Abstract
Heterotopic ossification in colorectal carcinoma is extremely rare. This report presents the case of a 57-year-old male who had undergone a low anterior resection following a diagnosis of rectal carcinoma. Histological examination showed heterotopic ossification in the tumor. The patient was referred to Ibaraki Medical Center, Tokyo Medical University, with a diagnosis of rectal carcinoma by a local physician. Abdominal computed tomography revealed thickening of the rectal wall with calcified deposits, and virtual colonoscopy showed stenosis with a mass in the rectum. The patient underwent a low anterior resection and diverting ileostomy in May 2014. Histological examination of the excised tumor showed moderately differentiated adenocarcinoma and an infiltration of spindle cells with numerous foci of osteoid and ossification, with osteoblastic rimming in the stroma. Immunohistochemical analysis of these spindle cells and osteoblasts revealed negative staining for AE1/AE3, suggesting a reactive change. There was metastasis in 1 of the 12 lymph nodes, and the tumor was diagnosed as stage IIIB (T4a, N1a, M0) rectal carcinoma. The patient had an uneventful recovery and was followed up at our outpatient clinic. In conclusion, the malignant potential of heterotopic ossification in rectal carcinoma has not been determined. However, heterotopic ossification is induced by tumor progression in a microenvironment, suggesting a high tumor malignity. The patient should be carefully monitored after surgery in terms of improved patient outcome.
Keywords: Colorectal carcinoma, Ossification, Calcified deposits, Prognosis, Histogenesis
Introduction
Calcification is often observed in thyroid, breast, and ovarian carcinomas as well as brain tumors; however, ossification is rare in gastrointestinal tract malignancies. Moreover, ossification in colorectal carcinoma is decidedly rare and has not been elucidated with regard to malignity and histogenesis. Here, we report the case of a 57-year-old male with heterotopic ossification in rectal carcinoma.
Case Presentation
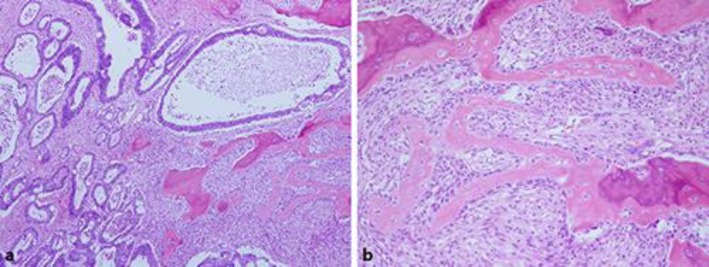
A 57-year-old male visited a local physician due to recurrent constipation and diarrhea. In the rectum, colonoscopy revealed the entire circumference of an elevated tumor with a central depression not passing through the tumor. A tumor biopsy indicated moderately differentiated adenocarcinoma. The following day, he was referred to Ibaraki Medical Center, Tokyo Medical University, with a diagnosis of rectal cancer. His medical history was otherwise unremarkable. The findings of a subsequent physical examination were also unremarkable, except for a rectal examination that detected a palpable mass. Laboratory data revealed an increase in the white blood cell (8,900/μL), C-reactive protein (1.76 mg/dL), and carcinoembryonic antigen (10.7 ng/mL) levels. Abdominal computed tomography revealed thickening of the rectal wall with calcified deposits, but no liver metastasis (Fig 1). A virtual colonoscopy revealed stenosis with a mass in the rectum, which was confirmed by colonoscopy (Fig 2). A diagnosis of rectal carcinoma was established, and a low anterior resection and diverting ileostomy were performed in May 2014. The resected tumor measured 8.0 × 7.0 cm, and a section of the tumor revealed solid gray, yellowish myxoid areas. Histological examination of the excised tumor showed moderately differentiated adenocarcinoma that had penetrated the rectal wall and exposed the surface of the serosa. The tumor contained an infiltration of spindle cells and numerous foci of osteoid and ossification, with osteoblastic rimming in the stroma (Fig 3). Immunohistochemical analysis of these spindle cells and osteoblasts revealed negative staining for AE1/AE3. There was cancer metastasis in 1 of the 12 lymph nodes. The tumor was diagnosed as stage IIIB (T4a, N1a, M0) according to the International Union against Cancer Tumor Node Metastasis Classification (7th edition) [1]. The patient had an uneventful recovery and was discharged on postoperative day 20. Subsequently, the patient was administered oral tegafur/uracil and leucovorin therapy for 6 months, and his progress is currently being followed up at our outpatient clinic.
Fig. 1.
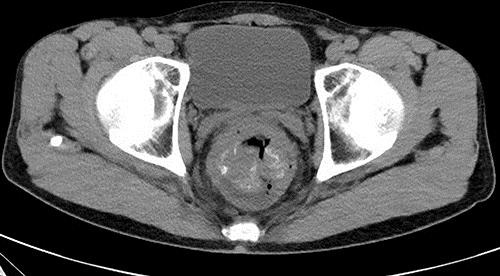
Abdominal computed tomography demonstrating thickening of the rectal wall with calcified deposits.
Fig. 2.
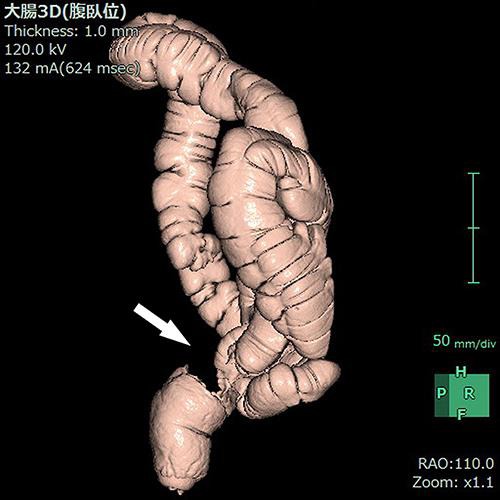
Virtual colonoscopy revealing stenosis with a mass in the rectum (arrow).
Fig. 3.
Histological analysis of the excised tumor. a Moderately differentiated adenocarcinoma with numerous foci of osteoid and ossification (hematoxylin and eosin stain. ×40). b Osteoblasts are linked by tight and gap junctions and integrated with the underlying osteocyte, namely osteoblastic rimming (hematoxylin and eosin stain. ×100).
Discussion
According to 2 review articles, the most common sites of heterotopic ossification in a gastrointestinal tract tumor were the colon and rectum [2, 3]. Dukes [4] mentioned a frequency of incidence below 0.4%. In the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/), there have been 11 reported cases of colorectal carcinoma, including the present case, with ossification described by the words “ossification”, “calcified”, and “colorectal carcinoma” between 1995 and 2015 (Table 1) [3, 5, 6, 7, 8, 9, 10, 11]. The average age of the patients in these reports was 58.8 years (range 46–79), and the male-to-female ratio was 7: 4. In most of these patients, the tumors mainly occurred in the rectum (63.6%). Although there were no data for 2 patients, the average maximum size of the tumor was 6.5 cm (range 2.1–9.0). Regarding the histological type, mucinous adenocarcinoma was seen in 4 patients (36.4%). Although there were no data for 4 patients, 6 of 7 cases (85.7%) had metastasis to the lymph nodes. The nature of heterotopic ossification in colorectal carcinoma is debatable. Dukes [4] reported that it had a slow growth and low malignant potential. On the other hand, Matsumoto et al. [10] reported a case of a rapidly progressive course after surgery. In the present case, the tumor penetrated the rectal wall, and cancer metastasis was detected in the regional lymph node. Taking this into consideration, ossification in colorectal carcinoma has a high progression potential and a poor prognosis.
Table 1.
Summary of heterotopic ossification in colorectal carcinoma
| First author [ref.] | Reported year | Age, years | Sex | Tumor location | Size, cm | Pathology | Lymph node metastasis | TNM classification |
|---|---|---|---|---|---|---|---|---|
| Haque [3] | 1996 | 78 | M | Rectum | 7.0 × 4.0 | Mode-AC and Muci-AC | N.D. | N.D. |
| 46 | F | Cecum | 1.9 × 2.1 | Muci-AC | N.D. | N.D. | ||
| 55 | F | Transverse colon | 8.0 × 5.0 × 3.0 | Muci-AC | N.D. | N.D. | ||
| Beauchamp [5] | 1997 | 64 | M | Rectum | 9.0 | Mode-AC | (+) | T4, N2, MX |
| Papadopoulos [6] | 1998 | 47 | F | Cecum | N.D. | Mode-AC | N.D. | N.D. |
| Alper [7] | 2000 | 56 | M | Rectum | N.D. | Muci-AC | (+): 2/12 | N.D. |
| Imai [8] | 2001 | 50 | F | Ascending colon | 4.0 × 4.0 × 6.5 | Mode-AC and Poor-AC | (+): 6/43 | N.D. |
| Szumiło [9] | 2004 | 79 | M | Rectum | 4.0 × 5.0 × 0.8 | Mode-AC | (+): 2/10 | T3, N1, MX |
| Matsumoto [10] | 2004 | 67 | M | Rectum | 9.0 × 8.5 × 7.0 | Well-AC | (+): 4/13 | N.D. |
| Badmos [11] | 2011 | 48 | M | Rectosigmoid | 4.0 × 4.0 × 2.5 | Well-AC | (−) | T3, N0, MX |
| Present case | 2016 | 57 | M | Rectum | 8.0 × 7.0 | Mode-AC | (+): 1/12 | T4a, N1a, M0 |
N.D., no data; Mode-AC, moderately differentiated adenocarcinoma; Muci-AC, mucinous adenocarcinoma; Poor-AC, poorly differentiated adenocarcinoma; Well-AC, well-differentiated adenocarcinoma; TNM, tumor node metastasis; T, primary tumor site; N, regional lymph node involvement; M, presence or otherwise of distant metastatic spread; (+), present; (−), absent.
In the histological examination of ossification in a tumor, osteosarcoma should be considered as a differential diagnosis. In the present case, stromal spindle cells showed little dysplasia and mitosis, and osteoblasts were linked by tight and gap junctions and integrated with an underlying osteocyte, namely osteoblastic rimming. Moreover, immunohistochemical staining of these stromal cells were negative for AE1/AE3. These histological findings supported the fact that ossification was induced by a reactive change of the tumor progression. The formation process of heterotopic ossification in a tumor remains debatable. Previous reports have demonstrated that bone morphogenetic proteins (BMPs) are implicated in heterotopic ossification [8]. BMPs are a group of transforming growth factors (TGF) that have the ability to induce bone and cartilage formation, and TGF-β activity also strongly enhances BMP-2 [12]. An immunohistological study demonstrated that BMP-2, BMP-4, BMP-5, and BMP-6 were present in tumor and stromal cells, and BMPs played an important role in heterotopic ossification [8]. Moreover, in another immunohistological study, the glioma-associated oncogene protein-2 showed positive staining for tumor and stromal cells, and α-smooth muscle actin showed negative staining for tumor cells but positive staining for stromal cells [13]. The TGF-β pathway has been shown to induce glioma-associated oncogene protein-2 and α-smooth muscle actin [14, 15]. These previous studies have indicated that the histogenesis of heterotopic ossification is mediated by BMPs and TGF-β from the stromal cells in the tumor microenvironment.
Conclusion
Heterotopic ossification in rectal carcinoma is decidedly rare, and the malignant potential has not been determined. However, heterotopic ossification is induced by tumor progression in a microenvironment, suggesting a high tumor malignity. The patient should be carefully monitored after surgery in terms of an improved patient outcome.
Statement of Ethics
The patient provided written informed consent for the publication of this case report and any accompanying images.
Disclosure Statement
The authors declare no conflicts of interest or competing financial interests with respect to the authorship and/or publication of this article.
Acknowledgement
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumors. ed 7. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 2.Ansari MQ, Sachs IL, Max E, Alpert LC. Heterotopic bone formation in rectal carcinoma. Case report and literature review. Dig Dis Sci. 1992;37:1624–1629. doi: 10.1007/BF01296512. [DOI] [PubMed] [Google Scholar]
- 3.Haque S, Eisen RN, West AB. Heterotopic bone formation in the gastrointestinal tract. Arch Pathol Lab Med. 1996;120:666–670. [PubMed] [Google Scholar]
- 4.Dukes CE. Ossification in rectal cancer. Proc R Soc Med. 1939;32:1489–1494. [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchamp NJ, Pizer E, Hruban RH, Fishman EK. Ossification of a rectal tumor: CT evaluation. J Comput Assist Tomogr. 1997;21:671–673. doi: 10.1097/00004728-199707000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos MC, Weston J, Ball AB. Osseous metaplasia in caecal adenocarcinoma. J R Soc Med. 1998;91:434–435. doi: 10.1177/014107689809100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alper M, Akyürek N, Patiroǧlu TE, Yüksel O, Belenli O. Heterotopic bone formation in two cases of colon carcinoma. Scand J Gastroenterol. 2000;35:556–558. doi: 10.1080/003655200750023859. [DOI] [PubMed] [Google Scholar]
- 8.Imai N, Iwai A, Hatakeyama S, Matuszaki K, Kitagawa Y, Kato S, Hokari R, Kawaguchi A, Nagao S, Miyahara T, Itoh K, Miura S. Expression of bone morphogenetic protein in colon carcinoma with heterotopic ossification. Pathol Int. 2001;51:643–648. doi: 10.1046/j.1440-1827.2001.01243.x. [DOI] [PubMed] [Google Scholar]
- 9.Szumiło J, Chrościcki A, Swatek J, Korobowicz E. Rectal adenocarcinoma with osseous metaplasia. Pol J Pathol. 2004;55:39–40. [PubMed] [Google Scholar]
- 10.Matsumoto T, Masuda T, Inomata M, Kitano S, Kashima K, Shibata K, Arinaga S. Heterotopic ossification of rectal adenocarcinoma: report of a case. Surg Today. 2004;34:167–169. doi: 10.1007/s00595-003-2657-5. [DOI] [PubMed] [Google Scholar]
- 11.Badmos KB, Seada LS, Faraj AA. Heterotopic ossification in a colorectal carcinoma. J Coll Physicians Surg Pak. 2011;21:626–627. doi: 10.2011/JCPSP.626627. [DOI] [PubMed] [Google Scholar]
- 12.Tachi K, Takami M, Sato H, Mochizuki A, Zhao B, Miyamoto Y, Tsukasaki H, Inoue T, Shintani S, Koike T, Honda Y, Suzuki O, Baba K, Kamijo R. Enhancement of bone morphogenetic protein-2 induced ectopic bone formation by transforming growth factor-β1. Tissue Eng Part A. 2011;17:597–606. doi: 10.1089/ten.TEA.2010.0094. [DOI] [PubMed] [Google Scholar]
- 13.Huang RS, Brown RE, Buryanek J. Heterotopic ossification in metastatic colorectal carcinoma: case report with morphoproteomic insights into the histogenesis. Ann Clin Lab Sci. 2014;44:99–103. [PubMed] [Google Scholar]
- 14.Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-β/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011;71:5606–5610. doi: 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue and myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]