Abstract
Anti-retroviral drugs and antibodies limit HIV-1 infection by interfering with the viral life-cycle. In addition, antibodies also have the potential to guide host immune effector cells to kill HIV-1 infected cells. Examination of the kinetics of HIV-1 suppression in infected individuals by passively administered 3BNC117, a broadly neutralizing antibody (bNAb), suggested that the effects of the antibody are not limited to free viral clearance and blocking new infection, but also include acceleration of infected cell clearance. Consistent with these observations, we find that bNAbs can target CD4+ T cells infected with patient viruses and decrease their in vivo half-lives by a mechanism that requires FcγR engagement in a humanized mouse model. The results indicate that passive immunotherapy can accelerate elimination of HIV-1 infected cells.
Main text
Broadly neutralizing antibodies (bNAbs) to HIV-1 can block acquisition and suppress viremia in chronically infected humanized mice and macaques (1, 2). In humans, a single infusion of 3BNC117, a bNAb that targets the CD4 binding site on the HIV-1 envelope glycoprotein gp160, led to a rapid but transient reduction in viral loads by an average 1.48 log10 copies/ml (3).
Antibodies differ from small molecule drugs that interfere with viral replication in that antibodies have the potential to impact the half-lives of both free virus and infected cells. Indeed, antibodies accelerate the clearance of free virions from the blood of macaques (4) and induce killing of infected cells in vitro by Fcγ receptor (FcγR)-mediated mechanisms (5, 6). However, the majority of infected cells die rapidly by apoptosis or pyroptosis (7, 8), and whether bNAbs can accelerate HIV-1 infected cell clearance in vivo has not been tested directly.
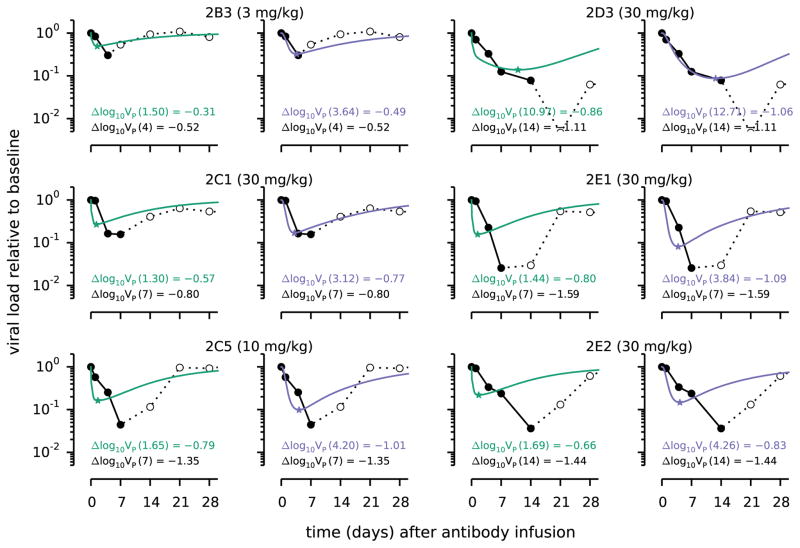
To examine the components that contribute to viral clearance in humans given a single infusion of 3BNC117, we adapted an existing model of HIV-1 viral dynamics (3, 9, 10). The model ((11), Fig. S1) includes virus-producing infected cells, as well as transport of free plasma virus to lymphoid tissues (LT) and vice versa. To this basic model, we added the feature that antibodies bind to virus particles, leading to virus neutralization and loss of antibody. Measurements of the decline of antibody concentrations in healthy humans were fitted to a two-compartment model (12, 13) to obtain the parameters characterizing the intrinsic antibody decay rates and transport between tissue and plasma over the time scale during which viral loads decay in patients treated with 3BNC117 (Fig. S2). The rate of free virus neutralization was fitted to the virus kinetics in 19 patients (Fig. S3), but we focused on patients showing an initial monophasic viral load decline (2B3, 2C1, 2C5, 2D3, 2E1, 2E2), which tended to coincide with those receiving a higher antibody dose (3).
This model is unable to recapitulate the kinetics of viral load decline for any of the 3BNC117-treated viremic patients (Fig. 1, green; Fig. S3). If we fit the overall extent of viral load decrease, the rate of viral load decay is predicted to be too fast. Conversely, matching the initial rate of viral load decline results in insufficient overall reduction of the viral load. Thus, we adjusted our model to incorporate a mechanism that includes antibodies acting to clear infected cells and explored if this provided additional reduction of virus over a longer timescale (11). The rates of free-virus neutralization and infected cell clearance are fit to the measured plasma viral load. Including cell clearance substantially improves the fit to patient data (Fig. 1, purple; Fig. S3; Table S3) because reducing the number of infected cells in tissues results in a second-order decay in the plasma viral load over a longer timescale.
Figure 1. Comparison of viral load measurements (filled circles, solid black lines) with best-fit model predictions (solid colored lines).
Each green line shows the predicted viral load over time, normalized by its initial amount, VP(t)/VP(0), in a model whereby antibody can only neutralize free virus particles. Each purple line shows a modified model whereby antibody can also lead to clearance of infected cells. Only those patients with a day 1 viral load lower than baseline are shown. Open circles and dashed black lines represent data points that were not used for parameter estimation. Within each subfigure, we note the quantity Δlog10VP(tmin) = log10(VP,min/VP(0)), i.e. the viral load at the nadir and the time in days at which this occurs for the data (black letters), and the predictions for a model with free virus clearance only (green) and a model that also includes infected cell clearance (purple). This predicted minimum for each patient and model is denoted with a star.
Our modeling clearly shows that the patient data cannot be explained if 3BNC117 acts only to neutralize free virions, thereby making them incapable of infecting target cells. Reasons for why including infected cell clearance improves the fit, but does not quantitatively recapitulate the data are noted in supplementary materials (11). Further evidence for such a mechanism is indicated by comparison of our modeling and clinical data for patients treated with a constant high level of entry-inhibitor drugs like maraviroc (14) ((11), Fig. S4).
To determine whether 3BNC117 can recognize the HIV-1 envelope (Env) trimer expressed on the surface of infected cells, we stained CD4+ T cells infected with HIVYU2 or primary isolates obtained from patients 2C1, 2C5, 2D3, and 2E5 before they were infused with 3BNC117 (3). Consistent with their neutralizing activity in TZM-bl assays, 3BNC117, PG16, and 10–1074 specifically stained HIVYU2-infected cells (Fig. S5A). 3BNC117 and 10–1074 also recognized nearly all Gag+ cells infected with primary isolates from patients 2C1, 2C5, 2D3 and 2E5; however, the mean fluorescence intensity of staining for Env was lower than for HIV-1YU2 (Fig. S5B), possibly due to lower levels of Env on the surface of cells infected with patient viruses, or variable levels of tetherin antagonism by HIV-1 accessory protein Vpu (15). We conclude that 3BNC117 recognizes the HIV-1 envelope glycoprotein on the surface of infected CD4+ T cells.
To examine whether bNAbs can accelerate clearance of infected cells in vivo, we performed adoptive transfer experiments using NOD Rag1−/−Il2rgnull (NRG) mice. To prevent spread of infection between human cells, animals and infected cell suspensions were treated with antiretroviral therapy (ART) before adoptive transfer. Anti-HIV-1 bNAbs or isotype control antibodies were administered 12 hours before infected cell transfer (Fig. S6).
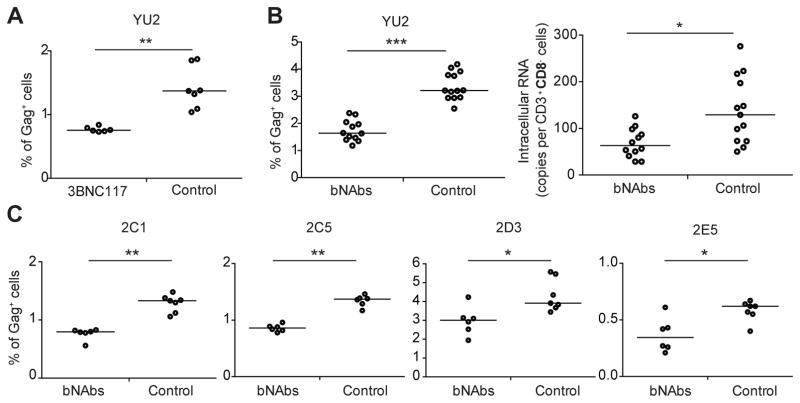
3BNC117 alone (P = 0.0012, Fig. 2A), or a combination of 3BNC117 and 10–1074, rapidly reduced the percentage of HIV-1YU2-infected cells among CD3+CD8− cells compared to an isotype control (P < 0.0001, Fig. 2B). Concomitant with reduction in the percentage of infected cells, cell-associated HIV-1 RNA levels were lower in bNAb treated mice than isotype controls (P = 0.0054, Fig. 2B). These data indicate that bNAbs can accelerate clearance of HIV-1YU2-infected cells in vivo.
Figure 2. bNAbs accelerate clearance of HIV-1-infected cells in vivo.
(A) Scatter plot showing the percentage of Gag+ cells among CD3+CD8− cells in 3BNC117 (600 μg) or isotype control treated mice 5 hours after HIVYU2-infected cell transfer.
(B) Percentage of Gag+ cells among CD3+CD8− cells in bNAbs (3BNC117 + 10–1074, 300 μg each) or isotype control treated mice 5 hours after HIVYU2-infected cell transfer (left panel). Cell-associated HIV-1 RNA was measured in enriched human cells extracted from the spleen of mice 5 hours after transfer, plotted as the ratio of HIV-1 RNA to the number of CD3+CD8− cells for each mouse (right panel).
(C) Graphs represent transfer experiments with cells infected by HIV2C1, HIV2C5, HIV2D3, or HIV2E5. Each dot represents one mouse. Lines represent median values. Data represent 2–4 independent experiments with a total of 6–13 mice per condition. *P<0.05; **P<0.005; ***P<0.001, two-tailed Mann–Whitney U test.
To determine whether anti-HIV-1 antibodies can accelerate clearance of cells infected with primary HIV-1 isolates, we repeated the adoptive transfer experiment described above using human CD4+ T cells infected with HIV2C1, HIV2C5, HIV2D3, or HIV2E5. As with HIV-1YU2, bNAbs accelerated clearance of cells infected with patient isolates (Fig. 2C). We conclude that bNAbs can accelerate clearance of CD4+ T cells infected with primary HIV-1 isolates.
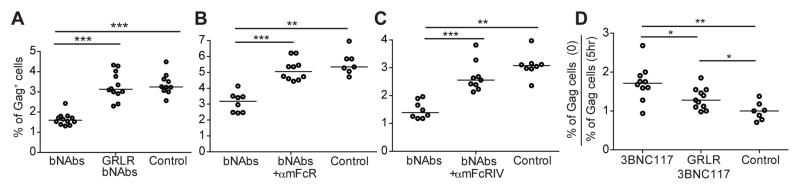
To determine whether enhanced clearance of HIV infected cells by antibodies depends on their ability to engage FcγR expressing cells, we repeated the adoptive transfer experiments using bNAbs which carry mutations that specifically abrogate mouse FcγR binding (G236R/L328R; GRLR) (16) (Fig. S7). Although GRLR-bNAbs show normal levels of neutralizing activity in TZM-bl assays (17), they do not interact with cytotoxic or phagocytic cells, and should therefore fail to accelerate clearance of HIV-1 infected cells in vivo. The frequency of infected cells remaining after treatment with GRLR-bNAbs was comparable to that found in mice receiving the isotype control (Fig. 3A), and significantly higher than that found in mice receiving wild-type bNAbs (P < 0.0001, Fig. 3A). In addition to testing GRLR-bNAbs, we also blocked wild-type bNAb Fc-FcγR interactions using a combination of antibodies 2.4G2 and 9E9, targeting mouse FcγRs II/III and IV, respectively (Fig. S7). Mice receiving FcγR-blocking antibodies failed to accelerate clearance of HIV-1-infected cells in response to bNAbs (Fig. 3B).
Figure 3. FcγR engagement is required to facilitate clearance of HIV-1-infected cells.
(A) Percentage of Gag+ cells among CD3+CD8− cells in bNAbs, GRLR-bNAbs, or isotype control treated mice.
(B) NRG mice were injected with mouse FcγRII/III/IV blocking antibodies or isotype control 6 hours before injection of bNAbs. Graphs show the percentage of Gag+ cells among CD3+CD8− cells.
(C) Percentage of Gag+ cells among CD3+CD8− cells in mice receiving FcγRIV blocking antibody, or isotype control.
(D) Infected cell clearance in chronically HIV-1YU2-infected hu-mice. Scatter plot shows the ratio of the percentage of Gag+ cells among CD3+CD8− cells before and 5 hours after 3BNC117, GRLR-3BNC117, or isotype control injection. Each dot represents one mouse. Lines represent median values. Data represent 2–4 independent experiments for each condition with a total of 7–12 mice per condition. *P<0.05; **P<0.005; ***P<0.001, two-tailed Mann–Whitney U test.
Human IgG1, the isotype of 3BNC117 and 10–1074, binds with highest affinity to mouse FcγRI and FcγRIV (mFcγRI and mFcγRIV) (18). These receptors are murine orthologues of human FcγRI and FcγRIII (hFcγRI and hFcγRIII) (19), which are expressed on human monocytes and NK cells that can perform antibody-dependent cell-mediated phagocytosis (ADCP) or cytotoxicity (ADCC) in vitro (5). To examine the role of mFcγRIV in clearance of HIV-1-infected cells in the NRG mouse, we blocked this receptor specifically with a monoclonal anti-mFcγRIV antibody. Mice treated with bNAbs plus anti-mFcγRIV were comparable to isotype-treated mice (Fig. 3C), indicating that mFcγRIV is essential for accelerating clearance of HIVYU2-infected cells in vivo. We conclude that bNAbs accelerate infected cell clearance in NRG mice by a mechanism that requires mFcγRIV engagement.
We repeated adoptive transfer experiments in FcγR-humanized (hFcγR) mice that express only the human FcγRs (20). Similar to NRG mice, HIV-1YU2-infected cells were reduced in bNAb-treated hFcγR mice (P = 0.0064, Fig. S8). This data suggests that bNAbs can utilize human FcγRs, and not just murine FcγRs, to accelerate clearance of HIV-1YU2-infected cells in vivo.
To examine whether 3BNC117 accelerates clearance of HIV-1 infected cells in the context of chronic viral infection, we performed hemi-splenectomy experiments in chronically infected humanized mice (Fig. S9). The frequency of HIV-1YU2-infected cells before treatment was comparable in all groups of mice (Fig. S10). As expected, mice treated with the Fc mutant antibody GRLR-3BNC117, which neutralizes HIV-1 but does not interact with effector cells, had lower frequencies of infected cells compared with isotype-treated controls (P = 0.0219, Fig. 3D). However, mice treated with wild-type 3BNC117, which can mediate ADCC, had substantially fewer infected cells compared with mice treated with GRLR-3BNC117 (P = 0.0167, Fig. 3D). These data suggest that 3BNC117 can accelerate clearance of HIV-1YU2-infected cells in the context of chronic viral infection.
In contrast to ART, antibodies have the potential to engage host immune cells in defense against the virus. They do so by binding to cell-free virions, thereby accelerating clearance and preventing their entry into target cells. Antibodies can also bind to HIV-1 Env on the surface of infected cells to induce ADCC. Finally, immune complexes can activate antigen-presenting dendritic cells to elicit adaptive immune responses (19).
Both neutralizing and non-neutralizing antibodies support anti-HIV-1 ADCC activity in vitro (5, 21), and Fc receptor binding is essential for optimal protection, post exposure prophylaxis, and therapy by bNAbs in animal models (17, 18, 22–24). Moreover, ADCC has been indirectly associated with both control of and protection against infection (15, 25–28). However, the rapid death of HIV-1 infected cells has made it difficult to establish that antibodies can accelerate the clearance of infected cells in vivo. Our mathematical analysis of patient data, and the antibody-mediated reduction in infected cells seen in adoptive transfer experiments establish that bnAbs alter the half-life of infected cells. This observation may help explain why post-exposure prophylaxis with bNAbs is more effective than ART in hu-mice (23).
Experiments with human cells in mice cannot fully recapitulate the human host; nevertheless, these experiments establish that antibodies can accelerate clearance of infected cells in vivo, and do so by an FcγR-dependent mechanism. The finding that antibodies can clear infected cells in vivo has significant implications for therapies aimed at HIV prevention and viral reservoir reduction or elimination.
Supplementary Material
Acknowledgments
We thank all study participants who devoted time to our research. We thank the Rockefeller University Hospital Clinical Research Support Office and nursing staff for patient care and recruitment and members of the Nussenzweig lab for helpful discussions. We thank T. Eisenreich for help with mouse colony management, K. Yao, S. Hinklein for technical help, P. Smith for FcγR humanized mice, CellDex Therapeutics for providing 3BNC117 and 10–1074, and all members of the Nussenzweig Laboratory for helpful discussion and advice. The data reported in this paper are tabulated in the main paper and in the supplementary materials. This work was supported by Collaboration for AIDS Vaccine Discovery Grant OPP1033115 (M.C.N. and J.V.R.). This work was also supported, in part, by grant # 8 UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, NIH Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) 1UM1 AI100663-01 (M.C.N), Bill and Melinda Gates Foundation grant OPP1092074 and OPP1124068 (M.C.N), the Robertson Foundation to M.C.N., German Research Foundation postdoctoral fellowship SCHO 1612/1-1 (T.S.), NIH grant F31 AI118555-01 (J.A.H.), the American foundation for AIDS research (amfAR) Mathilde Krim Fellowship in Basic Biomedical Research (108977-57-RKVA) (S.B.), the Ragon Institute of MGH, MIT, & Harvard (A.K.C.), a National Science Foundation Graduate Research Fellowship under Grant No. #1122374 (D.K.M.), and National Institute Of Allergy and Infectious Diseases of the NIH Grants AI100148-02 and AI081677-05 (M.C.N. and J.V.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. M.C.N. is a Howard Hughes Medical Institute Investigator. There are pending patent applications on the 3BNC117 and 10-1074 antibody by Rockefeller University on which Michel Nussenzweig is an inventor. The patents are not licensed by any companies.
References and Notes
- 1.Mouquet H. Antibody B cell responses in HIV-1 infection. Trends in immunology. 2014;35:549–561. doi: 10.1016/j.it.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.West AP, Jr, et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igarashi T, et al. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat Med. 1999;5:211–216. doi: 10.1038/5576. [DOI] [PubMed] [Google Scholar]
- 5.Kramski M, Parsons MS, Stratov I, Kent SJ. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr HIV Res. 2013;11:388–406. doi: 10.2174/1570162x113116660061. [DOI] [PubMed] [Google Scholar]
- 6.Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS Res Hum Retroviruses. 2015;31:13–24. doi: 10.1089/aid.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perelson AS, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 9.Muller V, Maree AF, De Boer RJ. Release of virus from lymphoid tissue affects human immunodeficiency virus type 1 and hepatitis C virus kinetics in the blood. J Virol. 2001;75:2597–2603. doi: 10.1128/JVI.75.6.2597-2603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boer RJ, Ribeiro RM, Perelson AS. Current estimates for HIV-1 production imply rapid viral clearance in lymphoid tissues. PLoS Comput Biol. 2010;6:e1000906. doi: 10.1371/journal.pcbi.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Supplementary analysis is available as supplementary materials on Science Online.
- 12.Joos B, et al. Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5) Antimicrob Agents Chemother. 2006;50:1773–1779. doi: 10.1128/AAC.50.5.1773-1779.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibaldi PDM. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. [Google Scholar]
- 14.Fatkenheuer G, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11:1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 15.Kramski M, Stratov I, Kent SJ. The role of HIV-specific antibody-dependent cellular cytotoxicity in HIV prevention and the influence of the HIV-1 Vpu protein. Aids. 2015;29:137–144. doi: 10.1097/QAD.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 16.Horton HM, et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010;116:3004–3012. doi: 10.1182/blood-2010-01-265280. [DOI] [PubMed] [Google Scholar]
- 17.Pietzsch J, et al. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A. 2012;109:15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bournazos S, et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bournazos S, DiLillo DJ, Ravetch JV. The role of Fc-FcgammaR interactions in IgG-mediated microbial neutralization. The Journal of experimental medicine. 2015;212:1361–1369. doi: 10.1084/jem.20151267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su B, Moog C. Which Antibody Functions are Important for an HIV Vaccine? Front Immunol. 2014;5:289. doi: 10.3389/fimmu.2014.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 23.Halper-Stromberg A, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boesch AW, Brown EP, Ackerman ME. The role of Fc receptors in HIV prevention and therapy. Immunol Rev. 2015;268:296–310. doi: 10.1111/imr.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollara J, et al. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr HIV Res. 2013;11:378–387. doi: 10.2174/1570162X113116660059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis GK. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology. 2014;142:46–57. doi: 10.1111/imm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 28.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012;109:E3268–3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abboud N, et al. A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. The Journal of experimental medicine. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta P, Dart M, Roenneburg DA, Torrealba JR, Burlingham WJ. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant. 2011;11:1296–1301. doi: 10.1111/j.1600-6143.2011.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwitz JA, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P, Vu QD. A simple method for identifying parameter correlations in partially observed linear dynamic models. BMC Syst Biol. 2015;9:92. doi: 10.1186/s12918-015-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase AT, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.