Abstract
Background:
The quality of semen is one of the major parameters in male infertility. Pentoxifylline, a methylxanthine derivative, is an agent primarily used in the treatment of intermittent claudication and other vascular disorders. Studies have shown that pentoxifylline enhances the quality and quantity of sperms. In this study, we have investigated the in vitro effects of pentoxifylline on viability and motility of spermatozoa in samples of infertile oligoasthenozoospermic males.
Materials and Methods:
In this observer-blinded clinical trial, semen samples of 25 infertile oligoasthenozoospermic males were collected in Alzahra Educational Medical Center of Tabriz University of Medical Sciences from August 2010 to August 2012. After the isolation of spermatozoa by the swim-up method, they were randomized into four groups in ISM1 environment: The controls treated normally: Group 1 treated by pentoxifylline at a dose of 50 μg/ml, Group 2 treated by pentoxifylline at a dose of 100 μg/ml, and Group 3 treated by pentoxifylline at a dose of 200 μg/ml. Sperm viability and motility were compared among the groups on 45 min, 24 h, 36 h, and 48 h intervals.
Results:
Mean percentages of live sperms were 98.40%, 51.40%, 20.60%, and 6.00% in control group and 98.40%, 69.20%, 38.60%, and 14.60% in Group 3 on the mentioned intervals, respectively. This mean percentage decrease of live sperms was significantly lower in Group 3 comparing with that of other groups (P = 0.01). Mean percentages of motile sperms were 54%, 8.40%, 2.80%, and 0% in control group; and 54%, 16%, 4.80%, and 1.40% in Group 3 on the mentioned intervals, respectively. There was not a significant difference between the four groups in this regard (P = 0.19).
Conclusion:
Pentoxifylline can enhance the viability of sperm of infertile oligoasthenozoospermic males with no significant effect on its motility.
Keywords: Asthenozoospermia, pentoxifylline, sperm motility
INTRODUCTION
Infertility means failure in pregnancy in spite of frequent sexual intercourse without contraception for 1 year which shows decrease in the ability of fertility and breeding.1 Based on studies, 15–20% of couples suffer from infertility.2 About 45–50% of infertility reasons resulted from male problems and simultaneous male female problems only exists in some cases.3
The primary male examination should be rapid, noninvasive, and inexpensive, including history taking, physical examination, estimating the size of testicles, seminal, and hormonal analysis.4,5 Low sperm motility or low sperm number, or both of them, consist the most important infertility cases of men.6 Three major influential factors on men infertility are decrease in sperm number, including azoospermia and oligospermia, asthenospermia, and abnormal sperm morphology.7
Sperm examination should be done macroscopically in terms of seminal volume and microscopically in terms of concentration and motility and morphology.8 In sperm motility investigation, clinically speaking percentage of rapid progressive motile sperms is more valuable.9
Assisted reproductive technology (ART) is used to increase fertility through approximating spermatozoa and ovule.10 It is possible to mention to controlled ovarian hyperstimulation combined with intrauterine insemination, in vitro fertilization, and intracytoplasmic sperm injection (ICSI) of ART methods which most of the infertile couples use.11 In a meta-analysis study, the success rate of fertility for couples in a cycle of using ART was 42.7% and in three cycles of using ART, it was 57.9%.12 Disadvantages of ART methods include multiple pregnancies, and also increase in the possibility of premature birth, low birth weight, and perinatal mortality. In addition, possibilities of genital, musculoskeletal, cardiovascular, and gastrointestinal disorders increase while implementing ART methods.13
Pentoxifylline is a derivative of xanthine, which decreases blood viscosity by increasing deformability of red blood cells and is used to symptomatic relief of intermittent thrombosis,14 and its usage has been effective in therapy of cardiovascular diseases, cerebrovascular diseases, other diseases related to local blood flow abnormalities (vascular),15,16 and reduction of ischemic effects.17 Common side effects of using pentoxifylline are nausea, vomiting, headache, dizziness, and rare complications such as angina, arrhythmias, shortness of breath, and hypotension. The incidence rate of complications has been reported as one-third in studies.18
In patients suffering oligoasthenozoospermia, implementing hormonal therapies had low effects, suggesting the need for more discussions.19 It is possible to increase sperm motility using pentoxifylline in vitro conditions.20 Results of the previous studies show that pentoxifylline has a desirable effect on sperm motility,21 but to reach definite results in this field, we need further investigations. The objective of this study is to investigate the effect of pentoxifylline in infertile men suffering oligoasthenozoospermia on motility and viability of sperm in the culture medium.
MATERIALS AND METHODS
In this observer-blinded clinical trial registered on Iranian Registry of Clinical Trials (IRCTs) site (IRCTID: IRCT201110175942N1), semen samples of 25 infertile oligoasthenozoospermic males were collected in Alzahra Educational Medical Center of Tabriz University of Medical Sciences – where is the main referral center of infertility in Northwest of Iran - from August 2010 to August 2012. Inclusion criteria were: Age among 25–40, primary infertility during recent 1–2 years, sperm motility <50% in the progressive type or <25% in rapidly progressive type, not receiving oral pentoxifylline for 9 h before sampling (considering drug half-life), sperm number <20 million in a milliliter, and lack of sexual relationship during recent 3–7 days. Exclusion criteria were azoospermia, seminal infections, history of contact with chemicals and radiation, history of trauma to testicles, varicocele, hydrocele, undescended testis or its corrective surgery and vasectomy reversal surgery, other mental or physical diseases, and also implementing other ART methods.
In this study, sample volume determined as 25 specimens based on sperm motility and considering α = 0.05 and minimum 5% increase in motility of rapidly progressive sperms in studied samples and 80% power. After a complete explanation of objectives and methods of study and privacy of information and getting written informed consent, 25 people were randomly included into the study using Randlist Software version 1.2 (Randlist Ins., Chicago, USA).
Samples were collected in sterile containers by masturbation method. Only one seminal sample was taken from each person. In case of spoiling or loss of sample for any reason, the person was asked to refer again to give another sample in next 3–7 days without performing any sexual intercourse. After getting the samples, they were rapidly put in the incubator 37°C for complete liquefaction of samples. After preparing the sample using standard method and isolation of spermatozoa with swim up method, samples were randomly divided into four groups in ISM1 environment: Samples in three groups influenced by three different pentoxifylline doses whereas one group (control group) was kept without pentoxifylline. In Group 1, pentoxifylline 50 μg/ml; in Group 2, pentoxifylline 100 μg/ml; and in Group 3, pentoxifylline 200 μg/ml was added. A code was assigned for each group, and samples were kept in same conditions in all four groups and were investigated based on recommended guidelines according to the World Health Organization manual.22 The person evaluating samples reported results based on the code and was unaware of grouping method. The percentage of live and motile rapidly progressive sperms was determined and compared in 45th min and 24th, 36th, and 48th h. The desirable result in this study was considered as vitality of sperm and increasing minimum 5% sperm motility in rapidly progressive type.
The collected information was analyzed via SPSS™ version 15 statistical software (SPSS Ins., Chicago, USA) and intra- and inter-group comparisons were done by repeated-measures analysis. Data have been presented as mean ± standard deviation and frequency and percentage. Statistical significance level was considered as P ≤ 0.05.
RESULTS
Mean age of participants in this study was 31.52 ± 2.4 years. The percentage of live sperms in our studied groups in mentioned time intervals is presented in Table 1.
Table 1.
Rate of alive sperm in study groups at different time of sperm analysis
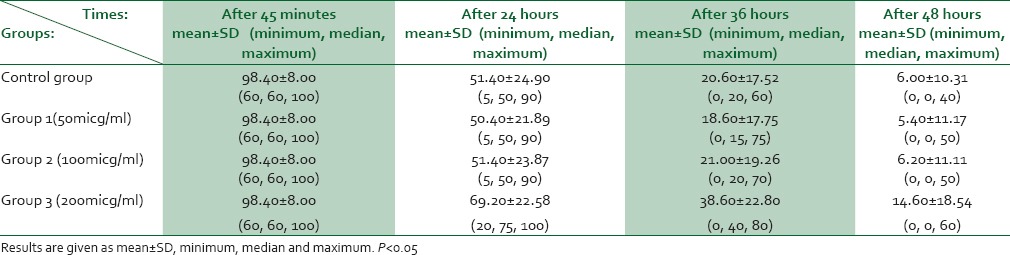
Mean percentage of live sperms had reduced significantly in all four groups through time (P < 0.05). Mean reduction rate of live sperm percentage during investigation interval was significantly lower in 200 μg group than that of other three groups (P = 0.01). This rate did not show statistically significant difference among other groups (P = 0.12).
The percentage of motile rapidly progressive sperms in studied groups during investigated time intervals is shown in Table 2.
Table 2.
Rate of motile (rapid progressive) sperm in study groups at different time of sperm analysis
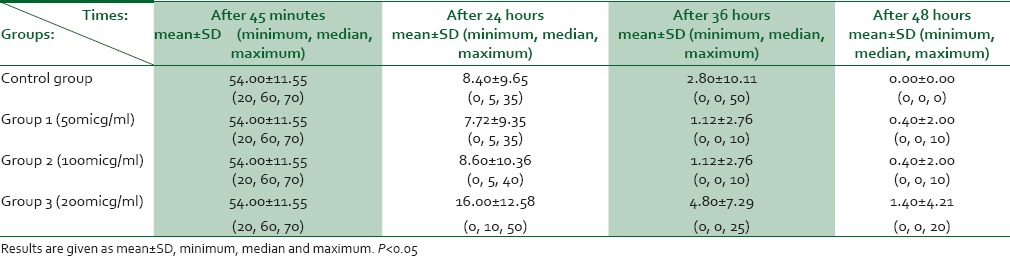
Mean of motile sperms during investigated time interval did not have statistically significant difference among four groups (P = 0.19).
DISCUSSION
In this study, the effect of various pentoxifylline doses (50, 100, 200 μg in ml) on sperm viability and mortality rate in infertile men suffering oligoasthenozoospermia was investigated in culture medium. Based on this, pentoxifylline with 200 μg/ml dose significantly increased viability rate of sperms in infertile men suffering oligoasthenozoospermia. During 45 min to 48 h after incubation, different doses of pentoxifylline did not have a significant effect on sperm motility rate (percentage of rapidly progressive cases), and there was not a significant difference with control group from this perspective.
In a study about the effect of pentoxifylline on viability and motility rate of sperm before and after freezing, using pentoxifylline before freezing has led to a reduction in viability rate, and increase in motility rate of sperm and after freezing has led to increase in viability and decrease in motility of sperm.23 In another study on semen of dog, the highest viability rate of sperm has been in freezing condition alongside with 1 mM pentoxifylline.24
Since the reduction in seminal lifetime is one of the destructive factors on sperm fertility, by increasing sperm viability time, fertility power also increases. In another study, the effect of pentoxifylline on increasing horse seminal lifetime has been shown.25 In another study, rate of sperms with normal morphology had increased with pentoxifylline at 400 mg twice a day and acrosomal reaction has also increased.26
In the current study, pentoxifylline leads to increase the viability of sperms, but no significant effect was observed on motility rate of sperms. Also in a study, adding 3 mM pentoxifylline during incubation has not caused any change in sperm motility.27 In a study on horse, 3.5 mM pentoxifylline has not had any effect on motility of sperms but has significantly increased ratio of live and powerful sperms.28
In the current study, pentoxifylline did not have a significant effect on sperm motility. Also in another study, in low doses such as 0.01 mM, pentoxifylline had a positive effect on sperm motility but in higher doses such as 10 mM had reverse effect on sperm motility.29 In another study, pentoxifylline at 1.76 mM dose significantly increased sperm motility rate and also fertility rate and clinical pregnancy were significantly higher in the intervention group.30 Furthermore, in another study adding pentoxifylline has led to an increase in sperm motility.21
A study on effect of pentoxifylline on sheep sperms has mentioned to increase in sperm viability and preserving spermatic membrane after 30 min and has not had significant effect on sperm motility.31
Totally, most of the studies have shown that using pentoxifylline lead to an increase in fertility ability of oligoasthenozoospermic persons through increasing stability and viability of sperm, increasing motility of sperm, or both factors. Therefore, it seems that it is possible to use pentoxifylline as an adjuvant drug in therapy of infertility.32,33,34,35,36
CONCLUSION
In this study, pentoxifylline at 200 μg/ml dose significantly increased sperm viability in infertile men suffering oligoasthenozoospermia. Therefore, implementing it in testicular sperm extraction or percutaneous epididymal sperm aspiration methods in ICSI helps the improvement of results of this therapy, especially in cases where ovules were immature and would remain in culture medium for hours before microinjection for maturation of ovule. In this study, pentoxifylline did not have statistically significant effect on sperm motility rate of infertile men suffering oligoasthenozoospermia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The international committee for monitoring assisted reproductive technology (ICMART) and the World Health Organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod. 2009;24:2683–7. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 2.Turchi P. Prevalence, Definition, and Classification of Infertility. InClinical Management of Male Infertility. Springer International Publishing; 2015. pp. 5–11. [Google Scholar]
- 3.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European Association of Urology guidelines on male infertility: The 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 4.Stahl PJ, Stember DS, Goldstein M. Contemporary management of male infertility. Annu Rev Med. 2012;63:525–40. doi: 10.1146/annurev-med-051810-101036. [DOI] [PubMed] [Google Scholar]
- 5.Centola GM. Semen assessment. Urol Clin North Am. 2014;41:163–7. doi: 10.1016/j.ucl.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Alaa I, Nasr A. Semen analysis: Indispensable, yet non-ideal. Middle East Fertil Soc J. 2013;18:287–8. [Google Scholar]
- 8.Baker HG, Clarke GN, De Yi Liu TM, Garrett C. Semen Analysis and Sperm Function Testing. Male Reproductive Dysfunction: Pathophysiology and Treatment. 2007;22:271. [Google Scholar]
- 9.Mortimer D, Mortimer ST. Manual methods for sperm motility assessment. Spermatogenesis: Methods and Protocols. 2013:61–75. doi: 10.1007/978-1-62703-038-0_7. [DOI] [PubMed] [Google Scholar]
- 10.Tournaye H. Male factor infertility and ART. Asian J Androl. 2012;14:103–8. doi: 10.1038/aja.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandes M, Hamilton CJ, van der Steen JO, de Bruin JP, Bots RS, Nelen WL, et al. Unexplained infertility: Overall ongoing pregnancy rate and mode of conception. Hum Reprod. 2011;26:360–8. doi: 10.1093/humrep/deq349. [DOI] [PubMed] [Google Scholar]
- 12.Gameiro S, Verhaak CM, Kremer JA, Boivin J. Why we should talk about compliance with assisted reproductive technologies (ART): A systematic review and meta-analysis of ART compliance rates. Hum Reprod Update. 2013;19:124–35. doi: 10.1093/humupd/dms045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganju N, Petrikovsky B, Zeitoun K. Do reproductive technologies cause fetal anomalies? Neonatal Intensive Care. 2013;26:19. [Google Scholar]
- 14.Magnusson M, Gunnarsson M, Berntorp E, Björkman S, Höglund P. Effects of pentoxifylline and its metabolites on platelet aggregation in whole blood from healthy humans. Eur J Pharmacol. 2008;581:290–5. doi: 10.1016/j.ejphar.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 15.Ali FN, Carman TL. Medical management for chronic atherosclerotic peripheral arterial disease. Drugs. 2012;72:2073–85. doi: 10.2165/11640810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. The Cochrane Library. 2014 doi: 10.1002/14651858.CD000990.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Brasileiro JL, Inoye CM, Aydos RD, Silva IS, Falcão GR, Marks G, et al. Ischemia and reperfusion of rat small intestine using pentoxyfilline and prostaglandin E1. Acta Cir Bras. 2013;28:767–73. doi: 10.1590/s0102-86502013001100004. [DOI] [PubMed] [Google Scholar]
- 18.Lu D, Song H, Li Y, Clarke J, Shi G. Pentoxifylline for endometriosis. Cochrane Database Syst Rev. 2012;1:CD007677. doi: 10.1002/14651858.CD007677.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Cavallini G. Male idiopathic oligoasthenoteratozoospermia. Asian J Androl. 2006;8:143–57. doi: 10.1111/j.1745-7262.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Dujaily SS, Malik K. In Vitro Sperm Preparation by Progesterone, Pentoxifylline and Glycyrrhiza Glabra for Asthenozoospermic Men. Global Journal of Medical Research. 2013;13(1):22–8. [Google Scholar]
- 21.Archer SL, Roudebush WE. Enhancement of sperm motility using pentoxifylline and platelet-activating factor. Spermatogenesis: Methods and Protocols. 2013:241–5. doi: 10.1007/978-1-62703-038-0_21. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 23.Esteves SC, Spaine DM, Cedenho AP. Effects of pentoxifylline treatment before freezing on motility, viability and acrosome status of poor quality human spermatozoa cryopreserved by the liquid nitrogen vapor method. Braz J Med Biol Res. 2007;40:985–92. doi: 10.1590/s0100-879x2006005000118. [DOI] [PubMed] [Google Scholar]
- 24.Ji DY, Kim CK, Lee JH, Park SJ, Ryu LS, Ryu JW, et al. Studies on Frozen Semen Characteristics Following Pentoxifylline Treatment and Artificial Insemination in Dog. Journal of Animal Science and Technology. 2005;47(6):925–36. [Google Scholar]
- 25.Stephens TD, Brooks RM, Carrington JL, Cheng L, Carrington AC, Porr CA, et al. Effects of pentoxifylline, caffeine, and taurine on post-thaw motility and longevity of equine frozen semen. Journal of Equine Veterinary Science. 2013;33(8):615–21. [Google Scholar]
- 26.Safarinejad MR. Effect of pentoxifylline on semen parameters, reproductive hormones, and seminal plasma antioxidant capacity in men with idiopathic infertility: A randomized double-blind placebo-controlled study. Int Urol Nephrol. 2011;43:315–28. doi: 10.1007/s11255-010-9826-4. [DOI] [PubMed] [Google Scholar]
- 27.Stanic P, Sonicki Z, Suchanek E. Effect of pentoxifylline on motility and membrane integrity of cryopreserved human spermatozoa. Int J Androl. 2002;25:186–90. doi: 10.1046/j.1365-2605.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- 28.Ortgies F, Klewitz J, Görgens A, Martinsson G, Sieme H. Effect of procaine, pentoxifylline and trolox on capacitation and hyperactivation of stallion spermatozoa. Andrologia. 2012;44(Suppl 1):130–8. doi: 10.1111/j.1439-0272.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 29.Mirshokraei P, Köfer J, Schobesberger H. Proceedings of the XVth International Congress of the International Society for Animal Hygiene. Vol. 2. Vienna, Austria: Tribun EU; 2011. In vitro Effects of Pentoxifylline on Kinematic Parameters of Ram Epididymal Sperm. Animal Hygiene and Sustainable Livestock Production. [Google Scholar]
- 30.Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl. 2006;27:45–52. doi: 10.2164/jandrol.05079. [DOI] [PubMed] [Google Scholar]
- 31.Silva SV, Batista AM, Maia VN, Ramalho FC, Guerra MM, Carneiro GF. Effect of Pentoxifylline on viability of ovine frozen semen. InCiência Animal. 2012;22(Suppl 1):362–5. Universidade Estadual do Ceará, Faculdade de Veterinaria. [Google Scholar]
- 32.Hosseini SE, Mehrabani D, FatemehAlSadat R. The Effect of Palm Pollen Extract on Sexual Hormones and the Numbers of Spermatozoa Dynastic Cell in Adult Male Mice. Medical Journal of Tabriz University of Medical Sciences and Health Services. 2015;37(5):20–5. [Google Scholar]
- 33.Mostafalou S, Abdollahi M, Eghbal MA, Kouzehkonani NS. Protective effect of NAC against malathion-induced oxidative stress in freshly isolated rat hepatocytes. Adv Pharm Bull. 2012;2(1):79–88. doi: 10.5681/apb.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammadnejad D, Abedelahi A, Soleimani-rad J, Mohamadi-roshandeh A, Rashtbar M, Azami A. Degenerative effect of cisplatin on testicular germinal epithelium. Adv Pharm Bull. 2012;2(2):173–7. doi: 10.5681/apb.2012.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oskouei BS, Ghanbar AA, Roshangar L, Khaki AA, Rad JS. The Effects of Amlodipine Administration and In Vitro Addition of Pentoxifylline on Sperm Parameters in Mice. Medical Journal of Tabriz University of Medical Sciences and Health Services. 2012;33(6):16–19. [Google Scholar]
- 36.Zahedi A, Fathiazad F, Khaki A, Ahmadnejad B. Protective effect of ginger on gentamicin-induced apoptosis in testis of rats. Adv Pharm Bull. 2012;2(2):197–200. doi: 10.5681/apb.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


