Abstract
Background
As a symptom of pyomyositis, sepsis usually follows local inflammation signs. Here, we report pyomyositis with lymphedema of upper extremity in which septic shock and poor local findings initially presented during chemotherapy for breast cancer.
Case Report
An 80-year-old woman presented with chronic right shoulder pain during chemotherapy for the recurrent disease. She had a history of postmastectomy lymphedema, diabetes mellitus, and repeated hyaluronic acid injections to the shoulder joint. The pain suddenly worsened with septic shock and no apparent local signs. Magnetic resonance imaging revealed myonecrosis, and no pus was yielded by ultrasound-guided needle aspiration. After 2 weeks of recovery by conservative medical management, surgical drainage was performed. Late formulated massive intramuscular pus showed severe neutrophil infiltration and myonecrosis.
Conclusion
Pyomyositis can develop into septic shock with poor local signs. Myelosuppression after chemotherapy can cause myonecrosis without macroabscess, and magnetic resonance imaging was useful for the diagnosis of this condition. When unspecified local pain appears during cancer chemotherapy we should consider this disease, too.
Keywords: Diabetes mellitus, Intraregional injections, Magnetic resonance imaging, Necrosis, Neutropenia, Septic shock, Shoulder pain, Upper extremity
Introduction
Pyomyositis is an infectious disease causing intramuscular abscess of the large skeletal muscles, which was originally described to be common in tropical areas and referred to as pyomyositis tropicans [1]. Pyomyositis is recognized increasingly in temperate climates, associated with an immunocompromised host, particularly due to human immunocompromised virus infection and hematological malignancy [2, 3]. The most common organism implicated is Staphylococcus aureus [1, 2, 3].
The clinical picture is divided into invasive, suppurative, and late stages; sepsis as symptom of the late stage follows abscess formation in the suppurative stage [2]. However, pyomyositis without macroabscess at the time of septic shock has rarely been reported. Here, we report the disease in a patient with lymphedema of upper extremity. Septic shock and poor local findings initially presented during breast cancer chemotherapy.
Case Presentation
The case was an 80-year-old Japanese woman presenting with right shoulder pain. She had undergone a right modified radical mastectomy for breast cancer 19 years prior, and local recurrence appeared 10 years prior. After excision of the recurrence, she received adjuvant hormonal therapy, but distant metastasis recurrences were found in her right lung and pleura. Furthermore, right supraclavicular lymph node metastases made postmastectomy lymphedema worse in the same side. She also had had diabetes mellitus for 9 years. She did not have chronic kidney disease. Five months prior to admission, she had fallen, hit her right shoulder, and had repeatedly been injected to the joint with hyaluronic acid as a treatment of frozen shoulder. She did not report any physical activity and did not work due to general weakness but had received biweekly nab-paclitaxel as a palliative chemotherapy for 3 months. The chemotherapy was so effective that her right pleural effusion decreased in size.
She was admitted to our hospital because the chronic right shoulder pain worsened suddenly. The pain was accompanied by catecholamine-resistant shock with bradycardia at 60/– mm Hg and 36 beats per minute. Electrocardiography showed atrioventricular junction rhythm, so we inserted a temporary pacemaker. On admission she had no fever. Her right upper arm was not hot or fluctuant without erythema. WBC and absolute neutrophil counts were normal (3,400 and 2,900/μL, respectively) but higher than her usual levels during the chemotherapy without infection. The absolute neutrophil count in her nadir period was around 500–1,000/μL. C-reactive protein increased significantly to 19.9 mg/dL (reference range: 0.0–0.5). Creatine kinase was slightly increased to 234 IU/L (reference range: 40–170). Hemoglobin concentration and hemoglobin A1c were 8.2 g/dL and 8.2%, respectively. Serum creatinine level increased to 1.6 mg/dL (reference range: 0.4–0.8). Computed tomography (CT) scans showed swelling, increased density, and small air bubbles in the muscles surrounding her right shoulder joint (Fig 1). On day 2 of admission, she developed a high fever of up to 38°C, erythema, tenderness, and progressive edema in the arm (Fig 2). She was found to have methicillin-sensitive S. aureus bacteremia. Magnetic resonance imaging (MRI) revealed necrosis of several right shoulder joint muscles, showing abnormally high signal intensity in the muscles on T2-weighted and short tau inversion recovery sequence images, abnormal enhancement in some parts of the fascia, and no enhancement in the muscles (Fig 3). She received intravenous antimicrobial treatment with meropenem (500 mg every 12 h with dose adjustment for renal impairment) and clindamycin (600 mg every 8 h), and intensive insulin therapy. No pus was yielded by ultrasound-guided needle aspiration from the shoulder muscles or its joint, and the patient responded well to the antimicrobial therapy, so we were able to stop catecholamine. It was difficult to rule out necrotizing fasciitis, but we decided not to perform immediate debridement. Debridement for necrotizing fasciitis would have meant removal of large amounts of tissue from her arm, and the patient would have spent the rest of her limited time (by metastatic breast cancer with carcinomatous pleurisy) bedridden with a major wound. Thus, we continued conservative medical management considering her quality of life. Local inflammation signs persisted. Transthoracic echocardiography indicated no verruca or abscess around the heart valves. Whole-body CT scans showed no abscess, either.
Fig. 1.
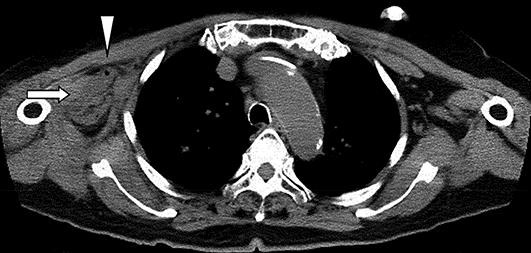
Computed tomography scan of scapular lesion showing swelling (arrow) and a small air bubble (arrowhead) in the muscles surrounding the right shoulder joint.
Fig. 2.
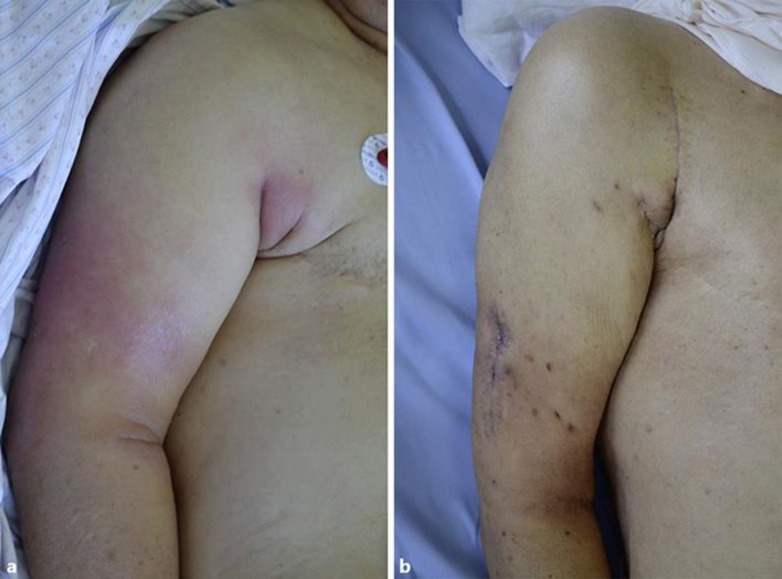
Visual findings of the right upper arm. a On day 2 of administration, erythema developed and swelling increased. b After 2 weeks of surgical drainage, erythema disappeared and swelling diminished.
Fig. 3.
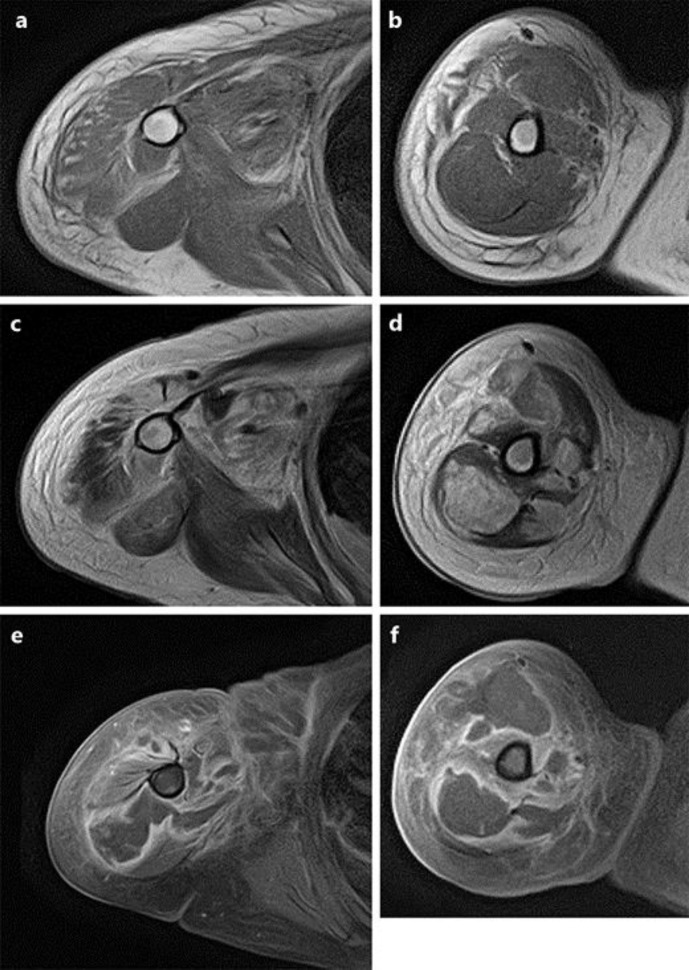
Magnetic resonance imaging showing necrosis of deltoid, triceps, and biceps brachii muscles with edematous subcutaneous tissue: coronal (a) and axial (b) T1-weighted images, coronal (c) and axial (d) T2-weighted images, and coronal (e) and axial (f) fat-suppressed T2-weighted images.
Due to skin disintegration and pus outflow after 2 weeks, we performed surgical drainage. Massive pus and necrotic tissue erupted from the axilla and lateral side of the distal upper arm. Coracoid process was palpable through the axial cavity. Histological examination confirmed pyomyositis, showing severe necrosis of striated muscle and severe neutrophil infiltration. No apparent bacterial cells were identified. Pyomyositis was cured after definitive antimicrobial therapy for 3 weeks from the drainage. She was able to restart the chemotherapy for her breast cancer which was discontinued due to the infection.
Discussion
This patient course highlighted 2 important clinical issues. Pyomyositis associated with cancer chemotherapy can rapidly develop into septic shock without macroabscess, especially in breast cancer patients with lymphedema. MRI was useful in the diagnosis of this case with myonecrosis and no macroabscess.
First, pyomyositis can rapidly develop into septic shock without macroabscess during cancer chemotherapy, especially in breast cancer patients with lymphedema. Sepsis usually follows fever and abscess formation in the disease. The clinical picture is divided into 3 stages: the invasive, suppurative, and late stage [2]. The initial “invasive stage” is characterized by painful, firm swelling and low-grade fever. After 1–2 weeks, high spiky fever with abscess marks the beginning of the “suppurative stage” followed by the “late stage” defined by bacteremia [2]. However, the patient did not have macroabscess when she was in septic shock due to pyomyositis. No pus was yielded by ultrasound-guided needle aspiration at the time of the shock. Moreover, local redness and heat as inflammation signs seemed to be hidden by lymphedema. Myelosuppression after cancer chemotherapy most likely delayed the formation of intramuscular abscess. The onset of infection was just neutrophil nadir period after the latest chemotherapy. Cancer chemotherapy decreases not only the neutrophil count, but also its function [4]. As a similar example, only 8% of pneumonia patients produce purulent sputum in a neutropenic state under 0.1 × 109/L [5]. In addition, in as much as 40% of cases with pneumonia caused by gram-negative bacilli no lung shadows are seen on chest radiography in the neutropenic state [6]. This is why the patient had presented with no macroabscess and poor local inflammation signs until the septic shock developed [5]. Breast cancer with lymphedema is most likely one of the initiating factors of pyomyositis. These factors include trauma such as blunt injuries, vigorous exercise, falls, and injections, which were noted in 43% of human immunocompromised virus-negative pyomyositis patients in the United States [3]. Diabetes mellitus, hematologic malignancies, and solid malignancies accounted for 19, 11, and 9%, respectively; pyomyositis associated with solid malignancy was not common [3]. Others were rheumatologic disorders, cirrhosis, renal insufficiency, benign hematologic diseases, skin disorders, organ transplants and administration of immunosuppressive agents including corticosteroids, and so on [3]. However, lymphedema has never been reported as an initiating factor of the disease. Additionally, to the best of our knowledge, pyomyositis associated with breast cancer was reported in only 3 cases [7, 8, 9]. Fluid congestion in intercellular space may cause infections like cellulitis in patients with lymphedema [1]. This time the condition resulted in abscesses in multiple muscles, which is unusual in this disease; pyomyositis usually affects only a single muscle. This is the first reported case of pyomyositis associated with lymphedema.
Second, MRI was useful in pyomyositis diagnosis with myonecrosis and no macr-abscess during cancer chemotherapy. It may be the best available imaging technique for the diagnosis of the disease [10]. At times, CT alone may be unreliable in distinguishing abscess or necrosis from swollen muscles [11]. MRI can provide detailed information regarding the location and regional extent of infection, and the presence or absence of abscess or necrosis [12]. Abscess is characterized by peripheral rim of increased signal intensity on T1-weighted images, and central region of instance signal on T2-weighted and short tau inversion recovery sequence images [10]. The rim surrounding the abscess is hypointense on T2-weighted images and enhances after intravenous administration of contrast, whereas necrotic tissue and purulent material show no enhancement [10, 12]. Abscess may be mistaken for myonecrosis, but it is generally differentiated on T2-weighted images by a presence of central high signal intensity and a mass effect [13]. In this case, macroabscess was not formed on admission; ultrasound-guided needle aspiration yielded no pus at the time.
Mechanical damage to the skeletal muscle including fall and repeated injection most likely initiated pyomyositis. The pathogenesis of pyomyositis is still unclear, but some initiating factor is necessary to develop the disease [14]. The skeletal muscle tissue is intrinsically resistant to bacterial infections under normal circumstances [14]. Mechanical damage to the skeletal muscle was suggested to be largely associated with development of the disease [15]. The etiologic agent was S. aureus, and other infection routes were not detected. We suspected trauma and repeated injections to the shoulder as the most likely causes of the infection. In addition, diabetes mellitus and advanced age contributed to the aggressive progression.
In conclusion, pyomyositis associated with cancer chemotherapy can rapidly develop into septic shock without macroabscess, especially in breast cancer patients with lymphedema, and MRI was useful in the diagnosis of this condition with myonecrosis. When unspecified local pain appears during cancer chemotherapy, after mechanical damage, or lymphedema, we should lower the testing threshold of imaging techniques including MRI to diagnose or rule out pyomyositis. Local findings may mimic cellulitis like in this case. If necrosis, even without macroabscess is detected, surgical drainage should be considered. Clinical awareness of this issue is important for rapid diagnosis and treatment, so that treatment-related death be avoided and full neuromuscular recovery be attained.
Statement of Ethics
The patient was informed that data from the case would be submitted for publication and gave her consent.
Disclosure Statement
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the patient for her kind cooperation, and Mr. David Hochman for reviewing the language of our article.
References
- 1.Chauhan S, Jain S, Varma S, Chauhan S. Tropical pyomyositis (myositis tropicans): current perspective. Postgrad Med J. 2004;80:267–270. doi: 10.1136/pgmj.2003.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiedozi LC. Pyomyositis. Review of 205 cases in 112 patients. Am J Surg. 1979;137:255–259. doi: 10.1016/0002-9610(79)90158-2. [DOI] [PubMed] [Google Scholar]
- 3.Crum NF. Bacterial pyomyositis in the United States. Am J Med. 2004;117:420–428. doi: 10.1016/j.amjmed.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 4.Mendonça MA, Cunha FQ, Murta EF, Tavares-Murta BM. Failure of neutrophil chemotactic function in breast cancer patients treated with chemotherapy. Cancer Chemother Pharmacol. 2006;57:663–670. doi: 10.1007/s00280-005-0086-4. [DOI] [PubMed] [Google Scholar]
- 5.Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med. 1975;135:715–719. [PubMed] [Google Scholar]
- 6.Valdivieso M, Gil-extremera B, Zornoza J, Rodriquez V, Bodey GP. Gram-negative bacillary pneumonia in the compromised host. Medicine (Baltimore) 1077;56:241–254. doi: 10.1097/00005792-197705000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Keith BD, Bramwell VH. Pyomyositis after chemotherapy for breast cancer. Am J Clin Oncol. 2000;23:42–44. doi: 10.1097/00000421-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Chiu SK, Lin JC, Wang NC, Peng MY, Chang FY. Impact of underlying diseases on the clinical characteristics and outcome of primary pyomyositis. J Microbiol Immunol Infect. 2008;41:286–293. [PubMed] [Google Scholar]
- 9.McRae M, Sharma S. Forearm pyomyositis in a breast cancer patient on chemotherapy. J Plast Reconstr Aesthet Surg. 2010;63:e737–e739. doi: 10.1016/j.bjps.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 10.Theodorou SJ, Theodorou DJ, Resnick D. MR imaging findings of pyogenic bacterial myositis (pyomyositis) in patients with local muscle trauma: illustrative cases. Emerg Radiol. 2007;14:89–96. doi: 10.1007/s10140-007-0593-1. [DOI] [PubMed] [Google Scholar]
- 11.Falasca GF, Reginato AJ. The spectrum of myositis and rhabdomyolysis associated with bacterial infection. J Rheumatol. 1994;21:1932–1937. [PubMed] [Google Scholar]
- 12.Yu CW, Hsiao JK, Hsu CY, Shih TT. Bacterial pyomyositis: MRI and clinical correlation. Magn Reson Imaging. 2004;22:1233–1241. doi: 10.1016/j.mri.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Theodorou DJ, Theodorou SJ, Kakitsubata Y, Sartoris DJ, Resnick D. Imaging characteristics and epidemiologic features of atypical mycobacterial infections involving the musculoskeletal system. AJR Am J Roentgenol. 2001;176:341–349. doi: 10.2214/ajr.176.2.1760341. [DOI] [PubMed] [Google Scholar]
- 14.Gransden WR, Eykyn SJ, Phillips I. <italic>Staphylococcus aureus</italic> bacteraemia: 400 episodes in St Thomas's Hospital. Br Med J (Clin Res Ed) 1984;288:300–303. doi: 10.1136/bmj.288.6413.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith IM, Vickers AB. Natural history of 338 treated and untreated patients with staphylococcal septicaemia (1936–1955) Lancet. 1960;1:1318–1322. [PubMed] [Google Scholar]