Abstract
Selective serotonin reuptake inhibitors (SSRIs) are widely prescribed to treat anxiety and depression, yet they paradoxically increase anxiety during initial treatment. Acute administration of these drugs prior to learning can also enhance Pavlovian cued fear conditioning. This potentiation has been previously reported to depend upon the bed nucleus of the stria terminalis (BNST). Here, using temporary inactivation, we confirmed that the BNST is not necessary for the acquisition of cued or contextual fear memory. Systemic administration of the SSRI citalopram prior to fear conditioning led to an upregulation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) in the oval nucleus of the BNST, and a majority of these neurons expressed the 5-HT2C receptor. Finally, local infusions of a 5-HT2C receptor antagonist directly into the oval nucleus of the BNST prevented the fear memory-enhancing effects of citalopram. These findings highlight the ability of the BNST circuitry to be recruited into gating fear and anxiety-like behaviors.
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed to treat anxiety disorders and depression (Kent et al., 1998; van der Kolk et al., 1994). However, they paradoxically increase anxiety in humans when they are given acutely (Mir and Taylor, 1997), and can increase the risk of suicidal ideation (Teicher et al., 1990). Rodent models of anxiety including the elevated plus maze, social interaction task and novelty suppressed feeding task reveal a similar anxiogenic effect of acute SSRI administration (Griebel et al., 1999; Bodnoff et al., 1989; Dekeyne et al., 2000).
Previous research has revealed that acute SSRI administration prior to fear conditioning enhances the consolidation of fear memories (Burghardt et al., 2004; Ravinder et al., 2013). One advantage of using fear conditioning to investigate the actions of SSRIs is that it is a model of emotional learning for which the underlying neural circuitry has been characterized in great detail (Johansen et al,. 2011; Orsini and Maren, 2012; Pape and Pare, 2010). Fear conditioning engages fear circuits as well as mechanisms involved in learning and memory. Moreover, many anxiety disorders in humans can be characterized as abnormalities in the acquisition or extinction of conditioned fear (Grillon, 2002; Milad et al., 2008).
The enhancing effects of SSRIs on fear conditioning appear to involve neural activity within the bed nucleus of the stria terminalis (BNST), as systemic injections or intra-BNST infusions of the SSRI fluoxetine potentiate fear learning (Ravinder et al., 2013). Systemic administration of SSRIs also lead to upregulation of the immediate early gene Arc (activity-regulated cytoskeleton associated protein) in the oval nucleus of the BNST (BNSTov; Ravinder et al., 2013). The BNSTov, which is a subregion of the anterolateral BNST (BNST-AL), is one of a dozen defined cell groups within the BNST (Alheid 2003). In general, the BNST has been implicated in processing both adaptive and pathological anxiety, with the majority of studies focusing on its contribution to an animal’s response to unpredictable stressful events and anxiety (Alheid 2003; Dunn and Williams, 1995).
Lesions of the BNST do not interfere with fear conditioning (LeDoux et al., 1988; Sullivan et al., 2004). Instead, they disrupt the expression of longer “anxiety-like” states (Walker et al., 2003). This has led to the idea that short duration cues (such as a 30 second tone) recruit amygdalar circuits, whereas long-duration cues, including contextual cues, recruit the BNST (Lee and Davis, 1997; Walker e, 2009). However, there is also evidence that BNST activity can modulate fear conditioning even when short duration cues are used. A subset of BNST-AL neurons develops inhibitory responses to a short duration conditioned stimulus (CS), whereas a separate group of neurons in the anteromedial BNST develop positive CS responses (Haufler et al., 2013). As described above, local infusions of SSRIs into the BNST prior to fear conditioning enhance fear memory consolidation (Ravinder et al., 2013).
Systemic injections of SSRIs enhance both the consolidation and the expression of fear responses, and this latter effect is blocked by the co-administration of a 5-HT2C antagonist (Burghardt et al., 2007). Several lines of evidence suggest that 5-HT2C receptors within the BNST might play a role in the fear enhancing effects of SSRIs. Systemic activation of 5-HT2C receptors increases c-fos expression in the BNST as well as anxiety-like behavior (Bagdy et al., 2001; Singewald et al., 2003). Conversely, 5-HT2C knockout mice show decreased anxiety (Heisler et al., 2007). Importantly, 5-HT2C receptor antagonists block the anxogenic effects of different SSRIs, including fluoxetine and citalopram (Bagdy et al., 2001; Dekeyne et al., 2000).
The goal of the present study was to determine if the fear-enhancing effects of SSRI administration depend on 5-HT2C receptors in the BNST. We first confirmed that temporary inactivation of the BNST does not interfere with the acquisition of cued or contextual fear conditioning. We then characterized the neurons in the BNSTov that show upregulation of the immediate-early gene Arc following SSRI administration plus fear conditioning and determined that the majority of these neurons express 5-HT2C receptors. Finally, enhanced fear conditioning by SSRI administration was blocked by local infusions of a 5-HT2C antagonist into the BNSTov. Together, these experiments support the idea that recruitment of BNST activity can modulate the consolidation of fear memories.
Methods
Subjects
Adult male Sprague Dawley rats (Charles River Laboratories; 250–325g) were housed individually with ad libitum access to food and water and maintained on a 12 hour light/dark cycle. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Columbia University’s Animal Care and Use Committee.
Surgery
Rats were anesthetized with a mixture of isoflurane and oxygen and mounted in a stereotaxic apparatus. Betadine was applied to the scalp and a local anesthetic (bupivacaine, s.c.) was injected under the scalp. The scalp was incised and small burr holes were made in the skull to insert cannulas into the BNST (−0.12 AP, +/− 1.5 ML; Paxinos and Watson, 2009). To avoid placing the cannulas within the lateral ventricles, they were inserted at a 10 degree angle. 26-gauge guide cannula (Plastics One) were inserted just dorsal to the BNSTov (−6.0 DV) and cemented, with skull screws, to the skull. Dummy cannulas were inserted to prevent clogging. Rats received an analgesic (carprofen, 5 mg/kg, i.p.) and 5 ml of lactated ringer (s.c.). Animals recovered for one week before behavioral testing.
Behavior
On the first day, rats were habituated to the training context for 20 min. 24 hours later, rats were placed in a rodent conditioning chamber with a metal grid floor (Coulbourn Instruments). In Experiment 1, rats received 5 tone-shock pairings: CS = 5 kHz tone, 80dB, 30 sec; US = 0.5mA shock, 1 sec, tone coterminating with the shock. Five CS-US pairings were given to elicit sufficient contextual fear learning and robust freezing to the CS on testing day. In Experiments 2 and 3, rats received 1 tone-shock pairing such that animals receiving saline injections exhibited 50% freezing to the CS on testing day. This allowed us to measure increased fear learning in citalopram-treated animals, and was consistent with previous research (Ravinder et al., 2013; Burghardt et al., 2004). 24 hours later rats were placed in a different context with a black plexiglass floor washed with peppermint soap, different light placements and with walls made of a different material (either metal or clear plastic). They received 10 or 20 CS tones (30 sec duration; 60–120 sec inter-tone intervals). Animals which were tested for contextual fear conditioning were exposed to the conditioning chamber 24 hours after training for 10 minutes with no CS tones. Behavior was recorded by video camera and analyzed off-line. Time spent freezing to each CS or context (immobility with the exception of breathing) was manually scored for each animal by an observer blind to group assignment. At the end of behavioral experiments, animals were sacrificed by carbon dioxide inhalation, their brains removed and stored in 4% paraformaldehyde in phosphate buffer (PB). Brains were sectioned at a thickness of 100 µm. Nissl staining and light microscopy were used to verify cannula placements within the amygdala. Animals with cannula placements outside the BNST were excluded from analysis.
Drug administration
Experiment 1: 15 min prior to fear conditioning training, animals received bilateral intra-BNST infusions of vehicle (0.5 µL/side; 0.9% sterile saline) or muscimol (4.4 nmol in 0.5 µL/side). This dose has been used previously to temporarily inactivate the BNST (Fendt et al., 2003). Experiment 2: 1 hour prior to fear conditioning training, animals received systemic injections of either 0.9% saline vehicle or citalopram hydrobromide (Sigma Aldrich) dissolved in vehicle (10mg/kg; i.p). Experiment 3: 1 hour prior to fear conditioning training, animals received systemic injections of either 0.9% saline vehicle or citalopram hydrobromide (Sigma Aldrich) dissolved in vehicle (10mg/kg; i.p). 15 minutes prior to fear conditioning training, animals received bilateral intra-BNST infusions of vehicle (40% DMSO and 60% saline solution, 0.25µL), or the 5-HT2C antagonist, RS-102221, 0.5µg per side in 0.25µl of a 40% DMSO and 60% saline solution. Solutions were infused at a rate of 0.1 µL/min through infusion cannula extending 1mm from the tip of the guide cannula that were attached to 1 µL Hamilton syringes with polyethylene tubing. The cannulae were left in place for 2 min after infusion to ensure the entire drug dose was delivered.
5-HT2C receptor and Arc dual-labeling experiments
Animals received systemic injections of either saline or citalopram as described above. 45 minutes after fear conditioning, animals were given an overdose of sodium pentobarbital (100 mg/kg), and then perfused transcardially with 0.9% saline and 4% paraformaldehyde in 0.1M phosphate buffer (PB). Tissue was post-fixed for 4 hours then transferred to a 20% sucrose solution. Tissue was sliced into 80 µM coronal sections using a Vibratome. Every other slice was collected, so there was no need to correct for double counting. 5 or 6 slices per animal were collected, covering the rostro-caudal extent of the BNSTov. Tissue was washed in 0.1M PB 3 times, then in PB with 1% Triton (PBT) 3 times for 5 min each wash. Slices were blocked in 1% bovine serum albumin (BSA; Sigma Fraction V, A-3059) in PBT for 1 hr and incubated for 48 hours at 4 degrees C in primary antibodies in BSA. Primaries used were a mouse monoclonal anti Arc/Arg3.1 antibody (1:250; Santa Cruz Biotechnology, sc-17839) and a goat polyclonal antibody against the 5HT2C receptor (1:1000; Santa Cruz Biotechnology, sc-15081). Slices were washed in PBT and incubated in secondary antibodies in PBT for 1 hr. Secondary antibodies used were donkey anti-mouse Alexa Fluor-488, and donkey anti-goat Alexa-594 (1:200; Life Technologies). Slices were washed in PBS, mounted on slides and coverslipped.
Microscopy
Double-labeling was assessed by confocal microscopy using a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss, Thornwood, NY) with LSM 510-META scanning confocal attachments. Stacked images were collected as 1 µm multitract optical sections. LSM 510 software (Carl Zeiss) was used to visualize doubly-labeled fluorescent slices at 25× and to capture images. Manual counting was performed on images of the BNSTov; 2 samples per oval nucleus were counted and summed together.
Specificity of antibodies
We used a goat polyclonal anti-5-HT2C receptor antibody (1:1000, Santa Cruz Biotechnology; sc-15081) that is directed toward a 19 amino acid sequence at the Nterminus of the receptor. This specific antibody does not react with the closely related 5-HT2A receptors, as 5HT2C knockout animals (in which the 5-HT2A receptors are intact) show no immunoreactivity of this antibody while 5-HT2A immunoreactivity is unaltered (Bubar et al., 2005). Primary antibody only and secondary antibody only controls contained a complete absence of specific labeling of cells. Arc protein expression was revealed using the anti-Arc/Arg3.1 antibody (mouse monoclonal; Santa Cruz Biotechnology, sc-17839) which has been used extensively to label Arc protein in the amygdala and BNST (Ploski et al., 2008; Ravinder et al., 2013).
Results
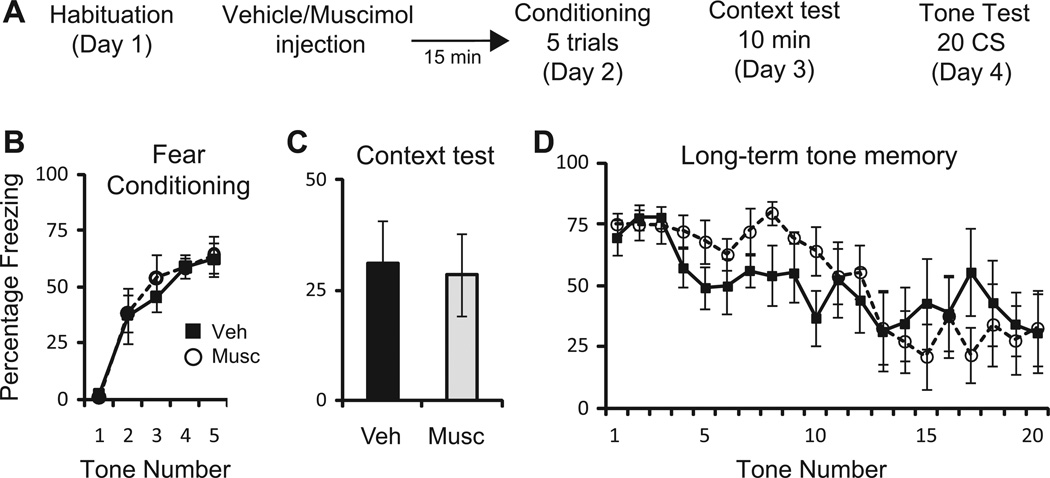
To confirm that the BNST does not normally contribute to the acquisition of either context or cued fear conditioning, rats were prepared with bilateral cannulas aimed at the BNST. After recovery, rats were habituated to the training context. 24 hours later, rats received local infusions of saline vehicle (0.5 µL/side; n=7) or muscimol (4.4 nmol in 0.5 µL/side; n=6). 30 minutes later, rats were presented with 5 CS tones each coterminating with a 1 sec US. A oneway repeated measures ANOVA revealed a main effect of tone (F(4,44) = 27.1, p<0.001); but no effect of drug, (F(1,11) = 0.89; p=0.77) or interaction (F(4,44) = 0.16; p=0.96). 24 hours later, animals were placed in the training context for 10 minutes. An unpaired Student’s t-test revealed no significant difference in the amount of freezing to the training context 24 hours after training (p=0.86). 48 hours after training, animals were placed in a novel context and presented with 20 CS tones. A one-way repeated measures ANOVA revealed a main effect of tone (F(19,209) = 5.52, p<0.001); but no effect of drug, (F(1,11) = 0.15; p=0.7) or interaction (F(19,209) = 1.36; p=0.15). Thus, in agreement with previous lesion studies (LeDoux et al., 1988; Duvarci et al., 2009), temporary inactivation of the BNST prior to training had no effect on the acquisition of cued or contextual fear memories (Figure 1).
Figure 1.
Temporary inactivation of the BNST does not impair fear conditioning. A: Schematic of behavioral protocol. B: Mean ± SE percent freezing to 5 CS tones during fear conditioning in rats receiving saline vehicle (0.5 µL; n=7) or muscimol (4.4 nmol in 0.5 µL; n=6) 15 min before fear conditioning. C: Mean ± SE percent freezing to the training context for 10 min, 24 hours later. D: Mean ± SE percent freezing to 20 CS tones 48 hours after training.
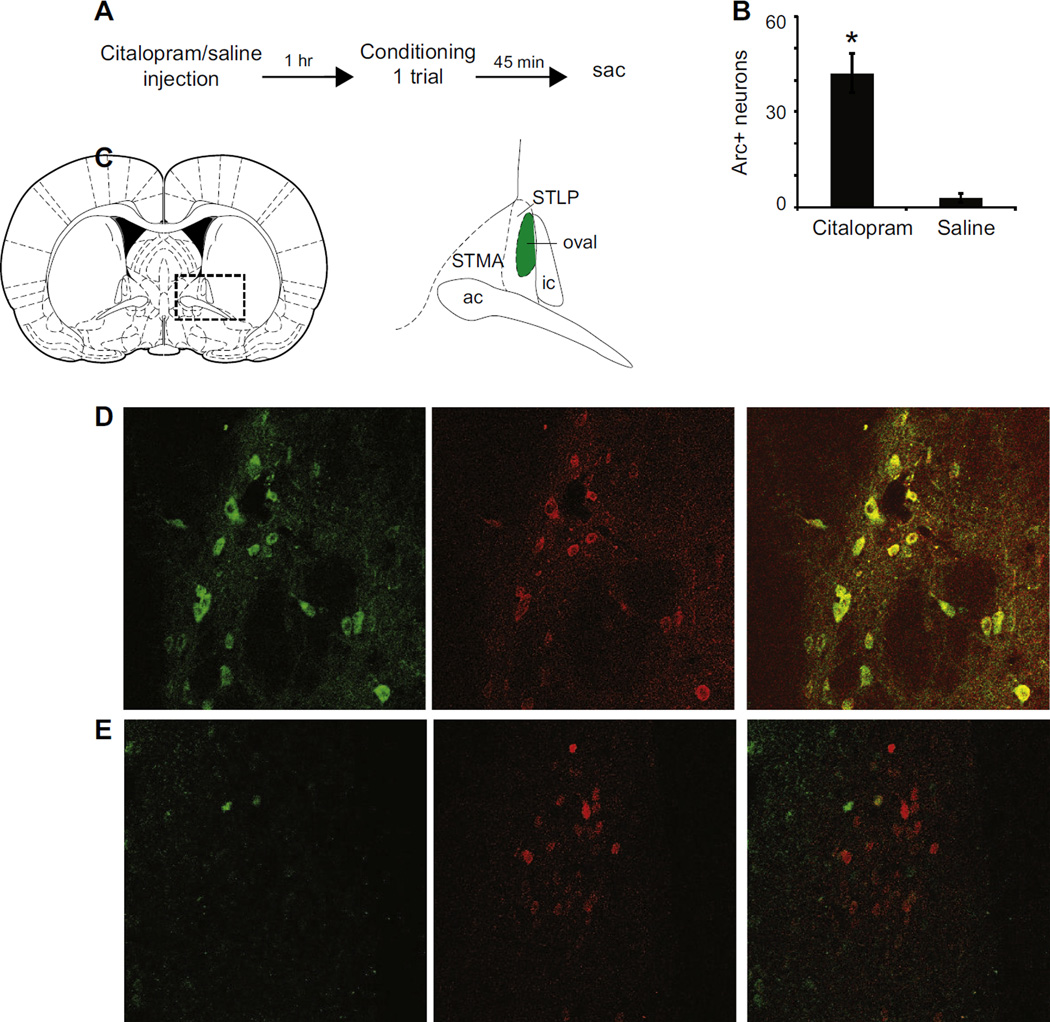
Acute administration of the SSRI fluoxetine enhances fear conditioning and Arc protein expression in the BNST (Burghardt et al., 2004; Ravinder et al., 2013). The SSRI citalopram also inhibits reuptake of serotonin from the synapse where it can interact with serotonin receptors. Thus, we determined whether neurons within the BNSTov showed increased Arc expression following citalopram administration and fear conditioning, and if those neurons also expressed the 5-HT2C receptor. 24 hours after habituation to the training context, animals were administered citalopram (10mg/kg, i.p. in saline) or saline. 1 hour later they received 1 CS-US pairing. 45 min later, they were perfused and their brains collected for immunohistochemistry (Figure 2A). Animals receiving citalopram (n=6) before conditioning had significantly higher levels of Arc protein expression than those receiving saline (n=5; p<0.001; Figure 2B). We then determined whether Arc-expressing neurons also expressed 5-HT2C receptors. The percentage of Arc-expressing BNSTov neurons which co-express 5-HT2C receptors in animals receiving citalopram was 74.3 ± 4.3% (Figure 2C–E).
Figure 2.
Systemic citalopram injections increase Arc expression in 5-HT2C containing neurons of the BNSTov. A: Schematic of behavioral protocol. B: Quantification of Arc-expressing cells in the BNSTov following injection of citalopram (n=6) or saline (n=5) followed by fear conditioning, *P<0.001. C: Left, the dotted box shows the location of the BNST at 0.00 Bregma (left, Paxinos and Watson, 2009). Schematic of the principal anterior dorsal BNST subregions (right). ac: anterior commissure, ic: internal capsule, STMA: BNST medial-anterior, STLP: BNST lateral-posterior. All images and cell counts were taken from the shaded oval nucleus. D: Colocalization of Arc-expressing cells (green) and 5-HT2C receptor-expressing cells (red). Merging of the green and red channels reveals overlap: 74.3 ± 4.3% of Arc-positive cells expressed 5-HT2C receptors. E: Animals receiving saline injections have few Arc-expressing cells, but many 5-HT2C receptor-expressing cells (red) and little overlap.
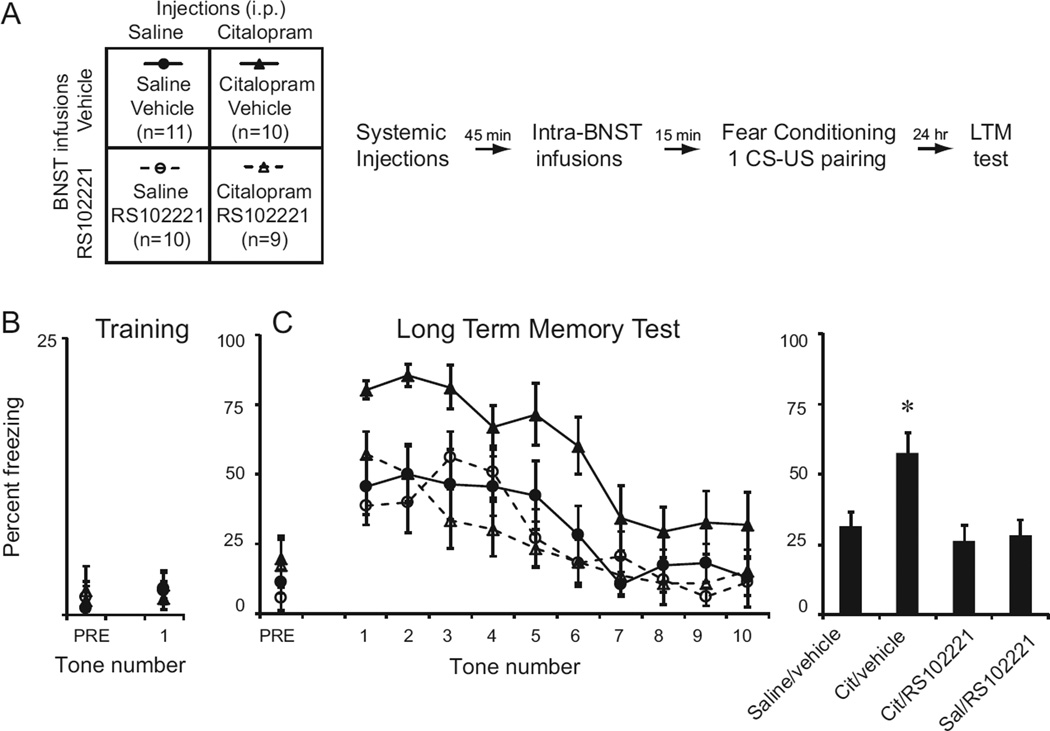
To determine whether 5-HT2C receptors in the BNSTov mediate the enhancing effects of SSRIs on fear conditioning, we implanted bilateral cannulas aimed at the BNSTov. A week later, animals were habituated to the training context. 24 hours later, they were assigned to one of four groups (Figure 3A). Animals received a systemic injection of either saline vehicle or citalopram dissolved in saline (10mg/kg, i.p.) 1 hour prior to fear conditioning. 15 minutes prior to fear conditioning, animals received infusions of either vehicle (0.25µL; 80% saline, 20% DMSO) or the 5-HT2C receptor antagonist RS102221 (1 µg/side dissolved in 0.25µL 80% saline, 20% DMSO) such that four groups of animals were established (Figure 3A). Rats were then conditioned with one CS-US pairing (Figure 3B). A two-way ANOVA found no significant effects of the injection, BNST infusion or interaction on freezing during the 30 seconds before the CS (pre-CS) (Injection: F (1,36)=0.21; p=0.65; Infusion: F(1,36) = 0.48; p=0.49; Interaction: F(1,36) = 0.004; p=0.95) or to freezing to the CS (Injection: F(1,36) = 0.02; p=0.89; Infusion: F(1,36)=0.2; p=0.66; Interaction: F(1,36) = 0.41; p = 0.53). 24 hours later, animals received 10 CS tones (Figure 3C). A two-way repeated measures ANOVA revealed a significant interaction between systemic injections and local infusions (F(1,36)=4.45; p<0.05). Averaging freezing scores across all 10 tones, a one-way ANOVA across all four groups found a significant effect of group (F(1,36) = 4.96; p<0.01), with post-hoc tests revealing that the group receiving systemic citalopram and local infusions of saline showed significantly higher freezing levels than all other groups (p<0.05 for all comparisons; Bonferroni post-hoc tests). Thus, systemic injections of the SSRI citalopram enhance fear conditioning, which can be blocked by local infusions of 5-HT2C antagonists into the BNST.
Figure 3.
Intra-BNST infusions of the 5-HT2C receptor antagonist RS-102221 reduce the enhancement of fear learning produced by systemic injections of the SSRI citalopram. A: Schematic of behavioral protocol. Animals received i.p. injections of either 0.9% saline or 10mg/kg citalopram 1 hr prior to fear conditioning and then intra-BNST infusions of vehicle or RS-102221 (1 µg/side) 15 min prior to fear conditioning. 24 hr later they were tested for long term memory (LTM). B: Mean ± SE percent freezing to the pre CS period (30 sec; PRE) and to the CS during fear conditioning. C: Mean ± SE percent freezing to the pre-CS and 10 CS tones 24 hr after training (left). Mean ± SE percent freezing of each group averaged across all 10 tones (right), *P<0.05.
Discussion
Consistent with earlier reports (LeDoux et al., 1988; Duvarci et al., 2009), we found that temporary inactivation of the BNST did not affect the acquisition of cued or context fear suggesting that the BNST contributes to the expression rather than the acquisition of contextual fear memories. Systemic injections of the SSRI citalopram did, however, enhance fear learning when given prior to fear conditioning. Citalopram also upregulated the expression of Arc protein within the BNSTov, and a majority of those cells (74%) expressed the 5-HT2C receptor, suggesting a role for this receptor in the behavioral effects of citalopram. Accordingly, intra-BNSTov infusion of a 5-HT2C receptor antagonist eliminated the effects of systemic citalopram injections. Together these results suggest that citalopram acts on a subset of neurons expressing the 5-HT2C receptor within the BNSTov to enhance fear memory consolidation.
Studies using electrolytic or neurotoxic lesions of the BNST have demonstrated that the BNST is not necessary for cued fear conditioning (LeDoux et al., 1988; Gewirtz et al.,1998; Sullivan et al., 2004; Duvarci et al., 2009). The timing of irreversible lesions or reversible inactivation of the BNST can reveal the contribution of this structure to either the acquisition and/or expression of contextual fear. If lesions are made before training (Duvarci et al., 2009; LeDoux et al., 1988), and contextual fear conditioning is impaired, it is unclear whether BNST activity was necessary for acquisition or expression of contextual fear. However if lesions or reversible inactivation are performed after training (Resstel et al., 2008; Sullivan et al,. 2004), the reduction in the amount of freezing to the conditioning context suggests that BNST activity is necessary for the expression of fear memory. Here, we inactivated the BNST before training; the animals were tested drug free. Since there was no difference in freezing behavior on testing day, this suggests that BNST activity during training is not necessary for the learning or acquisition of contextual fear memory. However, it should be noted that more recent studies in which specific regions or cell types of the BNST are manipulated reveal that the BNST is functionally heterogeneous (Jennings et al., 2013; Kim et al., 2013). When activity of individual neurons within the BNST-AL is recorded during fear conditioning, a subset of neurons acquire inhibitory responses to a short-duration CS. These responses persist when animals are tested days later, at which time BNST-AM neurons have acquired excitatory responses to the CS (Haufler et al., 2013). Moreover, there are intrinsic BNST networks which could be disrupted by large infusions of muscimol (Turesson et al., 2013). Thus, while the data presented here are in agreement with past lesion and inactivation studies, future work should take into account the functional differences between adjacent regions of the BNST by making more targeted manipulations.
Acute SSRI administration before fear conditioning led to an upregulation of Arc protein in the BNSTov. The majority of these neurons express the 5-HT2C receptor. Within the BNST-AL, serotonin elicits complex postsynaptic responses (Guo et al., 2009), suggesting the existence of multiple networks within this structure. Although the primary response to serotonin is inhibitory within the anterior BNST, one specific set of neurons (Type III) containing 5-HT2C receptors depolarizes in response to serotonin (Guo et al., 2009). The majority of neurons activated by SSRIs and fear conditioning co-expressed 5-HT2C receptors, suggesting that SSRIs might preferentially act on this subset of BNSTov neurons. Interestingly, the Type III neurons containing 5-HT2C receptors also express the neuropeptide corticotropin-releasing factor (CRF; Dabrowska et al., 2011). CRF neurons within the BNST pay a major role in an animal’s response to stressors (Lee and Davis, 1997; Sahuque et al., 2006; Dabrowska et al., 2013). Evidence suggests that activation of 5-HT2C receptors and CRF receptors in the BNST are both anxiogenic (Gibson et al., 1994; Campbell and Merchant 2003; Lee and Davis, 1997). Thus future work might determine whether manipulations of the BNST CRF system might mimic the effects of citalopram on fear conditioning.
There is evidence that SSRIs alone (in the absence of conditioning) can enhance activation of the BNST (Singewald et al., 2003). This could suggest that citalopram enhances baseline anxiety during conditioning, rather than recruiting the BNST into the fear learning circuit. However, we did not see increases in freezing behavior in these animals during the pre-CS or the CS period during training, nor during the pre-CS period during testing. This suggests that citalopram did not indiscriminately increase animals’ baseline anxiety. Nevertheless, future studies should determine the extent to which active neurons within the BNST during acquisition become a permanent part of the memory trace by addressing whether the BNST is also active during memory recall.
It is unclear why citalopram might preferentially act on a specific BNST circuit, if the majority of BNST neurons express 5-HT receptors. However, it should be noted that there is a large body of literature demonstrating that SSRIs interact with specific subtypes of 5-HT receptors as well as non-5-HT receptors and ion channels (for review see Bianchi 2008). Depending on the type of SSRI administered, the noradrenergic, dopaminergic and opioid systems can be modulated (Bymaster et al., 2002; Goodnick and Goldstein, 1998). These interactions suggest that SSRIs may differentially modulate fear learning and other behavioral paradigms.
Another possibility is that citalopram affects the activity of multiple BNST circuits, but only a subset of those interact with amygdalar circuitry. The central nucleus of the amygdala (CE) and the BNST are reciprocally connected to each other (Dong et al., 2001; Kretteck and Price, 1978). They share almost identical patterns of efferent targets including brainstem areas involved in responses to fear and anxiety, hypothalamic areas involved in autonomic motivation, the parabrachial nucleus and the substantia innominata (Radley et al., 2009; Dong et al., 2001; Petrovich and Swanson, 1999). BNST projections to the CE mostly originate in the BNST-AL and BNST-AM divisions (Sun and Cassell 1993; Dong et al., 2001; Dong and Swanson 2006). The current model of fear conditioning posits that short duration cues recruit CE circuits whereas long-duration cues recruit the BNST which then suppresses the CE (Lee and Davis, 1997; Walker et al., 2009). For there to be a seamless transition from the early to the late components of a sustained threat response, there must be some interaction and coordination between the two structures. The experiments here suggest that modulation of BNST activity by SSRI administration might alter the balance of this interaction. Recruitment of BNST networks during fear conditioning to short-duration CSs could then modulate fear memory consolidation. Overall, our findings suggest that the BNST can modulate fear memory formation and consolidation, specifically during acute SSRI administration. Future studies will be needed to address the precise role of specific subpopulations of BNST neurons in this effect and the connections between the BNST and amygdalar nuclei.
Figure 4.
Histological verification of cannula placements.
Highlights.
BNST inactivation does not impair acquisition of cued or context fear conditioning
SSRI injections enhance cued fear conditioning and Arc expression in the BNST
The majority of these Arc expressing neurons express 5-HT2C receptors
BNST infusions of 5-HT2C receptor antagonists block the effects of SSRIs on cued fear
Acknowledgments
This work was supported by NIH grant MH-095032-01 to E.P.B and by a grant to Barnard College from the Undergraduate Science Education Program of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Bianchi MT. Non-serotonin anti-depressant actions: direct ion channel modulation by SSRIs and the concept of single agent poly-pharmacy. Med Hypotheses. 2008;70:951–956. doi: 10.1016/j.mehy.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl) 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Seitz PK, Thomas ML, Cunningham KA. Validation of a selective serotonin 5-HT(2C) receptor antibody for utilization in fluorescence immunohistochemistry studies. Brain Res. 2005;1063(2):105–113. doi: 10.1016/j.brainres.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Denorme B, Monneyron S, Millan MJ. Citalopram reduces social interaction in rats by activation of serotonin (5-HT)(2C) receptors. Neuropharmacology. 2000;39:1114–1117. doi: 10.1016/s0028-3908(99)00268-3. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic and behavioral aspects of energy balance. J Comp Neurol. 2006;494(1):142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36(9):1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci. 2013;7:156. doi: 10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JD, Williams TJ. Cardiovascular responses to electrical stimulation of the bed nucleus of the stria terminalis. J Comp Neurol. 1995;352:227–234. doi: 10.1002/cne.903520206. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Pare D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23(1):23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(4):625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Barnfield AM, Curzon G. Evidence that mCPP-induced anxiety in the plus-maze is mediated by postsynaptic 5-HT2C receptors but not by sympathomimetic effects. Neuropharmacology. 1994;33:457–465. doi: 10.1016/0028-3908(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders—I. Basic pharmacology. J Psychopharmacol. 1998;12(3 Suppl B):S5–S20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- Griebel G, Cohen C, Perrault G, Sanger DJ. Behavioral effects of acute and chronic fluoxetine in Wistar-Kyoto rats. Physiol Behav. 1999;67:315–320. doi: 10.1016/s0031-9384(98)00298-4. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20(11):633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JM, Coplan JD, Gorman JM. Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiatry. 1998;44:812–824. doi: 10.1016/s0006-3223(98)00210-8. [DOI] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioral state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178(2):255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17(16):6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir S, Taylor D. The adverse effects of antidepressants. Curr Opin Psychiatry. 1997;10:88–94. [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 2009. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravinder S, Burghardt NS, Brodsky R, Bauer EP, Chattarji S. A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Transl Psychiatry. 2013;3:e209. doi: 10.1038/tp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Alves FH, Reis DG, Crestani CC, Correa FM, Guimaraes FS. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience. 2008;154(3):869–876. doi: 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacol (Berl) 2006;186(1):122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330(3):381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Glod C, Cole JO. Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry. 1990;147:207–210. doi: 10.1176/ajp.147.2.207. [DOI] [PubMed] [Google Scholar]
- Turesson HK, Rodriguez-Sierra OE, Pare D. Intrinsic connections in the anterior part of the bed nucleus of the stria terminalis. J Neurophysiol. 2013;109(10):2438–2450. doi: 10.1152/jn.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk BA, Dreyfuss D, Michaels M, Shera D, Berkowitz R, Fisler R, Saxe G. Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry. 1994;55:517–522. [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prof Neuropsychopharamcol Biol Psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]