Abstract
Recently, immunotherapeutic drugs, including PD-1 inhibitors (nivolumab, pembrolizumab), PD-L1 inhibitors (atezolizumab, avelumab), and CTLA4 inhibitors (ipiliumumab), have emerged as important additions to the armamentarium against certain malignancies and have been incorporated into therapeutic protocols for first-, second-, or third-line agents for these metastatic cancers. Immune checkpoint inhibitor nivolumab is currently FDA approved for the treatment of patients with metastatic malignant melanoma [Redman et al.: BMC Med 2016;14: 20], metastatic non-small cell lung cancer [Guibert and Mazières: Expert Opin Biol Ther 2015;15: 1789–1797], metastatic renal cell cancer [Farolfi et al.: Expert Opin Drug Metab Toxicol 2016;12: 1089–1096], and relapsed or refractory classic Hodgkin's lymphoma [Villasboas and Ansell: Expert Rev Anticancer Ther 2016;16: 5–12]. Given the current and increasing indications for these drugs, it is essential for all physicians to become well versed with their common adverse effects and to be observant for other less documented clinical conditions that could be unmasked with the use of such medications. A definite association between autoimmune hemolytic anemia and the immune checkpoint inhibitor nivolumab has not been clearly documented, although a few cases have been reported recently [Kong et al.: Melanoma Res 2016;26: 202–204; Schwab et al.: Case Rep Oncol 2016;9: 373–378; Tardy et al.: Hematol Oncol 2016, DOI: 10.1002/hon.2338]. We report a case of fatal autoimmune hemolytic anemia refractory to steroids in a patient treated with nivolumab for metastatic lung cancer, and reflect on the other reported cases of autoimmune hemolytic anemia after the use of nivolumab.
Keywords: Immune checkpoint inhibitors, Nivolumab, Autoimmune hemolytic anemia
Introduction
Immune checkpoint inhibitor nivolumab is a fully human IgG4 monoclonal antibody against programmed death-1 (PD-1) receptor that blocks interactions between PD-1 and its ligands on tumor cells to prevent T cell exhaustion [1] in patients with cancer. However, this release of check on the immune system can also unleash it against the body's own tissues leading to an autoimmune phenomenon which is the main mechanism for the adverse effects associated with nivolumab [2]. Common clinical manifestations due to this autoimmune phenomenon include pneumonitis, hepatitis, colitis, hypophysitis, pruritis, arthritis, and nephritis [2]. Autoimmune hemolytic anemia (AIHA) is recently being recognized as another autoimmune adverse effect of this treatment.
Case Presentation
A 70-year-old male was treated with nivolumab at 3 mg/kg every 2 weeks in the second-line setting for metastatic adenocarcinoma of lung. Three days after the second dose, he was admitted to the hospital with complaints of increased shortness of breath and new onset of atrial fibrillation with rapid ventricular response. His hemoglobin at baseline of 10.5 g/dL was noted to have decreased to 8.1 g/dL on admission and subsequently decreased to 7.0 g/dL. Further evaluation showed an LDH of 200 units/L and an elevated reticulocyte count of 0.1962 × 1012/L. Peripheral blood smear revealed severe normocytic normochromic anemia, occasional spherocytes, nucleated RBC, and polychromasia (Fig 1, Fig 2, Fig 3).
Fig. 1.
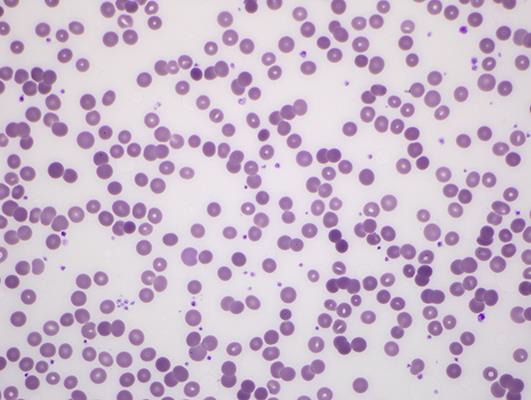
Peripheral blood smear showing spherocytosis due to warm autoimmune hemolytic anemia [6].
Fig. 2.

Peripheral blood smear showing nucleated RBC [6].
Fig. 3.
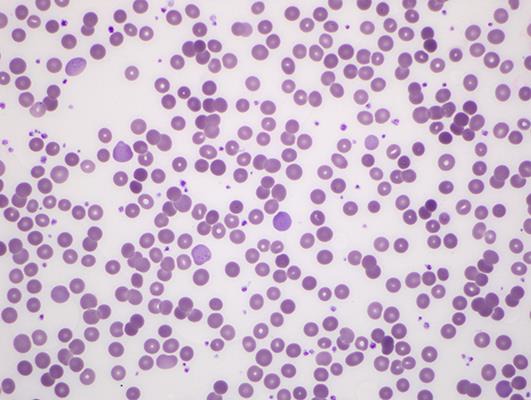
Peripheral blood smear showing polychromasia [6].
He was noted to have warm agglutinins, with Coombs test positive for C3, but negative for IgG. A cold agglutinin screen was negative on gel antibody screen but was found to be positive using tube methodology; however, the titer was <1: 64. No underlying alloantibodies were discovered on reference lab testing. Hemoglobin further decreased to 5.5 g/dL and LDH increased to 330 units/L (reference upper limit 225 units/L). Review of peripheral blood smear confirmed presence of agglutination at room temperature that resolved after refrigeration. A diagnosis of AIHA was made and he was started on prednisone initially at 1 mg/kg, which was later increased to 1.5 mg/kg. Since the patient was not on any other medications commonly implicated in the etiology of AIHA and given the timing of his anemia after initiation of nivolumab, we concluded that his AIHA was most likely due to the autoimmune effect of nivolumab. Despite the steroids, he continued to require frequent blood transfusions to maintain a hemoglobin level >7.0 g/dL. His total bilirubin peaked at 3.89 mg/dL and LDH peaked at 1,192 units/L. The patient continued to have issues with atrial fibrillation, and later also developed significant respiratory distress but did not want to be intubated and eventually expired.
Discussion
AIHA related to nivolumab therapy is not well documented, though few cases of this have recently been reported. Kong et al. [3] reported a case of AIHA in a patient who received nivolumab in the third line for metastatic malignant melanoma after progression with ipilimumab. AIHA developed after 5 cycles of nivolumab and responded to prolonged course of steroids. Schwab et al. [4] reported a case of AIHA in a patient who received nivolumab in the third line for cutaneous squamous cell carcinoma. AIHA developed after 8 cycles of nivolumab and responded to short course of steroids. Tardy et al. [5] reported a case of AIHA in a patient who received nivolumab in the third line for relapsed Hodgkin's lymphoma. AIHA developed after 2 cycles of nivolumab and responded to prolonged course of steroids (Table 1).
Table 1.
Details of cases of autoimmune hemolytic anemia after the use of nivolumab therapy reported thus far in the literature, including the case in focus
| Kong et al. [3] | Schwab et al. [4] | Tardy et al. [5] | Palla et al. [6] | |
| Age, gender | 85-year-old male | 82-year-old male | 75-year-old female | 70-year-old male |
| Indication for nivolumab | Metastatic melanoma (1st dacarbazine, 2nd ipilimumab, 3rd nivolumab) | Cutaneous squamous cell carcinoma (1st cisplatin/radiation, 2nd cetuximab and docetaxel, 3rd nivolumab) | Stage IIIB Hodgkin's lymphoma (1st ABVD ×2 cycles, 2nd bendamustine and brentuximab ×4 cycles, 3rd nivolumab) | Stage IV adenocarcinoma of the lung (pT4 pN2p pM1a) (1st carboplatin and pemetrexed ×3 cycles, 2nd nivolumab) |
| Cycles of nivolumab | 5 (3 mg/kg) | 8 (3 mg/kg) | 2 (3 mg/kg) | 2 (3 mg/kg) |
| Mediator | IgG | IgG, C3 | IgG | C3 |
| Clinical course | No mention of ICU; anemia recovered after ~2 weeks | No mention of ICU; anemia recovered after 2 weeks | No mention of ICU; anemia recovered after 10 days, but persistent subclinical hemolysis for total 1 month | Admitted with atrial fibrillation with RVR; received 10 U pRBCs, transferred to MICU for respiratory failure |
| Treatment | Prednisone (~110 mg ×2 weeks, tapered over a total of 80 days) | Prednisolone (80 mg/day ×2 weeks) | Prednisone (2 mg/kg ×10 days, then 1 mg/kg ×2 weeks, then tapered over 3 months) | Prednisone 1.5 mg/kg ×5 days |
| Outcome | Complete recovery of anemia | Complete recovery of anemia 1 month after stopping treatment; good response to cancer for at least 5 months | Complete recovery of anemia after 1 month; good response to treatment after 2 doses; 3 weeks after steroids discontinued, nivolumab re-introduced and received 6 more cycles without recurrence of hemolysis | Expired 10 days after admission |
| Caveats | Prior red cell antibodies in 2011 (c positive, d positive, e positive, DAT positive); no prior history of hemolysis, repeat DAT in 2012 was negative | Cold agglutinin screen positive, titer <1:64; no alloantibodies | ||
Drug-induced AIHA may be either “warm” or “cold” depending on the temperature at which the autoantibodies become active. Warm AIHA is generally mediated via IgG that binds the Fc receptor of phagocytic cells, but can also rarely be mediated by IgM, IgA, or a combination of these 3 antibodies or via C3 alone [6], and the autoantibodies are active at temperatures greater than 37°C [7]. Cold AIHA results from IgM activation of classic complement pathway and the autoantibodies are active at temperatures of 0–4°C [7]. In some cases, both types of autoantibodies may exist simultaneously and such cases are labeled as “mixed” AIHA [7].
AIHA post-nivolumab therapy appears to be a warm AIHA and is commonly mediated through IgG [3, 5], though IgG and C3 were both positive in one case [4]. Our case was exclusively mediated through C3, and IgG was negative. Another interesting observation in our case was that the cold agglutinin screen was positive, though the titer was very low (<1: 64). There was definite evidence of hemolysis via warm autoantibodies, thus suggesting that our case was possibly a drug-induced mixed AIHA.
In all cases reported, the patients received nivolumab either in the second- or third-line setting. The anemia occurred after 2 cycles of nivolumab in 2 cases [5], after 5 cycles in one case [3], and after 8 cycles in one case [4]. Thus AIHA associated with nivolumab therapy can occur with nivolumab given in any line, either after initiation of the drug within the first few cycles or later in the clinical course. Thus clinicians should be vigilant in diagnosing this entity, even if the patient tolerates nivolumab without hematologic complications in the first few cycles. Another interesting observation is that in all the cases reported, the dose of nivolumab used was 3 mg/kg [3, 4, 5], though the AIHA can likely occur independent of the dose.
In the previous cases reported, the patients did fairly well and the anemia responded to steroids. However, the patient in our case had steroid-refractory anemia and subsequently expired due to respiratory failure. Nonetheless, clinicians should have a low threshold for initiating steroids in patients with suspected nivolumab-related AIHA. Rituxan could be considered as a second-line agent early on in patients who do not respond to steroids.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Agrawal S, Statkevich P, Bajaj G, Feng Y, Saeger S, Desai DD, Park JS, Waxman IM, Roy A, Gupta M. Evaluation of immunogenicity of nivolumab monotherapy and its clinical relevance in patients with metastatic solid tumors. J Clin Pharmacol. 2016 doi: 10.1002/jcph.818. DOI: 10.1002/jcph.818. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11:e0160221. doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong BY, Micklethwaite KP, Swaminathan S, Kefford RF, Carlino MS. Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res. 2016;26:202–204. doi: 10.1097/CMR.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 4.Schwab KS, Heine A, Weimann T, Kristiansen G, Brossart P. Development of hemolytic anemia in a nivolumab-treated patient with refractory metastatic squamous cell skin cancer and chronic lymphatic leukemia. Case Rep Oncol. 2016;9:373–378. doi: 10.1159/000447508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tardy MP, Gastaud L, Boscagli A, Peyrade F, Gallamini A, Thyss A. Autoimmune hemolytic anemia after nivolumab treatment in Hodgkin lymphoma responsive to immunosuppressive treatment. A case report. Hematol Oncol. 2016 doi: 10.1002/hon.2338. DOI: 10.1002/hon.2338. [DOI] [PubMed] [Google Scholar]
- 6.Palla AR, Khimani F, Craig MD. Warm autoimmune hemolytic anemia with a direct antiglobulin test positive for C3 and negative for IgG: a case study and analytical literature review of incidence and severity. Clin Med Insights Case Rep. 2013;6:57–60. doi: 10.4137/CCRep.S11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SH. Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood Res. 2016;51:69–71. doi: 10.5045/br.2016.51.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]


