Abstract
Non-Hodgkin lymphomas (NHL) are a heterogeneous group of immune cell neoplasms that comprise molecularly distinct lymphoma subtypes. Recent work has identified high frequency promoter point mutations in the telomerase reverse transcriptase (TERT) gene of different cancer types, including melanoma, glioma, liver and bladder cancer. TERT promoter mutations appear to correlate with increased TERT expression and telomerase activity in these cancers. In contrast, breast, pancreatic, and prostate cancer rarely demonstrate mutations in this region of the gene. TERT promoter mutation prevalence in NHL has not been thoroughly tested thus far. We screened 105 B-cell lymphoid malignancies encompassing nine NHL subtypes and acute lymphoblastic leukemia, for TERT promoter mutations. Our results suggest that TERT promoter mutations are rare or absent in most NHL. Thus, the classical TERT promoter mutations may not play a major oncogenic role in TERT expression and telomerase activation in NHL.
Keywords: Telomerase, non-Hodgkin lymphoma, TERT promoter
1. Introduction
Non-Hodgkin lymphomas (NHL) are a heterogeneous group of B, T, and natural killer cell neoplasms that arise primarily in lymph nodes. Most NHL in the western hemisphere are B-cell derived and comprise a variety of lymphomas, with diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) being the most common [1]. Recent advances in molecular genetics have confirmed the molecular heterogeneity of NHL. Classically, NHL can be characterized by chromosomal translocation events that have been shown to occur frequently with different subtypes of NHL [2,3,4]. Whole exome sequencing has further expanded molecular characterizations of NHL. Parallel sequencing experiments with DLBCL patients [5] and FL patients [6] have identified recurrent mutations in functionally relevant genes as well as novel genes that have not been previously implicated. Despite these advances, NHL remains a heterogeneous group of malignancies, with many less characterized subtypes that remain difficult to diagnose and treat with current therapeutic strategies [7].
Recently, non-coding sequences have become an emerging field of active investigation in cancer research [8]. In 2013, specific high frequency promoter mutations in the telomerase reverse transcriptase (TERT) gene in melanoma were reported, and were associated with a two- to four-fold increase in transcriptional activity [9,10]. TERT encodes the catalytic subunit of telomerase, an enzyme that preserves chromosomal ends through telomere maintenance. The reported somatic transitions −124C>T and −146C>T in the TERT promoter region create a novel binding site for the ETS transcription factor GABP, which increases transcription of TERT [11]. Increased TERT expression may confer increased proliferative potential and cell survival, which are essential factors in tumorigenesis [12]. Strikingly, TERT promoter mutations are not unique to melanomas, but have been later found to be frequent in many other malignancies such as hepatocellular carcinoma, bladder cancer, and glioblastoma [13,14,15,16,17,18,19]. However, TERT promoter mutations are not universal. Mutations have been shown to be absent, or rarely observed, in other cancer types like breast, pancreatic, and prostate cancer [13,14,19].
Our lab has used the avian leukosis virus (ALV) as a tool to screen for common proviral integration sites in the host chicken genome to assess events involved in lymphoma development. By high-throughput sequencing and inverse PCR, we have previously shown that early chicken TERT (chTERT) expression through proviral integrations is associated with a similar two- to four-fold increase in transcriptional activity [20,21] and is likely important in lymphomagenesis. Although lymphocytes are known to be a cell type characterized by high telomerase activity throughout their life cycle, lymphoid malignancies are associated with elevated TERT expression like the majority of cancers, suggesting a requirement for persistent TERT activity in transformed cells [22,23].
We sought to investigate whether TERT promoter mutations play a role in TERT activation in human lymphomas. Presently, published work on the TERT promoter status of NHL is limited. Since the original reports in melanoma, we have found some published work that suggests TERT promoter mutations are absent in DLBCL and CLL [15,16]. In contrast, TERT promoter mutations were detected in primary central nervous system lymphoma [24]. Here, we report a TERT promoter mutation screen of a collection of 105 human B-cell malignancies encompassing nine different subtypes of NHL. Our results indicate that TERT promoter mutations are absent across all tested NHL. These findings suggest that TERT promoter mutations are not major drivers for TERT up-regulation in lymphomas in contrast to the aforementioned cancers.
2. Materials and Methods
2.1. Patients and Samples
Representative cases of a variety of B-cell neoplasms were obtained from archived formalin-fixed paraffin-embedded (FFPE) tissues as well as frozen cells and tissues previously banked as de-identified research samples after obtaining institutional review board approval (Johns Hopkins Institution Review Board no. NA_00028682). The FFPE archives were searched from 2000 to 2014 for cases of Burkitt lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, lymphoplasmacytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, myeloma/plasmacytoma, and plasmablastic lymphoma. Cases of glioblastoma and reactive lymph nodes were also queried as expected positive and negative control cases for formalin-fixed paraffin-embedded (FFPE) tissue. Representative cases with unambiguous pathologic diagnoses and sufficient material were selected for histologic re-review by a board-certified Pathologist (Rena R. Xian). Both tumor neoplastic cell content and tissue adequacy was assessed, and only cases with at least 25% neoplastic cells and sufficient tissue were selected for the study. FFPE tissue sections were then acquired from the respective archival cases for subsequent analysis. B-cell acute lymphoblastic leukemia (B-ALL) bone marrow aspirate samples with at least 15% lymphoblasts, and normal bone marrow aspirate samples without phenotypic abnormalities were consecutively collected over a two-month period from remnant material from routine clinical flow cytometric testing. Fresh aspirate material collected in EDTA blood tubes was obtained and frozen until further analysis.
2.2. DNA Isolation and Mutational Analysis
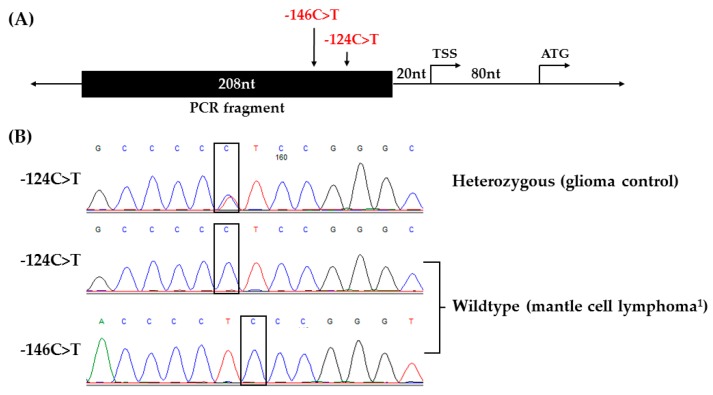
DNA extraction from bone marrow was performed using Qiagen DNeasy Blood and Tissue Kit (Valencia, CA, USA) according to manufacturer’s protocol. DNA extraction from FFPE tissue slides was performed using Pinpoint Slide DNA Isolation SystemTM (Irvine, CA, USA) according to manufacturer’s protocol. Primers with the sequences 5′-M13F-CGGGCTCCCAGTGGATTCGC-3′ and 5′-CGGGGCCGCGGAAAGGAA-3′ were used to PCR-amplify the proximal TERT promoter region containing −124C>T (chr5:1295228, NM_198253, GRCh37/hg19) and −146C>T (chr5:1295250, NM_198253, GRCh37/hg19). Amplified products were then sequenced using standard Sanger sequencing techniques (Louisville, KY, USA) with the universal sequencing priming site, M13F.
3. Results
Absence of TERT Promoter Mutations in NHLs
NHLs (Table 1) were screened for TERT promoter mutations. The PCR amplified region encompasses the two most commonly mutated nucleotides −124C>T (chr5:1295228G>A) and −146C>T (chr5:1295250G>A) upstream of the translational start site of TERT (Figure 1A). Altogether, 93 tumor samples were evaluated with at least 7 samples of each subtype in additional to a subset of gliomas as positive controls, and reactive lymph nodes and normal bone marrows as negative controls (Table 1). We confirmed TERT promoter mutations in glioblastomas (n = 2), which is lower than expected [13,15,16] but demonstrates that we can detect the mutation. A glioma control trace that is heterozygous for the −124C>T mutation is shown (Figure 1B). Glioblastoma samples (n = 7) previously identified with promoter mutations were used as additional positive controls. All 7 control samples were confirmed to have the −124C>T mutation. No TERT promoter mutations were detected in any NHL samples in the amplified promoter region. A representative NHL trace showing the wildtype TERT promoter sequences at both positions are shown from a mantle cell lymphoma tumor sample (Table 1 and Figure 1B).
Table 1.
Samples tested for telomerase reverse transcriptase (TERT) promoter mutations 1.
| Tumor Type | No. of Tumors | No. of Tumors Mutated |
|---|---|---|
| B-cell acute lymphoblastic leukemia | 12 | 0 |
| Burkitt lymphoma | 9 | 0 |
| Chronic lymphocytic leukemia | 11 | 0 |
| Diffuse large B-cell lymphoma | 9 | 0 |
| Follicular lymphoma | 13 | 0 |
| Lymphoplasmacytic lymphoma | 7 | 0 |
| Mantle cell lymphoma | 12 | 0 |
| Marginal zone lymphoma | 16 | 0 |
| Myeloma/plasmacytoma | 9 | 0 |
| Plasmablastic lymphoma | 7 | 0 |
1 Glioblastoma tissues were used as positive controls (n = 11); reactive lymph nodes (n = 13) were used as negative controls for formalin-fixed paraffin-embedded (FFPE) samples; normal bone marrow samples (n = 13) were used as negative controls for B-cell acute lymphoblastic leukemia (B-ALL) samples.
Figure 1.
Screening of TERT promoter mutations in Non-Hodgkin lymphomas (NHLs). (A) Schematic of the amplified region and the location of −124C>T and −146C>T in the TERT promoter. (B) Sequencing chromatographs of the TERT promoter locus in a glioma control that is heterozygous for −124C>T (top) and a representative NHL tumor sample that is wildtype at both positions (middle and bottom). 1 A representative trace for wildtype at both positions.
4. Discussion
As a terminally differentiated cell type, normal human lymphocytes have atypical telomere and telomerase biology. In contrast to other cell types, lymphocytes have above average telomere length and telomerase activity [22,24]. Despite the presence of longer telomeres, and enhanced telomerase activity, lymphocytes still experience division-dependent telomere shortening. Malignant transformation is associated with increased TERT expression and telomere length [25]. Furthermore, longer telomeres and higher telomerase activity are associated with more aggressive NHL than indolent ones, and have been suggested to be a prognostic risk factor for NHL [26]. Our lab has repeatedly observed TERT activation in chicken lymphomas via ALV integration into the chTERT promoter region as an early event in avian B-cell lymphomagenesis [20,21].
Taken together, to overcome the restriction of telomere shortening, and support higher proliferative potential and survival, we hypothesized that lymphocytes may acquire TERT promoter mutations in the process of malignant transformation, which can directly up-regulate TERT expression and drive telomerase function. However, our data suggest that the NHLs tested were free of the two most prevalent TERT promoter mutations [9,10]. This result does not exclude the possibility of promoter mutations further upstream of the area we investigated, and the small sample size does not exclude the presence of low-frequency TERT promoter mutations, which would require much larger screens to resolve.
Concurrent to this study, TERT promoter mutations were reported to be present in 33% of circulating mantle cell lymphoma [27]. In this study, a limited subset of other lymphoid neoplasms were reported to be free of TERT promoter mutations. While we detected no TERT promoter mutations in our set of mantle cell lymphoma, there are a few possible reasons for this apparent discordance. First, the small sample size of mantle cell lymphoma (n = 12) evaluated in the current study may be too small to detect a change that may be present in a small fraction of cases. Second, the source of neoplastic B-cells in our study was different from the concurrent study. The peripheral blood source of mantle cell lymphoma in the concurrent study indicates that all patients had circulating leukemic-phase disease, which is associated with advanced stage disease, and worse prognosis when coupled with nodal involvement [28]. This is compared to the node-based disease selected in our study, irrespective of circulating cells, which may harbor different clonal abnormalities commensurate with the stage of the lymphoma. TERT promoter mutations may be more prevalent in a particular stage in mantle cell lymphomagenesis. This has been previously shown to be true in melanoma, in which TERT mutations are associated with different histology types of the disease, and are more commonly found in melanoma without regression as compared to melanoma with regression [29].
Our findings suggest that activation of TERT expression by acquired TERT promoter mutations is not a major driver for TERT activation in NHL. As observed previously, the frequency of this phenomenon is perhaps associated with the intrinsic proliferative potential of the cell type, in which cells with higher proliferative potential like lymphocytes are less likely to have TERT promoter mutations [15]. In the case of lymphocytes, perhaps activation of TERT expression indirectly through the up-regulation of other genes like MYC is far more common, and supplants the requirement of other mechanisms of TERT activation like TERT promoter mutations. Despite our results, the non-coding sequences of NHL tissues remain an uncharted territory, as novel mutated regulatory sites are being discovered across many different cancers [30], and future analysis by whole-genome sequencing may lead to the discovery of novel mechanisms in lymphomagenesis. As whole-genome patient sequencing and clinical data becomes available, we can begin to explain these observations and apply them to enhance our understanding of cancer biology and the treatment of cancer.
5. Conclusions
Our results support the view that TERT promoter mutations are rare or absent in most NHL, and likely not major drivers of TERT activation.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 CA124596 and Training Grant T32GM007231. Rena R. Xian was partially supported by Sysmex Corporation.
Author Contributions
Rena R. Xian and Karen L. Beemon conceived the experiments. Gary Lam designed the experiments. Gary Lam and Yingying Li performed the experiments. Gary Lam analyzed the data. Rena R. Xian analyzed and collected human samples. Kathleen H. Burns reviewed and contributed materials. Gary Lam and Rena R. Xian wrote the paper. Karen L. Beemon and Kathleen H. Burns reviewed and edited the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Vardiman J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. [Google Scholar]
- 2.Biagi J., Seymour J. Insights into the molecular pathogenesis of follicular lymphoma arising from analysis of geographic variation. Blood. 2002;99:4265–4275. doi: 10.1182/blood.V99.12.4265. [DOI] [PubMed] [Google Scholar]
- 3.Barrans S.L., Evans P.A., O’Connor S.J., Kendall S.J., Owen R.G., Haynes A.P., Morgan G.J., Jack A.S. The t(14;18) is associated with germinal center-derived diffuse large B-cell lymphoma and is a strong predictor of outcome. Clin. Cancer Res. 2003;9:2133–2139. [PubMed] [Google Scholar]
- 4.Bertoni F., Rinaldi A., Zucca E., Cavalli F. Update on the molecular biology of mantle cell lymphoma. Hematol. Oncol. 2006;24:22–27. doi: 10.1002/hon.767. [DOI] [PubMed] [Google Scholar]
- 5.Lohr J.G., Stojanov P., Lawrence M.S., Auclair D., Chapuy B., Sougnez C., Cruz-Gordillo P., Knoechel B., Asmann Y.W., Slager S.L., et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl. Acad. Sci. USA. 2011;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okosun J., Bödör C., Wang J., Araf S., Yang C.Y., Pan C., Boller S., Cittaro D., Bozek M., Iqbal S., et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat. Genet. 2014;46:176–181. doi: 10.1038/ng.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansell S.M. Non-Hodgkin lymphoma: Diagnosis and treatment. Mayo Clin. Proc. 2015;90:1152–1163. doi: 10.1016/j.mayocp.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Diederichs S., Bartsch L., Berkmann J.C., Fröse K., Heitmann J., Hoppe C., Iggena D., Jazmati D., Karschnia P., Linsenmeier M., et al. The dark matter of the cancer genome: Aberrations in regulatory elements, untranslated regions, splice sites, non-coding RNA and synonymous mutations. EMBO Mol. Med. 2016;8:442–457. doi: 10.15252/emmm.201506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang F.W., Hodis E., Xu M.J., Kryukov G.V., Chin L., Garraway L.A. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn S., Figl A., Rachakonda P.S., Fischer C., Sucker A., Gast A., Kadel S., Moll I., Nagore E., Hemminki K., et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 11.Bell R.J., Rube H.T., Kreig A., Mancini A., Fouse S.D., Nagarajan R.P., Choi S., Hong C., He D., Pekmezci M., et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348:1036–1039. doi: 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y., Li H., Deb S., Liu J. TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene. 2002;21:3130–3138. doi: 10.1038/sj.onc.1205419. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich B., Rachakonda P.S., Hemminki K., Kumar R. TERT promoter mutations in cancer development. Curr. Opin. Genet. Dev. 2014;24:30–37. doi: 10.1016/j.gde.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Killela P.J., Reitman Z.J., Jiao Y., Bettegowda C., Agrawal N., Diaz L.A., Jr., Friedman A.H., Friedman H., Gallia G.L., Giovanella B.C., et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinhold N., Jacobsen A., Schultz N., Sander C., Lee W. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat. Genet. 2014;46:1160–1165. doi: 10.1038/ng.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinagre J., Almeida A., Pópulo H., Batista R., Lyra J., Pinto V., Coelho R., Celestino R., Prazeres H., Lima L., et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013 doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Wu G., Shan Y., Hartmann C., von Deimling A., Xing M. Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma. Cell Cycle. 2013;12:1637–1638. doi: 10.4161/cc.24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachakonda P.S., Hosen I., de Verdier P.J., Fallah M., Heidenreich B., Ryk C., Wiklund N.P., Steineck G., Schadendorf D., Hemminki K., et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by common polymorphism. Proc. Natl. Acad. Sci. USA. 2013;110:17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell B.J., Rube H.T., Xavier-Magalhães A., Costa B.M., Mancini A., Song J.S., Costello J.F. Understanding TERT promoter mutations: A common path to immortality. Mol. Cancer Res. 2016;14:315–323. doi: 10.1158/1541-7786.MCR-16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F., Xian R.R., Li Y., Polony T.S., Beemon K.L. Telomerase reverse transcriptase expression elevated by avian leucosis virus integration in B cell lymphomas. Proc. Natl. Acad. Sci. USA. 2007;104:18952–18957. doi: 10.1073/pnas.0709173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justice J.F., 4th, Morgan R.W., Beemon K.L. Common viral integration sites identified in avian leukosis virus-induced B-cell lymphomas. Am. Soc. Microbiol. 2015 doi: 10.1128/mBio.01863-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison G.M. Telomeres and telomerase in leukaemia and lymphoma. Transfus. Apher. Sci. 2007;37:43–47. doi: 10.1016/j.transci.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Lobetti-Bodoni C., Bernocco E., Genuardi E., Boccadoro M., Ladetto M. Telomeres and telomerase in normal and malignant B-cells. Hematol. Oncol. 2010;29:157–167. doi: 10.1002/hon.937. [DOI] [PubMed] [Google Scholar]
- 24.Bruno A., Alentorn A., Daniau M., Labussière M., Rahimian A., Tabouret E., Polivka M., Jouvet A., Adam C., Figarella-Branger D., et al. TERT promoter mutations in primary central nervous system lymphoma are associated with spatial distribution in the splenium. Acta Neuropathol. 2015;130:439–440. doi: 10.1007/s00401-015-1461-9. [DOI] [PubMed] [Google Scholar]
- 25.Machiela M.J., Lan Q., Slager S.L., Vermeulen R.C., Teras L.R., Camp N.J., Cerhan J.R., Spinelli J.J., Wang S.S., Nieters A., et al. Genetically predicted longer telomere length is associated with increased risk of B-cell lymphoma subtypes. Hum. Mol. Genet. 2016;25:1663–1676. doi: 10.1093/hmg/ddw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohyashiki J., Sashida G., Tauchi T., Ohyashiki K. Telomeres and telomerase in hematologic neoplasia. Oncogene. 2002;21:680–687. doi: 10.1038/sj.onc.1205075. [DOI] [PubMed] [Google Scholar]
- 27.Panero J., Alves-Paiva R.M., Roisman A., Santana-Lemos B.A., Falcão R.P., Oliveira G., Martins D., Stanganelli C., Slavutsky I., Calado R.T. Acquired TERT promoter mutations stimulate TERT transcription in mantle cell lymphoma. Am. J. Hematol. 2016;91:481–485. doi: 10.1002/ajh.24324. [DOI] [PubMed] [Google Scholar]
- 28.Pittaluga S., Verhoef G., Criel A., Maes A., Nuyts J., Boogaerts M., De Wolf Peeters C. Prognostic significance of bone marrow trephine and peripheral blood smears in 55 patients with mantle cell lymphoma. Leuk. Lymphoma. 1996;21:115–125. doi: 10.3109/10428199609067588. [DOI] [PubMed] [Google Scholar]
- 29.De Unamuno Bustos B., Estal R.M., Simó G.P., Martínez V.O., Ros M.L., Suela S.P., Estrada R.B. Lack of TERT promoter mutations in melanomas with extensive regression. J. Am. Acad. Dermatol. 2016;74:570–572. doi: 10.1016/j.jaad.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Melton C., Reuter J., Spacek D., Snyder M. Recurrent somatic mutations in regulatory regions of human cancer genomes. Nat. Genet. 2015;47:710–716. doi: 10.1038/ng.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]