Abstract
Prazosin, a potent and selective α1-adrenoceptor antagonist, displaces 25% of 11C-CUMI-101 ([O-methyl-11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione) binding in monkey cerebellum. We sought to estimate the percentage contamination of 11C-CUMI-101 binding to α1-adrenoceptors in human cerebellum under in vivo conditions. In vitro receptor-binding techniques were used to measure α1-adrenoceptor density and the affinity of CUMI-101 for these receptors in human, monkey, and rat cerebellum. Methods: Binding potential (maximum number of binding sites × affinity [(1/dissociation constant]) was determined using in vitro homogenate binding assays in human, monkey, and rat cerebellum. 3H-prazosin was used to determine the maximum number of binding sites, as well as the dissociation constant of 3H-prazosin and the inhibition constant of CUMI-101. Results: α1-adrenoceptor density and the affinity of CUMI-101 for these receptors were similar across species. Cerebellar binding potentials were 3.7 for humans, 2.3 for monkeys, and 3.4 for rats. Conclusion: Reasoning by analogy, 25% of 11C-CUMI-101 uptake in human cerebellum reflects binding to α1-adrenoceptors, suggesting that the cerebellum is of limited usefulness as a reference tissue for quantification in human studies.
Keywords: autoradiography, 5-HT1A receptor, CUMI-101, cerebellum, α1-adrenoceptor, cross-reactivity
Having a reference region in the brain typically makes PET quantitation of receptors both easier and more precise than measuring the concentration of parent radioligand in arterial plasma. The quantitation is easier because it requires only brain imaging and because it eliminates the need for an arterial line to measure the radioligand and its metabolites in plasma. A reference region should contain little or no specific (i.e., high affinity [1/dissociation constant]) binding to reflect nondisplaceable uptake: the sum of nonspecific binding and free radioligand in brain. However, measuring the amount of specific binding in a proposed reference region can be challenging, especially if the radioligand binds with high affinity to more than one receptor subtype.
11C-CUMI-101 ([O-methyl-11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione) was initially proposed as a novel agonist radioligand selective for subtype 1A of the 5-hydroxytryptamine (5-HT1A) receptor (1). However, previous studies from our laboratory found that 11C-CUMI-101 is an antagonist that nonselectively binds with high affinity to both 5-HT1A and α1-adrenoceptors (2). The percentage contamination of binding to α1-adrenoceptors is low enough to allow 11C-CUMI-101 to predominantly measure 5-HT1A receptors in many target regions of the brain such as the neocortex and hippocampus. However, it is unclear whether the cerebellum, which contains few 5-HT1A receptors (3) but a relatively high density of α1-adrenoceptors (4,5), can still be used as a reference region, as is commonly done for other 5-HT1A receptor PET radioligands.
In monkeys, we previously reported that 25% of the cerebellar 11C-CUMI-101 was bound to α1-adrenoceptors, as assessed via blockade by prazosin, a potent and selective α1-adrenoceptor antagonist (2). The present study sought to estimate the percentage contamination in human cerebellum under in vivo conditions, with the limitation that comparable displacement by prazosin would be difficult, if not impossible, because of cardiovascular side effects. Thus, to indirectly answer this question, we used in vitro receptor-binding techniques to measure both the density of α1-adrenoceptors and the affinity of CUMI-101 for these receptors in human, monkey, and rat cerebellum. To the best of our knowledge given the extant data in this area, when these two in vitro parameters are the same for two species, their in vivo cross-reactivity—in this case, for 11C-CUMI-101 and α1-adrenoceptors—should also be similar.
MATERIALS AND METHODS
Animal and human postmortem tissue was obtained from either the National Institute of Mental Health or Columbia University. All animals received standard laboratory care, including daily feedings and access to water ad libitum. The study was approved by the Animal Care and Use Committee of the National Institute of Mental Health.
Membrane Homogenization
Cerebellar tissue (comprising primarily gray matter but also containing some white matter) homogenates were prepared from rats, monkeys, and humans using a Polytron (Kinematica AG) in 50 mM Tris-HCl (pH 7.4) and centrifuged at 25,000g for 30 min at 4°C. The supernate was discarded, and the pellet was resuspended in the same buffer so that the final concentration was about 100 mg of wet tissue per milliliter. Tissues were stored at −80°C until the day of the experiment.
Radioligand and Drugs
3H-prazosin was purchased from PerkinElmer. Prazosin, WAY-100635 (N-(2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl-N-(2-pyridinyl)cyclohexanecarboxamide), demethylated WAY-100635 (DWAY), and fluorocyclohexanecarboxylic acid WAY-100635 (FCWAY) were purchased from Sigma. N-{2-[4-(2-methoxyphenyl)piperazinyl]ethyl}-N-(2-pyridyl)-N-(4-fluoromethylcyclohexane)carboxamide (mefWAY [trans]) was purchased from Isoflex. CUMI-101 was purchased from Alpha Biopharmaceuticals. All other reagents were purchased from Quality Biological.
3H-Prazosin Binding
Radioligand binding assays were performed as previously described (6). Briefly, brain tissues were thawed on ice and diluted in binding buffer (α1-adrenoceptors: 20 mM Tris-HCl, 145 mM NaCl; pH 7.4) to a final concentration of 6 mg of wet tissue per milliliter. Binding was performed by sequentially adding to each borosilicate vial, first, 100 μL of radioligand (radioactivity concentration for 3H-prazosin was 0.05–0.1 nM so that the final concentration was below the dissociation constant value (7)); second, 100 μL of displacer; and third, 800 μL of tissues mixed in the binding buffer. This step was followed by a 1.5-h incubation in a light-shielded shaker at either 37°C or 23°C because the affinity of receptors may increase or decrease with temperature. We chose 23°C because it was close to the temperature used for the initial characterization of CUMI-101 (25°C) (8), and we chose 37°C because it reflects in vivo body temperature. Reactions were terminated by rapid filtration under a vacuum and washing with ice-cold binding buffer through a GF/B glass fiber filter (Whatman) presoaked for 30 min in 0.5% polyethyleneimine.
Filters and collected radioactivity were measured (5 min per vial) in a liquid scintillation Ultima-Gold cocktail (4 mL per vial) in a β-counter (PerkinElmer). Protein concentration was determined using the Micro BCA protein assay kit (Thermo Scientific).
RESULTS
As expected, CUMI-101 showed nanomolar affinity to α1-adrenoceptors. These affinities were similar in cerebellar tissue from humans, monkeys, and rats (Table 1). In addition, the density of α1-adrenoceptor receptors was similar across species.
TABLE 1.
3H-Prazosin Binding to α1-Adrenoceptors in Human, Monkey, and Rat Cerebellum at 37°C and 23°C
|
Bmax (fmol/mg) |
Prazosin KD (nM) |
CUMI-101 Ki (nM) |
Bmax/Ki |
|||||
| Species | 37°C | 23°C | 37°C | 23°C | 37°C | 23°C | 37°C | 23°C |
| Human | 104 ± 34 | 50 ± 22 | 0.27 ± 02 | 0.04 ± 02 | 2.80 ± 50 | 0.64 ± 12 | 3.7 | 7.8 |
| Monkey | 81 ± 28 | NA | 0.34 ± 09 | 0.06 ± 03 | 3.50 ± 67 | 0.40 ± 07 | 2.3 | NA |
| Rat | 172 ± 42 | 74 ± 9 | 0.34 ± 01 | NA | 5.00 ± 06 | 0.80 ± 02 | 3.4 | NA |
Bmax = maximum number of binding sites; KD = dissociation constant; Ki = (half-maximal inhibitory concentration)/(1 + [radioligand]/KD).
Data are mean ± SD. Concentration-dependent displacement by nonradioactive prazosin was analyzed as homologous saturation curve to estimate Bmax and KD. Concentration-dependent displacement by nonradioactive CUMI was analyzed to provide Ki values, which correct for concentration of 3H-prazosin.
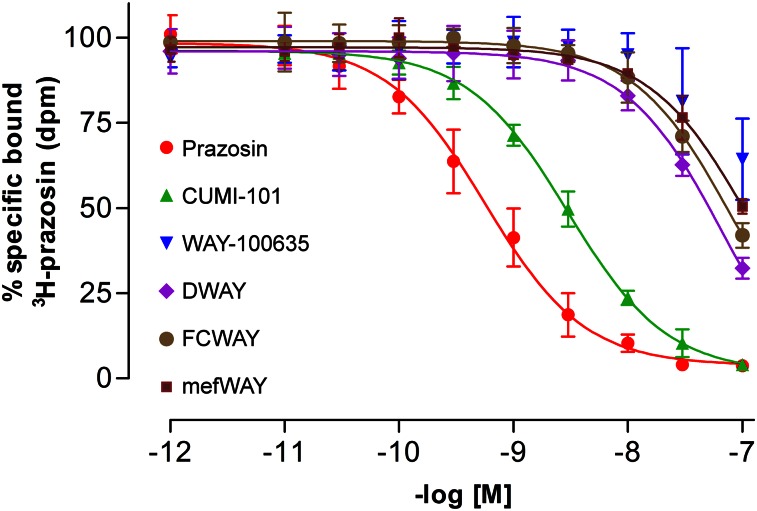
At 37°C, the concentration required for 50% displacement (inhibition constant, or Ki) of 3H-prazosin binding to α1-adrenoceptors by CUMI-101 was 2.8 ± 0.5 nM, which was only 10-fold lower than that for prazosin (0.3 ± 0.02 nM) (Table 2). In contrast, the displacement of 3H-prazosin by the current PET radioligands, which are WAY-100635 analogs, showed a greater difference in the shift of the binding curves (Fig. 1). The Ki for WAY-100635 analogs ranged from 80 to 120 nM (Table 2), suggesting that CUMI-101 would be expected to have 30- to 40-fold higher binding to α1-adrenoceptors than that for WAY-100635 analogs.
TABLE 2.
Binding Affinity of WAY-100635 Analogs vs. 11C-CUMI-101 to α1-Adrenoceptors in Human Cerebellum
| Compound | Ki (nM) | SD (n = 3) |
| Prazosin | 0.27 | 0.02 |
| CUMI-101 | 2.80 | 0.50 |
| WAY-100635 | 120.68 | 35.15 |
| DWAY | 78.96 | 29.33 |
| FCWAY | 87.11 | 22.85 |
| MefWAY (trans) | 116.60 | 15.70 |
For prazosin, value is KD (dissociation constant) instead of Ki.
FIGURE 1.
Unlike WAY-100635 analogs, CUMI-101 demonstrates nanomolar affinity to α1-adrenoceptors in human cerebellum. n = 3; error bars represent SD.
DISCUSSION
Using in vitro receptor-binding techniques, we found that the density of α1-adrenoceptors, as well as the affinity of CUMI-101 for these receptors, were similar in human, monkey, and rat cerebellum. The product of these two parameters (i.e., density and affinity) is the binding potential of 11C-CUMI for α1-adrenoceptors. Analogous to the in vitro measurements, we would expect the in vivo binding potential of 11C-CUMI for α1-adrenoceptors to be similar in human, monkey, and rat cerebellum. Moreover, as the total uptake (i.e., total distribution volume, or VT) of 11C-CUMI in cerebellum was similar in monkeys and humans, we would expect—on the basis of prior studies conducted on monkeys (2)—that about 25% of the uptake of 11C-CUMI in human cerebellum would be displaced by prazosin if this selective α1-adrenoceptor antagonist could be safely administered to humans. This moderate cross-contamination confounds the use of the cerebellum as a reference region. Use of the cerebellum would require proof that binding to α1-adrenoceptors is the same between individuals or groups.
Both a true reference region (i.e., devoid of receptor binding) and a pseudo-reference region (i.e., one that contains receptor binding) can be used in the denominator as a normalization factor for regions with higher receptor binding. When individuals are being compared, however, either a reference region or a pseudo-reference region must be the same in all subjects. This is typically the case if the reference region contains only nonspecific binding (i.e., low-affinity adsorption of the radioligand to a wide variety of lipids and proteins in brain) because such brain components are typically the same between individuals and even between species (9). Nevertheless, a reference region containing specific binding (i.e., high-affinity binding to a particular protein) can still be used either if the specific binding is a small component of total radioactivity or if the specific binding in the pseudo-reference region is the same in all subjects. Here, we demonstrate one way to reasonably predict the percentage of specific receptor binding in such a region when blockade studies are not possible in human subjects. Specifically, definitive blockade experiments can be performed in animals, and the relative binding potential of the radioligand for this target can subsequently be measured in vitro in human and animal brain.
With regard to measuring parent radioligand, a reference tissue analysis not only is much easier but also may significantly increase sensitivity. Our prior studies imaging translocator protein, a biomarker of inflammation, in Alzheimer's disease demonstrate this point (10). Furthermore, in this particular study, we could use the cerebellum as a pseudo-reference region because we previously demonstrated that binding of the radioligand 11C-PBR28 in this region was the same in patients and controls. In addition, the cerebellum was a useful pseudo-reference region despite the fact that its binding was only slightly lower than that in target neocortical regions. For example, the VT of 11C-PBR28 was 3.9 mL/cm3 in temporal cortex and 3.5 in cerebellum, with a ratio of only 1.11. Although that ratio was only slightly greater than 1, its variance in the population was significantly less than that of VT in the target region: 10% coefficient of variation for ratio of target to cerebellum compared with 38% coefficient of variation for VT. This decreased variance markedly increased the sensitivity and statistical power to detect differences between patients and controls. In this case, the VT of temporal cortex differed between groups, with a P value of 0.02, whereas the ratio of this region to cerebellum had a P value of 0.0005. Clearly, a reference region or pseudo-reference region has significant utility for brain imaging, but as shown here, the adequacy of this region must be carefully examined for the proposed purpose.
This paper primarily sought to assess what percentage of 11C-CUMI uptake in human cerebellum reflects binding to α1-adrenoceptors. We estimated this percentage on the basis of in vitro binding affinity and in vivo imaging in monkeys. Nevertheless, the most direct answer would come from 3H-prazosin blockade of 11C-CUMI uptake in humans. We initially thought such a study would cause intolerable cardiovascular effects and be associated with risks unlikely to be approved by an institutional review board. However, partial blockade of α1-adrenoceptors may be achievable with tolerable side effects and still provide an adequate displaceable signal from the brain regions to be analyzed with the Lassen/occupancy plot (11). Nevertheless, we do not know the occupancy-versus-response curve for α1-adrenoceptors versus cardiovascular side effects. If a pharmacologic dose of 3H-prazosin can be well tolerated, a blocking study of 11C-CUMI could be performed safely in humans. Such an experiment would provide a definitive and direct answer as to whether the cross-reactivity of α1-adrenoceptors with 11C-CUMI in human cerebellum is quantitatively significant.
CONCLUSION
This study found that both the density of α1-adrenoceptors and the affinity (1/Ki) of CUMI-101 for these receptors were similar in monkey and human cerebellum. We previously found that 25% of 11C-CUMI-101 was displaced by prazosin in monkey cerebellum. Taken together and reasoning by analogy, these findings suggest that using cerebellum as a reference tissue for quantification in human studies is problematic because a similar cross-reactivity between 11C-CUMI-101 and human α1-adrenoceptors would be expected.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was funded by the Intramural Research Program of the National Institute of Mental Health (NIH project ZIAMH002795). No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Drs. Ramin Parsey and Victoria Arango for providing human brain tissues and Ioline Henter (NIMH) for providing invaluable editorial assistance.
REFERENCES
- 1.Kumar JS, Majo VJ, Hsiung SC, et al. Synthesis and in vivo validation of [O-methyl-11C]2-{4-[4-(7-methoxynaphthalen-1-yl)piperazin-1-yl]butyl}-4-methyl-2H-[1,2,4]triazine-3,5-dione: a novel 5-HT1A receptor agonist positron emission tomography ligand. J Med Chem. 2006;49:125–134. [DOI] [PubMed] [Google Scholar]
- 2.Shrestha SS, Liow JS, Lu S, et al. 11C-CUMI-101, a PET radioligand, behaves as a serotonin 1A receptor antagonist and also binds to α1 adrenoceptors in brain. J Nucl Med. 2014;55:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar JS, Parsey RV, Kassir SA, et al. Autoradiographic evaluation of [3H]CUMI-101, a novel, selective 5-HT1AR ligand in human and baboon brain. Brain Res. 2013;1507:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Murad AK, Wakelin LP, Denny WA, Griffith R, Finch AM. α1-adrenoceptor and serotonin 5-HT(1A) receptor affinity of homobivalent 4-aminoquinoline compounds: an investigation of the effect of linker length. Biochem Pharmacol. 2013;85:1534–1541. [DOI] [PubMed] [Google Scholar]
- 5.Kalaria RN. Characterization of [125I]HEAT binding to alpha 1-receptors in human brain: assessment in aging and Alzheimer’s disease. Brain Res. 1989;501:287–294. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EW, Woods SW, Zoghbi S, McBride BJ, Baldwin RM, Innis RB. Receptor binding characterization of the benzodiazepine radioligand 125I-Ro16-0154: potential probe for SPECT brain imaging. Life Sci. 1990;47:1535–1546. [DOI] [PubMed] [Google Scholar]
- 7.Daly RN, Sulpizio AC, Levitt B, et al. Evidence for heterogeneity between pre- and postjunctional alpha-2 adrenoceptors using 9-substituted 3-benzazepines. J Pharmacol Exp Ther. 1988;247:122–128. [PubMed] [Google Scholar]
- 8.Kumar JS, Prabhakaran J, Majo VJ, et al. Synthesis and in vivo evaluation of a novel 5-HT1A receptor agonist radioligand [O-methyl-11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H, 4H)dione in nonhuman primates. Eur J Nucl Med Mol Imaging. 2007;34:1050–1060. [DOI] [PubMed] [Google Scholar]
- 9.Salinas CA, Searle GE, Gunn RN. The simplified reference tissue model: model assumption violations and their impact on binding potential. J Cereb Blood Flow Metab. 2015;35:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyoo CH, Ikawa M, Liow JS, et al. Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med. 2015;56:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab. 2010;30:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]