Abstract
The neuropeptide corticotropin-releasing factor (CRF) is released during periods of anxiety and modulates learning and memory formation. One region with particularly dense concentrations of CRF receptors is the basolateral nucleus of the amygdala (BLA), a critical structure for both Pavlovian fear conditioning and fear extinction. While CRF has the potential to modify amygdala-dependent learning, its effect on fear extinction has not yet been assessed. In the present study, we examined the modulatory role of CRF on within-session extinction and fear extinction consolidation. Intra-BLA infusions of the CRF binding protein ligand inhibitor CRF(6-33) which increases endogenous levels of free CRF, or intra-BLA infusions of exogenous CRF made prior to fear extinction learning did not affect either fear expression or within-session extinction learning. However, when these animals were tested twenty-four hours later, drug free, they showed impairments in extinction memory. Conversely, intra-BLA infusions of the CRF receptor antagonist α-helical CRF(9-41) enhanced memory of fear extinction. These results suggest that increased CRF levels within the BLA at the time of fear extinction learning actively impair the consolidation of long-term fear extinction.
Keywords: Fear extinction, amygdala, corticotropin-releasing factor, anxiety, fear learning
Introduction
Human subjects with anxiety disorders exhibit abnormalities in how they acquire and/or extinguish conditioned fear responses [1, 2]. Understanding how anxiety and conditioned fear interact at the neuronal level may thus yield fundamental insights into the causes of anxiety disorders and provide a foundation for clinical investigation. Corticotropin-releasing factor (CRF) is a key neuropeptide for initiating behavioral, endocrine and autonomic responses to stress [3–5]. It is released by neurons in the paraventricular nucleus of the hypothalamus to regulate pituitary ACTH secretion, thus triggering a key component of the hypothalamic-pituitary-adrenal (HPA) axis [6]. Growing evidence suggests that the CRF system plays a significant role in the regulation of anxiety [7, 8]. For example, chronic hyperactivation of the CRF system has been linked to several anxiety disorders and depression [9, 10]. Levels of CRF are enhanced in patients with post-traumatic stress disorder (PTSD) [11], while single nucleotide polymorphisms in the CRF gene have been associated with childhood risk factors for panic disorders [12].
Traditionally, stress-related behavior was thought to be mediated solely by activation of the HPA axis. However, growing evidence suggests that the extrahypohalamic CRF system plays a significant role in the regulation of anxiety. Both the basolateral nucleus of the amygdala (BLA) and central nucleus of the amygdala (CE) are rich in CRF immunoreactive cell bodies, terminals and receptors [13–15]. Acute stress elevates extracellular CRF levels in the amygdala [16–17]. A large number of studies have implicated the amygdala as a key player in mediating anxiety responses [18–20]. Moreover, altered amygdala function has been implicated in several anxiety disorders such as generalized anxiety disorder as well as in PTSD [21–23]. While the amygdala is only one of a constellation of structures involved in mediating anxiety [24], it plays a crucial and clearly defined role is Pavlovian fear conditioning and extinction.
In classical fear conditioning, an initially neutral stimulus, such as a tone (conditioned stimulus; CS) is paired with a noxious stimulus such as a brief electrical footshock (unconditioned stimulus; US). Afterwards, when presented alone, the CS elicits responses in the animal characteristic of fear [25]. Extinction of conditioned fear is a form of new learning in that the CS is repeatedly presented alone so that it ceases to elicit a fear response [26–27]. The original fear memory is inhibited, but not erased, as extinction actively suppresses fear responses in a context-dependent fashion [27]. Evidence suggests that the BLA is a critical site of plasticity for fear conditioning and is also required for the acquisition and storage of extinction memory [28–30].
A number of studies suggest that CRF may modulate both amygdala-dependent and amygdala-independent learning. CRF antagonists infused into the BLA disrupt contextual fear conditioning [31] and impair memory formation of an inhibitory avoidance task [32], suggesting that in general CRF may enhance learning. Moderate increases in CRF also enhance performance in a spatial learning task, visual discrimination paradigm and an inhibitory avoidance task [32–33]. However, a recent report suggests that infusions of CRF into the BLA might impair fear conditioning [34]. The effects of CRF or CRF antagonists on fear extinction learning have not been assessed. However, levels of CRF are enhanced in PTSD patients [35] and PTSD patients exhibit deficits in their ability to extinguish learned fear [36–38], suggesting that CRF in the amygdala might impair fear extinction learning as well.
Here we manipulated the CRF system within the BLA in several different ways prior to fear extinction learning to test the involvement of this neuropeptide on the extinction of fear memories. We took advantage of the fact that endogenous CRF binds to the high affinity binding protein (CRF-BP), a membrane-associated protein which sequesters and inhibits CRF [39]. Administration of CRF-BP ligand inhibitors displace CRF which is then free to act at available CRF receptors [40]. This increase in endogenous CRF is thought to be comparable to administration of a low concentration of exogenous CRF and has been shown to enhance performance in several learning tasks [32–33, 40]. In this study, we evaluated the effects of intra-BLA infusions of 1) a CRF-BP ligand inhibitor, 2) CRF itself and 3) a CRF receptor antagonist each administered prior to fear extinction learning. Our results suggest that increased levels of CRF within the BLA inhibit the formation of long-term memory of fear extinction.
Methods
Subjects
Adult male Sprague Dawley rats (Charles River Laboratories; 250–325g) were housed individually with ad libitum access to food and water and maintained on a 12 hour light/dark cycle. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Columbia University’s Animal Care and Use Committee.
Surgery
Rats were anesthetized with a mixture of isoflurane and oxygen and mounted in a stereotaxic apparatus. Betadine was applied to the scalp and a local anesthetic (bupivacaine, s.c.) was injected under the scalp. The scalp was incised and small burr holes were made in the skull above the BLA (−2.8 AP, +/− 5.3 ML) [41]. 26-gauge guide cannula (Plastics One) were inserted just dorsal to the BLA (−6.0 DV) and cemented, with skull screws, to the skull. Dummy cannulas were inserted to prevent clogging. Rats received an analgesic (carprofen, 5 mg/kg, i.p.) and 5 ml of lactated ringer (s.c.). Animals recovered for one week before behavioral testing.
Behavior
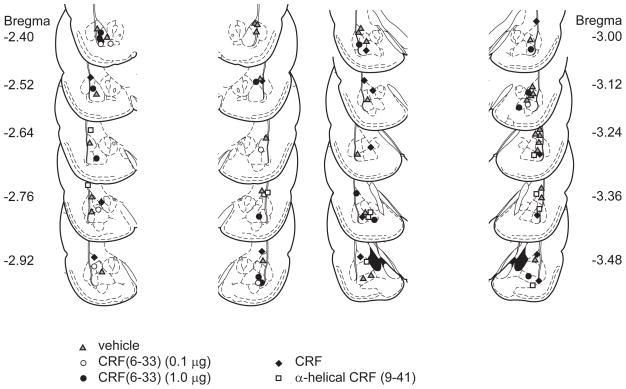
24 hours after habituation to the training context, rats were placed in a rodent conditioning chamber with a metal grid floor (Coulbourn Instruments). Rats received 3 tone-shock pairings: CS = 5 kHz tone, 80dB, 30 sec; US = 0.5mA shock, 1 sec, tone coterminating with the shock. 24 hours later rats were placed in a different context (B) with a black plexiglass floor washed with peppermint soap. They received 10 CS tones (30 sec duration; 60–120 sec inter-tone intervals). To assess extinction recall, rats were then placed 24 hours later in context B and received 1 CS tone. Behavior was recorded by video camera and analyzed off-line. Time spent freezing to each CS (immobility with the exception of breathing) was manually scored for each animal by an observer blind to group assignment. At the end of behavioral experiments, animals were sacrificed by carbon dioxide inhalation, their brains removed and stored in 4% paraformaldehyde in phosphate buffer. Brains were sectioned at a thickness of 100 μm. Nissl staining and light microscopy were used to verify cannula placements within the amygdala (Figure 1).
Figure 1.
Histological verification of cannula placements.
Infusions
15 min prior to fear extinction learning, animals received bilateral intra-BLA infusions of vehicle (saline, 0.5μL), one of two doses of the CRF-BP inhibitor rat/human CRF(6–33) dissolved in vehicle (0.1μg/side or 1.0μg/side in 0.5μL; Tocris Bioscience) or CRF dissolved in vehicle (15 ng/side in 0.5μL; Tocris Bioscience) or the non-selective CRF receptor antagonist α-helical CRF(9-41) (1μg/side in 0.5μL vehicle; Tocris Bioscience). Animals were infused at a rate of 1 μL/min and the infusion cannulas (extending 2 mmfrom the guide cannulas) were left in place for 1–2 min to allow drug diffusion away from the tip.
Results
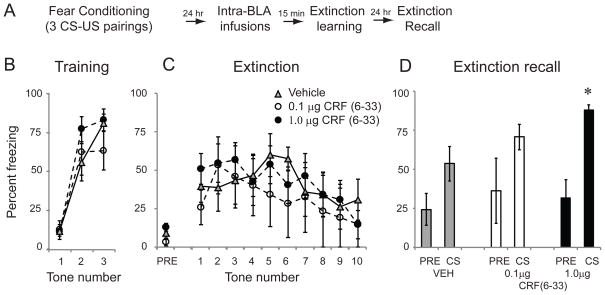
We first asked whether increasing endogenous levels of CRF in the BLA by inhibiting CRF-BP affects fear extinction. Rats were habituated to the training context on day 1 and conditioned with 3 tone-shock pairings on day 2 (Figure 2A; each CS was a 5kHz 30 sec tone coterminating with a footshock US = 0.5mA, 1 sec). Freezing to each tone was quantified (Figure 2B). A repeated measures ANOVA across all 3 tones found no significant difference between groups (F(2,16) = 0.86; p=0.44), ensuring that there were no a priori differences between groups.
Figure 2.
Intra-BLA infusions of the CRF binding protein ligand inhibitor CRF(6-33) impair fear extinction. A: Schematic of behavioral protocol. B: Mean ± SE % freezing to three CS tones during fear conditioning. C: Mean ± SE % freezing to 10 CS tones 24 hr after training in rats given intra-BLA infusions of saline vehicle (n=8), 0.1 μg CRF(6-33) (n=4) or 1.0 μg CRF (6-33) (n=8) 15 min prior to training. D: Mean ± SE % freezing to the 30 sec pre-CS period (PRE) and 1 CS tone (CS) 24 hr after extinction training, drug-free. Animals that previously received infusions of CRF(6-33) showed impaired extinction memory.
24 hours later, rats received intra-BLA infusions of vehicle (n=8), a low concentration of the CRF-BP inhibitor CRF(6–33) (0.1 μg/side; n=4) or a high concentration of CRF(6–33) (1.0 μg/side; n=7). These concentrations were chosen based on the literature [33, 42]. 15 minutes later, rats were placed in context B and presented with 10 CS tones (Figure 2C). A one-way ANOVA showed no difference between groups in freezing to the pre-tone period (defined as the 30 sec before the first CS tone) (F(2,16) = 1.18; p=0.33). To determine if CRF(6–33) affects extinction learning, a repeated measures ANOVA was performed across all 10 tones. A repeated measures ANOVA showed a significant effect of tone (F(9,153) = 2.4; p<0.05) no effect of drug (F(2,16) = 0.235; p=0.79) and no tone*drug interaction (F(18,153) = 0.53; p=0.94) These data suggest that animals successfully extinguished to the CS, but that CRF(6–33) does not affect fear expression or within-session fear extinction learning.
24 hours later, animals were again placed in context B to assess extinction recall (Figure 2D). To measure extinction recall in its purest form with no opportunity for further extinction, animals were tested with a single 30 second CS tone, and freezing during the pre-CS period and CS period was analyzed. A one-way ANOVA showed no difference between groups in freezing to the pre-tone period (defined as the 30 sec before the first CS tone) (F(2,16) = 0.21; p=0.82). However, when freezing to the tone CS was analyzed, a one-way ANOVA showed a significant main effect of group (F(2,16) = 4.35; p<0.05). Tukey’s post hoc t tests revealed that a significant difference existed between vehicle controls and animals receiving the high concentration (1.0 μg) of CRF(6–33) (p < 0.05). These data suggest that if endogenous levels of CRF within the BLA are increased at the time of extinction learning, long-term extinction memory is impaired.
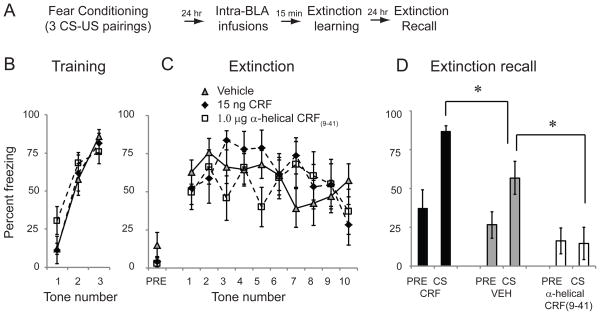
We next asked whether intra-BLA infusions of exogenous CRF also impair fear extinction and, conversely, whether intra-BLA infusions of a CRF receptor antagonist can enhance fear extinction. A pilot study revealed that high concentrations (30ng) of CRF infused into the BLA impaired post-shock freezing and fear expression, while a lower dose (15ng) did not impair footshock sensitivity or fear expression (Supplementary Figure 1). We therefore tested the effects of the lower concentration of CRF on fear extinction learning. This concentration is also consistent with the literature [34, 43–44]. Rats were habituated to the training context on day 1 and conditioned with 3 tone-shock pairings on day 2 (Figure 3A). Freezing to each tone was quantified (Figure 3B). A repeated measures ANOVA across all 3 tones found no significant difference between groups (F(2,25) = 0.47; p=0.63), ensuring that there were no a priori differences between groups.
Figure 3.
Intra-BLA infusions of CRF impair fear extinction, while infusions of a CRF receptor antagonist enhance fear extinction. A: Schematic of behavioral protocol. B: Mean ± SE % freezing to three CS tones during fear conditioning. C: Mean ± SE % freezing to 10 CS tones 24 hr after training in rats given intra-BLA infusions of saline vehicle (n=11), 15ng CRF (n=8) or 1.0 μg α-helical CRF (9-41) (n=8) 15 min prior to training. D: Mean ± SE % freezing to the 30 sec pre-CS period (PRE) and 1 CS tone (CS) 24 hr after extinction training, drug-free. Animals that previously received infusions of CRF showed impaired extinction memory; animals that had received a CRF receptor antagonist showed enhanced extinction memory.
24 hours later, rats received intra-BLA infusions of vehicle (n=11), CRF (15 ng/side; n=8) or the CRF receptor antagonist α-helical CRF(9-41) (1.0 μg/side; n=8). The concentration of antagonist was chosen based on the literature [42, 45]. 15 minutes later, rats were placed in context B and presented with 10 CS tones (Figure 3C). A one-way ANOVA showed no difference between groups in freezing to the pre-tone period (F(2,25) = 1.22; p=0.31). To determine if CRF or a CRF receptor antagonist affected extinction learning, a repeated measures ANOVA was performed across all 10 tones. A repeated measures ANOVA showed a significant effect of tone (F(9,216) = 2.16; p<0.05) no effect of drug (F(2,25) = 0.21; p=0.81) and no tone*drug interaction (F(18,216) = 1.62; p=0.11), suggesting no effect of these drugs on within-session fear extinction learning.
24 hours later, animals were again placed in context B and received one CS tone to assess extinction recall (Figure 3D). A one-way ANOVA showed no difference between groups in freezing to the pre-tone period (F(2,25) = 0.98; p=0.39). However, when freezing to the tone CS was analyzed, a one-way ANOVA showed a significant main effect of group (F(2,25) = 12.61; p < 0.001). Tukey’s post hoc t tests revealed that significant differences existed between vehicle controls and animals receiving CRF (p < 0.05), between vehicle controls and animals receiving α-helical CRF(9-41) (p<0.05) and between animals receiving CRF and animals receiving α-helical CRF(9-41) (p<0.001). Together, these data suggest that increased levels of CRF at the time of extinction learning impair the consolidation of long-term memory of fear extinction. Conversely, blocking CRF receptors with an antagonist enhances the formation of fear extinction memory.
Discussion
In this study we evaluated whether modulating the CRF system within the BLA affects fear extinction. Our data suggest that increasing the concentration of CRF within in the BLA impairs the consolidation of long-term fear extinction memories.
While this is the first study to examine the effects of CRF on fear extinction, it has been previously demonstrated that CRF enhances fear learning in the BLA. CRF antagonists infused into the BLA disrupt contextual fear conditioning [31] and impair memory formation of an inhibitory avoidance task [42]. Furthermore, inhibitors of CRF-BP indirectly increase endogenous levels of CRF and enhance performance on a spatial learning task, visual discrimination paradigm and inhibitory avoidance task [32–33]. Using specific CRF-1 receptor deletions in glutamatergic, GABAergic, dopaminergic or serotonergic cells, it has been shown that the selective deletion of forebrain CRF-1 receptors in forebrain glutamatergic neurons reduces anxiety [46]. Interestingly, neurotransmitter-specific deletion of CRF-1 receptors did not influence auditory fear conditioning, although fear extinction was not tested [46].
CRF administration has profound effects on the excitability of neurons within the BLA. When the CRF agonist urocortin is infused in vivo into the BLA for 5 days, rats develop anxiety. Brain slices collected from these animals reveal a decrease in spontaneous and stimulus-evoked IPSPs in the BLA leading to hyperexcitability of the principal excitatory neurons of the BLA [47]. Similarly, acute CRF in vitro increases the excitability of BLA neurons by reducing the slow afterhyperpolarization [48]. Mice in which CRF-1 receptors are deleted specifically on glutamatergic cells show decreased excitatory neurotransmission within the basolateral amygdala [43].
While long-term changes to the CRF system can have profound changes on amygdalar circuits, transient elevations in CRF are induced by acute stress and can modulate behavior. Immobilization stress elevates extracellular CRF levels in the amygdala [16–17] as does neonatal stress [49] and predator stress [50]. An aversive footshock can increase CRF levels in the CE [45]. Interestingly, the effects of stress on learning can be counteracted by manipulating the CRF system. Although immobilization stress impairs contextual fear memory, systemic injections of a CRF-1 receptor antagonist reverse the stress-induced memory impairment [51]. The source of endogenous CRF within the BLA is unclear: while the BLA contains large concentrations of CRF receptors, the CE is rich in CRF-expressing neurons [14–15, 52]. Yet neurons in the CE do not project to the BLA [53]. It is possible that increases in extracellular CRF in the CE are volume-conducted to the BLA [45]. However, this also raises the possibility that our pharmacological manipulations in this study affected not only neurons in the BLA, but neurons in the CE. Because fear learning is expressed, in part, by activity in the CE [54], future experiments should examine the role of CRF in the CE in modulating fear extinction.
The studies described above suggest that CRF enhances learning, possibly by increasing glutamatergic neurotransmission. Since fear extinction is a form of new learning dependent on the BLA, [26–27, 29], one might expect that CRF would enhance fear extinction, rather than impair it. Interestingly, a number of anxiety disorders as well as depression have been associated with increased levels of CRF in the CSF [55–56]. Crucially, these enhanced levels of CRF are also seen in patients with PTSD [35, 57]. It is well known that PTSD patients exhibit deficits in their ability to extinguish learned fear [36–38]. Moreover, PTSD patients display an overgeneralization of fear responses and/or deficits in learning to discriminate threat vs. safety cues [36, 58]. Thus it is possible that the deficits in fear extinction learning seen in PTSD may be directly related to increased CRF levels. Interestingly, increasing CRF within the amygdala and BNST, via deletion of GABA(A)α1 receptors specifically on CRF-containing neurons, had no effect on fear conditioning but impaired fear extinction [59]. The current study is in agreement with these results, suggesting that the CRF system might differentially modulate fear conditioning and fear extinction learning.
It should be noted that animals treated with CRF or CRF(6–33) showed enhanced freezing to the tone during extinction recall compared to the first CS tone during extinction training day. One possible explanation for this result relies on the fact that extinction involves not only the formation of a new memory, but weakening of the original CS-US association [29]. Moreover, fear extinction may involve activation and/or plasticity of inhibitory circuits within the amygdala [60]. If CRF administration leads to hyperexcitability of BLA principal excitatory neurons [47], one consequence would be a reduction in the influence of GABAergic inhibition. This might be manifested by increased freezing during extinction recall, although this possibility remains to be tested. A second possibility is that CRF infusions produced an aversive state, and that higher levels of freezing during the extinction recall test reflect new learning of the association between the context and CRF-induced aversion. However, this would also manifest itself as higher freezing levels during the pre-CS period, which was not seen in any drug group. Relatedly, the disruptive effects of CRF infusions on extinction recall might be explained by state-dependent effects. Although we did not explicitly test this possibility, infusions of the CRF receptor antagonist produced enhanced fear learning.
CRF-mediated hyperexcitability within the amygdala may underlie several anxiety disorders [61]. Here, we found that both endogenous and exogenous increases of CRF within the BLA impaired the consolidation of fear extinction memory, while treatment with CRF receptor antagonists increased fear extinction learning. These data add to our understanding of how CRF receptors contribute to anxiety-related psychological disorders.
Supplementary Material
Highlights.
Fear extinction memory is impaired by increases in endogenous CRF in the BLA
Fear extinction memory is impaired by intra-BLA infusions of CRF
Fear extinction memory is enhanced by intra-BLA infusions of a CRF receptor antagonist
Acknowledgments
This work was supported by the National Institute of Mental Health Grant R15-MH-095032.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- 2.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–327. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 4.Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale W. Corticotropin-releasing factor: Actions on the sympathetic nervous system and metabolism. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 6.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215(1):1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- 7.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 8.Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: Role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 9.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 10.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48(1):1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;45(12):1382–8. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 12.Smoller JW, Yarnaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry. 2005;57(12):1485–92. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaferi A, Pickel VM. Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience. 2009;159:526–539. doi: 10.1016/j.neuroscience.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439.47. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–66. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaada BR. Stimulation and regional ablation of the amygdala complex with reference to functional representation. In: Eleftheriou BE, editor. Neurobiology of the Amygdala. Plenum Press; NewYork: 1972. pp. 205–281. [Google Scholar]
- 19.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 20.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 21.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 22.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. A positron emission tomographic study of symptom provocation in PTSD. Ann N Y Acad Sci. 1997;821:521–523. doi: 10.1111/j.1749-6632.1997.tb48320.x. [DOI] [PubMed] [Google Scholar]
- 23.De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 24.Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: Implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 25.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 26.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 27.Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 28.Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: A cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- 29.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. J Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrichs SC, Vale EA, Lapsansky J, Behan DP, McClure LV, Ling N, De Souza EB, Schulteis G. Enhancement of performance in multiple learning tasks by corticotropin- releasing factor-binding protein ligand inhibitors. Peptides. 1997;18:711–716. doi: 10.1016/s0196-9781(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 34.Isogawa K, Bush DE, LeDoux JE. Contrasting effects of pretraining, posttraining and pretesting infusions of corticotropin-releasing factor into the lateral amygdala: attenuation of fear memory formation but facilitation of its expression. Biol Psychiatry. 2013;73(4):353–9. doi: 10.1016/j.biopsych.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50(4):550–61. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 37.Peri T, Ben-Shakhar G, Orr SP, et al. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 38.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann NY Acad Sci. 2003;1008:112–21. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 39.Behan DP, Linton EA, Lowry PJ. Isolation of the human plasma corticotophin-releasing factor-binding protein. J Endocrinol. 1989;122(1):23–31. doi: 10.1677/joe.0.1220023. [DOI] [PubMed] [Google Scholar]
- 40.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378(6554):284–7. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 42.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-related corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA. 2002;99(21):13908–13. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donatti AF, Leite-Panissi CR. Activation of corticotropin-releasing factor receptors from the basolateral or central amygdala increases the tonic immobility response in guinea pigs: an innate fear behavior. Behav Brain Res. 2011;225(1):23–30. doi: 10.1016/j.bbr.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100(1–2):207–15. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 45.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99(21):13908–13. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333(6051):1903–7. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 47.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Pharmacol Exp Ther. 1992;263:846–858. [PubMed] [Google Scholar]
- 49.Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increase corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675(1–2):297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- 50.Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82(4):751–62. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Blank T, Nijholt I, Vollstaedt S, Spiess J. The corticotropin-releasing factor receptor 1 antagonist CP-154,526 reverses stress-induced learning deficits in mice. Behav Bran Res. 2003;138(2):207–13. doi: 10.1016/s0166-4328(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 52.Uryu K, Okumura T, Shibasaki T, Sakanaka M. Fine structure and possible origins of nerve fibers with corticotropin-releasing factor-like immunoreactivity in the rat central amygdaloid nucleus. Brain Res. 1992;577(1):175–9. doi: 10.1016/0006-8993(92)90554-m. [DOI] [PubMed] [Google Scholar]
- 53.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20(11):517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 54.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–82. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 55.Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 1988;21:76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- 56.Lee R, Geracioti TD, Jr, Kasckow JW, Coccaro EF. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am J Psychiatry. 2005;162(5):995–7. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- 57.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154(5):624–9. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108(1):134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 59.Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci USA. 2012;109(40):16330–5. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25(2):502–6. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor and amygdala plasticity in chronic anxiety. Stress. 2005;8(4):209–19. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.