer incidence was relatively small, and thus some of the subgroup analyses were difficult to conduct. Second, in the dose-response analysis, the normal background uncontaminated by occupational dioxin exposure was different, and only McBride et al.24 study provided the New Zealand background level of 3.9 ppt. We didn’t add the background exposure level to our analysis for the limitation of original data. Third, the Steenland et al.16 used a 15-year lag time, whereas no lag was used in other cohorts. Although the Crump et al.’s analysis43 inferred that results based on cumulative exposure lagged 15 years should not differ greatly from those based on unlagged exposure, this could cause inaccuracy and heterogeneity. Thus, the individual participant data meta-analysis is needed to enhance future analysis. Fourth, the subgroup analysis for blood level of TCDD and all cancer mortality was limited in digestive system, respiratory system, lung cancer, prostate cancer and non-Hodgkin’s lymphoma. More studies with precise data of different cancer types are warranted to support the effects of TCDD on other cancers.
In conclusion, our findings suggest that external exposure and blood level of TCDD were both significantly associated with all cancer mortality. Higher external exposure of TCDD may significantly increase the mortality rate of esophagus cancer, larynx cancer, kidney cancer, non-Hodgkin’s lymphoma, myeloma, soft-tissue sarcoma and occupational exposure population. Of note, such relationship may be especially significant for non-Hodgkin’s lymphoma.
Additional Information
How to cite this article: Xu, J. et al. Association between dioxin and cancer incidence and mortality: a meta-analysis. Sci. Rep. 6, 38012; doi: 10.1038/srep38012 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (Grant Nos 81400371). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors wish to thank Dr. Xinqiang Zhu and Dr. Jun Zhang (Department of Toxicology, Zhejiang University School of Public Health) for valuable discussion and suggestion.
Footnotes
Author Contributions The Corresponding Authors (Drs. Y.W. and D.X.) have the right to grant on behalf of all authors and does grant on behalf of all authors. Drs. Y.W. and D.X. contributed to conception and design of the study; Drs. J.X. and Y.Y. contributed to conception, design, and editing the manuscript; Drs. F.H., H.C. and H.W. contributed to the data acquisition, analysis, interpretation of the data, and the statistical analysis; Drs. J.H. and J.H. contributed to conception, design, and editing the manuscript. All authors commented on drafts of the paper and have approved the final draft of the manuscript.
References
- Torre L. A. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65, 87–108, doi: 10.3322/caac.21262 (2015). [DOI] [PubMed] [Google Scholar]
- IARC. Working Group on the Evaluation of Carcinogenic Risks to Humans: Polychlorinated Dibenzo-Para-Dioxins and Polychlorinated Dibenzofurans. Lyon, France, 4–11 February 1997. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization, International Agency for Research on Cancer 69, 1–631 (1997). [PMC free article] [PubMed] [Google Scholar]
- Chemical agents and related occupations. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization, International Agency for Research on Cancer 100F, 9–562 (2012). [PMC free article] [PubMed]
- Safe S. Development of bioassays and approaches for the risk assessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Environmental health perspectives 101 Suppl 3, 317–325 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M., Patterson D., Huwe J. & Kahn H. Evaluation of background exposures of Americans to dioxin-like compounds in the 1990s and the 2000s. Chemosphere 77, 640–651, doi: 10.1016/j.chemosphere.2009.08.016 (2009). [DOI] [PubMed] [Google Scholar]
- Viel J. F., Arveux P., Baverel J. & Cahn J. Y. Soft-tissue sarcoma and non-Hodgkin’s lymphoma clusters around a municipal solid waste incinerator with high dioxin emission levels. American journal of epidemiology 152, 13–19 (2000). [DOI] [PubMed] [Google Scholar]
- Pirkle J. L. et al. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam Veterans of Operation Ranch Hand. Journal of toxicology and environmental health 27, 165–171, doi: 10.1080/15287398909531288 (1989). [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S. The mechanism of dioxin toxicity: relationship to risk assessment. Environmental health perspectives 102 Suppl 9, 157–167 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S. Developmental effects of dioxins and related endocrine disrupting chemicals. Toxicology letters 82–83, 743–750 (1995). [DOI] [PubMed] [Google Scholar]
- Michalek J. E., Wolfe W. H. & Miner J. C. Health status of Air Force veterans occupationally exposed to herbicides in Vietnam. II. Mortality. Jama 264, 1832–1836 (1990). [PubMed] [Google Scholar]
- Zober A., Messerer P. & Huber P. Thirty-four-year mortality follow-up of BASF employees exposed to 2,3,7,8-TCDD after the 1953 accident. International archives of occupational and environmental health 62, 139–157 (1990). [DOI] [PubMed] [Google Scholar]
- Collins J. J., Strauss M. E., Levinskas G. J. & Conner P. C. The mortatlity experience of workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a trichlorophenol process accident. Epidemiology (Cambridge, Mass.) 4, 7–13 (1993). [DOI] [PubMed] [Google Scholar]
- Kogevinas M. et al. Cancer incidence and mortality in women occupationally exposed to chlorophenoxy herbicides, chlorophenols, and dioxins. Cancer causes & control: CCC 4, 547–553 (1993). [DOI] [PubMed] [Google Scholar]
- Ott M. G. & Zober A. Cause specific mortality and cancer incidence among employees exposed to 2,3,7,8-TCDD after a 1953 reactor accident. Occupational and environmental medicine 53, 606–612 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogevinas M. et al. Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. American journal of epidemiology 145, 1061–1075 (1997). [DOI] [PubMed] [Google Scholar]
- Steenland K., Piacitelli L., Deddens J., Fingerhut M. & Chang L. I. Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Journal of the National Cancer Institute 91, 779–786 (1999). [DOI] [PubMed] [Google Scholar]
- Revich B. et al. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere 43, 951–966 (2001). [DOI] [PubMed] [Google Scholar]
- Bodner K. M., Collins J. J., Bloemen L. J. & Carson M. L. Cancer risk for chemical workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occupational and environmental medicine 60, 672–675 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M. et al. Did TCDD exposure or service in Southeast Asia increase the risk of cancer in air force Vietnam veterans who did not spray agent orange? Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine 47, 335–342 (2005). [DOI] [PubMed] [Google Scholar]
- Read D., Wright C., Weinstein P. & Borman B. Cancer incidence and mortality in a New Zealand community potentially exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin from 2,4,5-trichlorophenoxyacetic acid manufacture. Australian and New Zealand journal of public health 31, 13–18 (2007). [DOI] [PubMed] [Google Scholar]
- Consonni D. et al. Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. American journal of epidemiology 167, 847–858, doi: 10.1093/aje/kwm371 (2008). [DOI] [PubMed] [Google Scholar]
- Viel J. F. et al. Risk for non Hodgkin’s lymphoma in the vicinity of French municipal solid waste incinerators. Environmental health: a global access science source 7, 51, doi: 10.1186/1476-069x-7-51 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. J. et al. Mortality rates among workers exposed to dioxins in the manufacture of pentachlorophenol. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine 51, 1212–1219, doi: 10.1097/JOM.0b013e3181badd4e (2009). [DOI] [PubMed] [Google Scholar]
- McBride D. I. et al. Mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin at a trichlorophenol plant in New Zealand. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine 51, 1049–1056, doi: 10.1097/JOM.0b013e3181b571ae (2009). [DOI] [PubMed] [Google Scholar]
- Pesatori A. C., Consonni D., Rubagotti M., Grillo P. & Bertazzi P. A. Cancer incidence in the population exposed to dioxin after the “Seveso accident”: twenty years of follow-up. Environmental health: a global access science source 8, 39, doi: 10.1186/1476-069x-8-39 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M. et al. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environmental health perspectives 119, 1700–1705, doi: 10.1289/ehp.1103720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers D. et al. Plasma dioxin levels and cause-specific mortality in an occupational cohort of workers exposed to chlorophenoxy herbicides, chlorophenols and contaminants. Occupational and environmental medicine 69, 113–118, doi: 10.1136/oem.2010.060426 (2012). [DOI] [PubMed] [Google Scholar]
- Lin Y. S. et al. Environmental exposure to dioxin-like compounds and the mortality risk in the U.S. population. International journal of hygiene and environmental health 215, 541–546, doi: 10.1016/j.ijheh.2012.02.006 (2012). [DOI] [PubMed] [Google Scholar]
- Manuwald U., Velasco Garrido M., Berger J., Manz A. & Baur X. Mortality study of chemical workers exposed to dioxins: follow-up 23 years after chemical plant closure. Occupational and environmental medicine 69, 636–642, doi: 10.1136/oemed-2012-100682 (2012). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. Polychlorinated dibenzo-p-dioxins and dibenzofurans and their association with cancer mortality among workers in one automobile foundry factory. The Science of the total environment 443, 104–111, doi: 10.1016/j.scitotenv.2012.10.073 (2013). [DOI] [PubMed] [Google Scholar]
- Danjou A. M. et al. Estimated dietary dioxin exposure and breast cancer risk among women from the French E3N prospective cohort. Breast cancer research: BCR 17, 39, doi: 10.1186/s13058-015-0536-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardell L. Phenoxy herbicides, chlorophenols, soft-tissue sarcoma (STS) and malignant lymphoma. British journal of cancer 67, 1154–1156 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardell L. et al. Adipose tissue concentrations of dioxins and dibenzofurans, titers of antibodies to Epstein-Barr virus early antigen and the risk for non-Hodgkin lymphoma. Environmental research 87, 99–107, doi: 10.1006/enrs.2001.4295 (2001). [DOI] [PubMed] [Google Scholar]
- Floret N. et al. Dioxin emissions from a solid waste incinerator and risk of non-Hodgkin lymphoma. Epidemiology (Cambridge, Mass.) 14, 392–398, doi: 10.1097/01.ede.0000072107.90304.01 (2003). [DOI] [PubMed] [Google Scholar]
- Tuomisto J. T. et al. Soft-tissue sarcoma and dioxin: A case-control study. International journal of cancer. Journal international du cancer 108, 893–900, doi: 10.1002/ijc.11635 (2004). [DOI] [PubMed] [Google Scholar]
- De Roos A. J. et al. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin’s lymphoma. Cancer research 65, 11214–11226, doi: 10.1158/0008-5472.can-05-1755 (2005). [DOI] [PubMed] [Google Scholar]
- Reynolds P. et al. Adipose levels of dioxins and risk of breast cancer. Cancer causes & control: CCC 16, 525–535, doi: 10.1007/s10552-004-7840-5 (2005). [DOI] [PubMed] [Google Scholar]
- Zambon P. et al. Sarcoma risk and dioxin emissions from incinerators and industrial plants: a population-based case-control study (Italy). Environmental health: a global access science source 6, 19, doi: 10.1186/1476-069x-6-19 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel J. F. et al. Dioxin emissions from a municipal solid waste incinerator and risk of invasive breast cancer: a population-based case-control study with GIS-derived exposure. International journal of health geographics 7, 4, doi: 10.1186/1476-072x-7-4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S. et al. Occupation and occupational exposure to endocrine disrupting chemicals in male breast cancer: a case-control study in Europe. Occupational and environmental medicine 67, 837–844, doi: 10.1136/oem.2009.052175 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L., Chen X., Li C. P., Luo X. Y. & Tang N. J. 2,3,7,8-Tetrachlorodibezo-p-dioxin exposure and prostate cancer: a meta-analysis of cohort studies. Public health 128, 207–213, doi: 10.1016/j.puhe.2013.10.006 (2014). [DOI] [PubMed] [Google Scholar]
- Zendehdel R., Tayefeh-Rahimian R. & Kabir A. Chronic exposure to chlorophenol related compounds in the pesticide production workplace and lung cancer: a meta-analysis. Asian Pacific journal of cancer prevention: APJCP 15, 5149–5153 (2014). [DOI] [PubMed] [Google Scholar]
- Crump K. S., Canady R. & Kogevinas M. Meta-analysis of dioxin cancer dose response for three occupational cohorts. Environmental health perspectives 111, 681–687 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA: the journal of the American Medical Association 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. & Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 6, e1000097, doi: 10.1371/journal.pmed.1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology 25, 603–605, doi: 10.1007/s10654-010-9491-z (2010). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, doi: 10.1002/sim.1186 (2002). [DOI] [PubMed] [Google Scholar]
- Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Statistics in medicine 19, 1831–1847 (2000). [DOI] [PubMed] [Google Scholar]
- Akhtar F. Z., Garabrant D. H., Ketchum N. S. & Michalek J. E. Cancer in US Air Force veterans of the Vietnam War. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine 46, 123–136, doi: 10.1097/01.jom.0000111603.84316.0f (2004). [DOI] [PubMed] [Google Scholar]
- Baccarelli A. et al. Aryl-hydrocarbon receptor-dependent pathway and toxic effects of TCDD in humans: a population-based study in Seveso, Italy. Toxicology letters 149, 287–293, doi: 10.1016/j.toxlet.2003.12.062 (2004). [DOI] [PubMed] [Google Scholar]
- Becher H., Steindorf K. & Flesch-Janys D. Quantitative cancer risk assessment for dioxins using an occupational cohort. Environmental health perspectives 106 Suppl 2, 663–670 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzi A. et al. Cancer incidence in a population accidentally exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. Epidemiology (Cambridge, Mass.) 4, 398–406 (1993). [DOI] [PubMed] [Google Scholar]
- Bertazzi P. A. et al. Health effects of dioxin exposure: a 20-year mortality study. American journal of epidemiology 153, 1031–1044 (2001). [DOI] [PubMed] [Google Scholar]
- Bertazzi P. A. et al. Dioxin exposure and cancer risk: a 15-year mortality study after the “Seveso accident”. Epidemiology (Cambridge, Mass.) 8, 646–652 (1997). [PubMed] [Google Scholar]
- Bertazzi P. A. et al. Mortality of a young population after accidental exposure to 2,3,7,8-tetrachlorodibenzodioxin. International journal of epidemiology 21, 118–123 (1992). [DOI] [PubMed] [Google Scholar]
- Bertazzi P. A. et al. Ten-year mortality study of the population involved in the Seveso incident in 1976. American journal of epidemiology 129, 1187–1200 (1989). [DOI] [PubMed] [Google Scholar]
- Bond G. G., McLaren E. A., Lipps T. E. & Cook R. R. Update of mortality among chemical workers with potential exposure to the higher chlorinated dioxins. Journal of occupational medicine: official publication of the Industrial Medical Association 31, 121–123 (1989). [PubMed] [Google Scholar]
- Bueno de Mesquita H. B., Doornbos G., Van der Kuip D. A., Kogevinas M. & Winkelmann R. Occupational exposure to phenoxy herbicides and chlorophenols and cancer mortality in The Netherlands. American journal of industrial medicine 23, 289–300 (1993). [DOI] [PubMed] [Google Scholar]
- Cheng H. et al. TCDD exposure-response analysis and risk assessment. Risk analysis: an official publication of the Society for Risk Analysis 26, 1059–1071, doi: 10.1111/j.1539-6924.2006.00800.x (2006). [DOI] [PubMed] [Google Scholar]
- Cook R. R., Bond G. G. & Olson R. A. Evaluation of the mortality experience of workers exposed to the chlorinated dioxins. Chemosphere 15, 1769–1776 (1986). [Google Scholar]
- Fingerhut M. A. et al. Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. The New England journal of medicine 324, 212–218, doi: 10.1056/nejm199101243240402 (1991). [DOI] [PubMed] [Google Scholar]
- Flesch-Janys D. et al. Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. American journal of epidemiology 142, 1165–1175 (1995). [DOI] [PubMed] [Google Scholar]
- Flesch-Janys D., Steindorf K., Gurn P. & Becher H. Estimation of the cumulated exposure to polychlorinated dibenzo-p-dioxins/furans and standardized mortality ratio analysis of cancer mortality by dose in an occupationally exposed cohort. Environmental health perspectives 106 Suppl 2, 655–662 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heederik D., Hooiveld M. & Bueno-de-Mesquita H. B. Modelling of 2,3,7,8-tetrachlorodibenzo-p-dioxin levels in a cohort of workers with exposure to phenoxy herbicides and chlorophenols. Chemosphere 37, 1743–1754 (1998). [DOI] [PubMed] [Google Scholar]
- Hooiveld M. et al. Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. American journal of epidemiology 147, 891–901 (1998). [DOI] [PubMed] [Google Scholar]
- Ketchum N. S., Michalek J. E. & Burton J. E. Serum dioxin and cancer in veterans of Operation Ranch Hand. American journal of epidemiology 149, 630–639 (1999). [DOI] [PubMed] [Google Scholar]
- Kogevinas M. et al. Cancer mortality from soft-tissue sarcoma and malignant lymphomas in an international cohort of workers exposted to chlorophenoxy herbicides and chlorophenols. Chemosphere 25, 1071–1076 (1992). [Google Scholar]
- Manz A. et al. Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet (London, England) 338, 959–964 (1991). [DOI] [PubMed] [Google Scholar]
- Michalek J. E. & Pavuk M. Diabetes and cancer in veterans of Operation Ranch Hand after adjustment for calendar period, days of spraying, and time spent in Southeast Asia. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine 50, 330–340, doi: 10.1097/JOM.0b013e31815f889b (2008). [DOI] [PubMed] [Google Scholar]
- Ott M. G., Olson R. A., Cook R. R. & Bond G. G. Cohort mortality study of chemical workers with potential exposure to the higher chlorinated dioxins. Journal of occupational medicine: official publication of the Industrial Medical Association 29, 422–429 (1987). [PubMed] [Google Scholar]
- Pavuk M., Michalek J. E. & Ketchum N. S. Prostate cancer in US Air Force veterans of the Vietnam war. Journal of exposure science & environmental epidemiology 16, 184–190, doi: 10.1038/sj.jea.7500448 (2006). [DOI] [PubMed] [Google Scholar]
- Pesatori A. C. et al. Cancer morbidity in the Seveso area, 1976-1986. Chemosphere 25, 209–212 (1992). [Google Scholar]
- Pesatori A. C. et al. Cancer in a young population in a dioxin-contaminated area. International journal of epidemiology 22, 1010–1013 (1993). [DOI] [PubMed] [Google Scholar]
- Ramlow J. M. et al. Mortality in a cohort of pentachlorophenol manufacturing workers, 1940–1989. American journal of industrial medicine 30, 180–194, doi: (1996). [DOI] [PubMed] [Google Scholar]
- Salvan A., Thomaseth K., Bortot P. & Sartori N. Use of a toxicokinetic model in the analysis of cancer mortality in relation to the estimated absorbed dose of dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD). The Science of the total environment 274, 21–35 (2001). [DOI] [PubMed] [Google Scholar]
- Saracci R. et al. Cancer mortality in workers exposed to chlorophenoxy herbicides and chlorophenols. Lancet (London, England) 338, 1027–1032 (1991). [DOI] [PubMed] [Google Scholar]
- Steenland K., Deddens J. & Piacitelli L. Risk assessment for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) based on an epidemiologic study. American journal of epidemiology 154, 451–458 (2001). [DOI] [PubMed] [Google Scholar]
- Steenland K., Nowlin S., Ryan B. & Adams S. Use of multiple-cause mortality data in epidemiologic analyses: US rate and proportion files developed by the National Institute for Occupational Safety and Health and the National Cancer Institute. American journal of epidemiology 136, 855–862 (1992). [DOI] [PubMed] [Google Scholar]
- Vena J. et al. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environmental health perspectives 106, 645–653 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M. et al. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environmental health perspectives 110, 625–628 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardell L. & Sandstrom A. Case-control study: soft-tissue sarcomas and exposure to phenoxyacetic acids or chlorophenols. British journal of cancer 39, 711–717 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M., Hardell L., Berg N. O., Moller T. & Axelson O. Soft-tissue sarcomas and exposure to chemical substances: a case-referent study. British journal of industrial medicine 38, 27–33 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardell L. & Eriksson M. The association between soft tissue sarcomas and exposure to phenoxyacetic acids. A new case-referent study. Cancer 62, 652–656 (1988). [DOI] [PubMed] [Google Scholar]
- Eriksson M., Hardell L. & Adami H. O. Exposure to dioxins as a risk factor for soft tissue sarcoma: a population-based case-control study. Journal of the National Cancer Institute 82, 486–490 (1990). [DOI] [PubMed] [Google Scholar]
- Kogevinas M. et al. Soft tissue sarcoma and non-Hodgkin’s lymphoma in workers exposed to phenoxy herbicides, chlorophenols, and dioxins: two nested case-control studies. Epidemiology (Cambridge, Mass.) 6, 396–402 (1995). [PubMed] [Google Scholar]
- Hardell L., Eriksson M., Lenner P. & Lundgren E. Malignant lymphoma and exposure to chemicals, especially organic solvents, chlorophenols and phenoxy acids: a case-control study. British journal of cancer 43, 169–176 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg O. AhR signalling and dioxin toxicity. Toxicology letters 230, 225–233, doi: 10.1016/j.toxlet.2013.10.039 (2014). [DOI] [PubMed] [Google Scholar]
- Zudaire E. et al. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. The Journal of clinical investigation 118, 640–650, doi: 10.1172/jci30024 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Porta G., Dragani T. A. & Sozzi G. Carcinogenic effects of infantile and long-term 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment in the mouse. Tumori 73, 99–107 (1987). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.