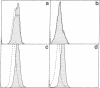
Abstract
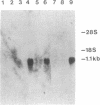
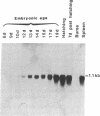
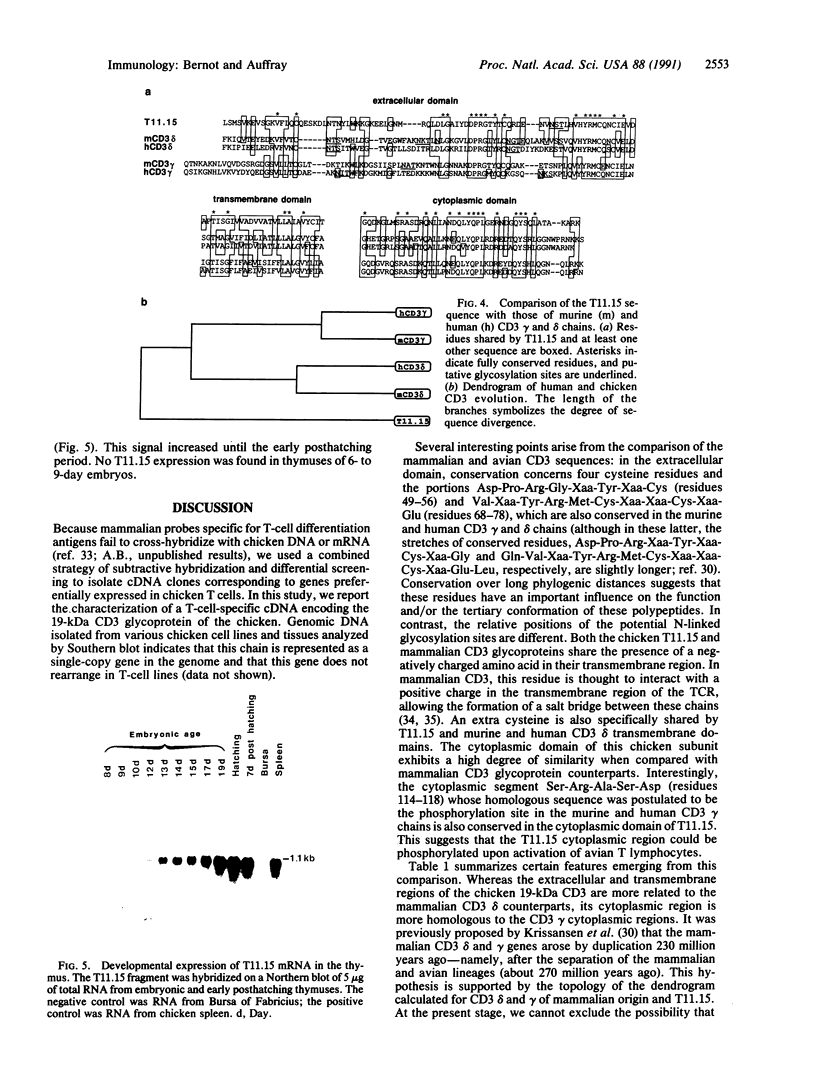
The structure of a chicken CD3 chain has been determined by isolating a cDNA clone (T11.15) that encodes a 175-amino-acid-long protein, including the NH2-terminal signal peptide. In Northern blot experiments, the earliest expression of the T11.15 transcript was detected in the thymus at embryonic day 10 (i.e., 1 day after cytoplasmic expression of a CD3 epitope recognized by a specific monoclonal antibody [CT3; Chen, C.L.H., Ager, L.L., Gartland, G.L. & Cooper, M.D. (1986) J. Exp. Med. 164, 375-380], but 2 days before the appearance of clonotypic components of the T-cell antigen receptor). Sequence similarity of this chicken protein sequence compared with that of the known mammalian CD3 gamma and delta polypeptides was 36-39% and 39-40%, respectively. Amino acid sequence alignments between avian and mammalian CD3 revealed maximum conservation in the transmembrane and cytoplasmic domains as well as in the regions flanking the cysteine residues in the extracellular domain, underlining their functional importance. The difficulty of unambiguously assigning this chain to a single mammalian CD3 subunit on the basis of sequence comparison raises the possibility that this polypeptide represents a derivative from an ancestral form of the gamma and delta chains. It is thus possible that a single chain may play the role of both CD3 gamma and delta subunits in the chicken CD3 complex or, alternatively, that gene duplications occurred independently in the avian and mammalian lineages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Cantrell D. A. Kinases and phosphatases in T-cell activation. Immunol Today. 1989 Jun;10(6):200–205. doi: 10.1016/0167-5699(89)90325-3. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Bonifacino J. S., Samelson L. E., Klausner R. D. Disulfide linkage of the zeta and eta chains of the T cell receptor. Possible identification of two structural classes of receptors. J Biol Chem. 1988 Jul 15;263(20):9874–9878. [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. H., Cooper M. D. Ontogeny of T cell receptors in the chicken thymus. J Immunol. 1990 Feb 15;144(4):1161–1168. [PubMed] [Google Scholar]
- Chen C. L., Ager L. L., Gartland G. L., Cooper M. D. Identification of a T3/T cell receptor complex in chickens. J Exp Med. 1986 Jul 1;164(1):375–380. doi: 10.1084/jem.164.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Cihak J., Lösch U., Cooper M. D. Differential expression of two T cell receptors, TcR1 and TcR2, on chicken lymphocytes. Eur J Immunol. 1988 Apr;18(4):539–543. doi: 10.1002/eji.1830180408. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Clayton L. K., Howard F. D., Koyasu S., Sieh M., Steinbrich R., Tarr G. E., Reinherz E. L. Molecular cloning of the CD3 eta subunit identifies a CD3 zeta-related product in thymus-derived cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3319–3323. doi: 10.1073/pnas.87.9.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S., Banting G. S., Goodfellow P. N., Owen M. J. Surface expression of the T cell receptor complex requires charged residues within the alpha chain transmembrane region. Eur J Immunol. 1989 Feb;19(2):335–339. doi: 10.1002/eji.1830190218. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. Use of statistical criteria for screening potential homologies in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):203–213. doi: 10.1093/nar/12.1part1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Fink P. J., Crumpton M. J. Molecular cloning of the cDNA encoding the T3 gamma subunit of the mouse T3/T cell antigen receptor complex. J Immunol. 1987 May 15;138(10):3513–3518. [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Verbi W., Crumpton M. J. Primary structure of the T3 gamma subunit of the T3/T cell antigen receptor complex deduced from cDNA sequences: evolution of the T3 gamma and delta subunits. EMBO J. 1986 Aug;5(8):1799–1808. doi: 10.1002/j.1460-2075.1986.tb04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Bernot A., Béhar G., Chaussé A. M., Gastinel L. N., Guillemot F., Park I., Thoraval P., Zoorob R., Auffray C. Molecular genetics of the chicken MHC: current status and evolutionary aspects. Immunol Rev. 1990 Feb;113:119–145. doi: 10.1111/j.1600-065x.1990.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Morley B. J., Chin K. N., Newton M. E., Weiss A. The lysine residue in the membrane-spanning domain of the beta chain is necessary for cell surface expression of the T cell antigen receptor. J Exp Med. 1988 Dec 1;168(6):1971–1978. doi: 10.1084/jem.168.6.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R. Molecular biology and function of CD4 and CD8. Adv Immunol. 1989;44:265–311. doi: 10.1016/s0065-2776(08)60644-6. [DOI] [PubMed] [Google Scholar]
- Rhyner T. A., Biguet N. F., Berrard S., Borbély A. A., Mallet J. An efficient approach for the selective isolation of specific transcripts from complex brain mRNA populations. J Neurosci Res. 1986;16(1):167–181. doi: 10.1002/jnr.490160116. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Sowder J. T., Chen C. L., Ager L. L., Chan M. M., Cooper M. D. A large subpopulation of avian T cells express a homologue of the mammalian T gamma/delta receptor. J Exp Med. 1988 Feb 1;167(2):315–322. doi: 10.1084/jem.167.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transy C., Moingeon P. E., Marshall B., Stebbins C., Reinherz E. L. Most anti-human CD3 monoclonal antibodies are directed to the CD3 epsilon subunit. Eur J Immunol. 1989 May;19(5):947–950. doi: 10.1002/eji.1830190525. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Baniyash M., Hou D., Samelson L. E., Burgess W. H., Klausner R. D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988 Feb 26;239(4843):1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Hou D., Orloff D. G., Modi W. S., Seuanez H., O'Brien S. J., Klausner R. D. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., Georgopoulos K., Shepley B. A., Orkin S., Terhorst C. Exon/intron organization of the genes coding for the delta chains of the human and murine T-cell receptor/T3 complex. Proc Natl Acad Sci U S A. 1986 May;83(9):2944–2948. doi: 10.1073/pnas.83.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Borst J., Coligan J. E., Markham A. F., Orkin S., Terhorst C. Isolation of cDNA clones encoding the 20K T3 glycoprotein of human T-cell receptor complex. 1984 Nov 29-Dec 5Nature. 312(5993):413–418. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]