Abstract
Background
Dystrophic calcifications may occur in patients with J uvenile Idiopathic Inflammatory Myopathy (JIIM) as well as other connective tissue and metabolic diseases, but a reliable method of measuring the volume of these calcifications has not been established. The purpose of this study is to determine the feasibility of low dose, limited slice, Computed Tomography (CT) to measure objectively in-situ calcification volumes in patients with JIIM over time.
Methods
Ten JIIM patients (eight JDM, two Overlap) with calcifications were prospectively recruited over a 2-year period to undergo two limited, low dose, four-slice CT scans. Calculation of the volume of calcifications used a CT post processing workstation. Additional patient data included: Disease Activity Scores (DAS), Childhood Myositis Assessment Scale (CMAS), myositis specific antibodies (MSA), and the TNFα-308 promoter region A/G polymorphism. Statistical analysis utilized the Pearson correlation coefficient, the paired t-test and descriptive statistics.
Results
Ten JIIM, mean age 14.54 ± 4.54 years, had a duration of untreated disease of 8.68 ± 5.65 months MSA status: U1RNP (1), PM-Scl (1), Ro (1, 4 indeterminate), p155/140 (2), MJ (3), Mi-2 indeterminate (1), negative (3). 4/8 JDM (50%) were TNF-α-308 A+. Overall, the calcification volumes tended to decrease from the first to the second CT study by 0.5 cm3 (from 2.79 ± 1.98 cm3 to 2.29 ± 2.25 cm3). The average effective radiation dose was 0.007 ± 0.002, 0.010 ± 0.005, and 0.245 mSv for the upper extremity, lower extremity and chest, respectively (compared to a standard chest x-ray-- 0.02mSV effective dosage).
Conclusion
We conclude: 1) the limited low dose CT technique provides objective data about volume of the calcifications in JIIM; 2) measuring the volume of calcifications in an extremity is associated with minimal radiation exposure; 3) This method may be useful to evaluate the efficacy of therapies for JIIM dystrophic calcification.
Keywords: Computed Tomography (CT), Calcification volume, Juvenile idiopathic inflammatory myopathy, Overlap syndrome, Calcification
Background
In children with JIIM, such as Juvenile Dermatomyositis (JDM) and Overlap Syndrome, dystrophic calcifications are a common and debilitating complication. The reported calcifications in JDM range from 71% [1] to 8% [2] with 40% most frequently cited [3]. These calcifications usually occur in children with chronic inflammation and hypoxia associated with JIIM, which includes JDM, Polymyositis and Overlap Syndromes as well as in patients with other rheumatic diseases such as Scleroderma [4] and Systemic Lupus Erythematosus [5, 6].
Plain radiography is effective for the detection of calcinosis and the categorization of morphological patterns of calcification [7]. Although radiography is recommended for the initial imaging of calcinosis, it fails to evaluate objectively the volume of calcifications. Case reports have employed different types of whole body scans (scintigraphy using Technetium methylene diphosphonate (Tc-99 m MDP) and Tc-99 m pyrophosphate and Strontium nitrate) in an attempt to identify the location of the calcifications and to provide a quantifiable assessment of their extent, as well as to develop a method to monitor the child’s therapeutic response [8]. Scintigraphic evaluation using Tc-99 m MDP can effectively delineate sites of dystrophic calcifications in JDM and it is more sensitive in detecting visceral calcifications than plain radiographs [9]. However, scintigraphy has failed to provide a quantitative estimation of the volume of the calcification. In contrast, micro CT and synchrotron x-ray diffraction studies of calcified deposit samples from four children with the diagnosis of JDM characterized the microstructure of calcinosis, and demonstrated excellent sensitivity with respect to quantitation of amount and spatial distribution of minerals in these calcifications samples [10]. These studies suggested that CT could be used to measure the calcifications occurring in the soft tissues of children with JIIM, as this method had been effective in the experimental mouse model [11].
The purpose of this pilot study was to determine the feasibility of the use of low dose, limited slice CT as an objective measure of in-situ calcification volume, over time, in patients with JIIM.
Methods
Patient population
Approval was obtained from the Ann & Robert H. Lurie Children’s Hospital of Chicago Institutional Review Board to perform this prospective study (IRB#2008-13316). Inclusion criteria for study enrollment consisted of a diagnosis of JIIM and documentation of moderate to severe calcifications as determined by the assessor on clinical evaluation. One hundred and fifty JIIM patients were followed in our clinic during the time of the study. Of those, 14 (9.3%) had moderate to severe calcification; ten of these children were available for study; they gave their consent and were enrolled in the study. Eight of these children had severe calcifications and fulfilled the Peter and Bohan [12] criteria for definite/possible JDM and two of the patients had Overlap Syndrome and moderate calcifications. The duration of untreated disease was given by the patient's family. Each of the ten cases had two scans over a 2 year period. For consistency purposes, a single individual, the principal investigator (PI), marked the specific accessible area of the calcification to be scanned. The PI performed a complete physical examination; anatomic landmarks of the area were noted as a reference point for future scans.
Laboratory assessment
TNF-α-308 promoter polymorphism
The A/G polymorphism in the −308 promoter region was determined by PCR, following established methodology [13].
Myositis Specific Antibody (MSA)
Each patient’s sera was analyzed at diagnosis and periodically thereafter, as new antibodies were recognized, by the Oklahoma Medical Research Foundation Clinical Immunology Laboratory, following methods previously outlined [14, 15].
Clinical assessment
Disease Activity Scores (DAS)
The patient’s status with respect to 1) total DAS score; 2) skin involvement (DAS-S) as well as, 3) muscle strength and endurance (DAS-M) was assessed at the time of every visit according to a standard protocol [16].
The Childhood Myositis Assessment Score (CMAS)
An estimate of performance was obtained at every visit by a physical therapist, who was an independent observer [17].
Imaging of calcifications
A limited four-slice CT was performed on a Siemens Somatom Sensation 64 CT scanner in the area of greatest calcium burden and utilized 100kVp, 100 mAs, 3 mm reconstructed slice thickness. Only the limb of interest was included in the scan field of view. For example, examination of the arm was performed in an image “arms-over-head” stance instead of alongside of the body to limit radiation exposure of the torso. The area of soft tissue calcifications was determined by thresholding the calcifications from the range of Hounsfield units of the calcifications (114–3017 Hounsfield units) using software on a Siemens CT workstation (MMWP, Siemens Medical Solutions, Malvern, PA). No sedation was required. There was a mean interval of 7.18 ± 2.33 months between the first scan and second scans. The computed tomography dose index (CTDI) and the dose length product (DLP) were reported by the scanner. The DLP was used to estimate the patient effective dose using published methods [18, 19] for the chest and lower extremities. Due to a paucity of data on the subject, estimates of the upper extremity effective dose were performed by a physicist based on published conversion factors for lower extremities [18, 19].
Statistical analysis
Presentation of demographic and baseline characteristics used descriptive statistics. A paired t-test analyzed volume changes of calcifications in JIIM patients over time. Pearson correlation coefficient was used to determine the correlation between change in calcification volume and duration of active disease.
Results
Demographics and disease state
The demographics of the ten patients enrolled in this proof of principle study are presented in Table 1. The patients had active inflammatory disease for a mean of 8.68 ± 5.65 months. At the time of both scans, the patients still displayed symptoms of moderately active JIIM. Their DAS-S and DAS-M at the first scan were 6 ± 2.3 and 3 ± 3.7 respectively; (maximal DAS-M = 11, maximal DAS-S = 9; normal = 0 for both DAS-S and DAS-M) with a CMAS of 43 ± 7.4 (normal CMAS = 52). At the time of the second scan, their disease activity demonstrated very little improvement (DAS-S = 5 ± 2.2, DAS-M = 3 ± 2.7, CMAS = 44 ± 9.5). Two of the ten patients had Overlap Syndrome, one each positive for either Pm/SCL or U1RNP indeterminate. The remainder of the group was classified as JDM (4 Ro indeterminate; 1 Mi-2 indeterminate; three MJ positive; two p155/140 positive, one Ro positive; three negative for MSA). Of the ten subjects, eight had JDM and 4/8 (50%) were positive for TNF-α-308 GA polymorphism, the rest were GG, which was consistent with our previous findings [20]. Before entering the study, the patients had been given a variety of medications including; methotrexate (n = 7), hydroxychloroquine (n = 6), cyclosporine (n = 4), mycophenolate mofetil (MMF) (n = 4), prednisone (n = 6), intravenous methylprednisolone (n = 2) and intravenous gamma globulin (IVIG) (n = 2). During the study, the following medications were added: prednisone (n = 2), intravenous methylprednisolone (n = 3) and MMF (n = 3). All the children with calcifications were placed on at least two medications, but only five had been given a high dose intermittent pulse methylprednisolone. By end of the study, a total of six children had been given MMF, which did improve their rash [21]. Of note, the four patients for whom cyclosporine was proscribed stated that they were non-compliant with respect to the cyclosporine and had low serum levels of the drug.
Table 1.
Clinical features of patients with juvenile idiopathic inflammatory myopathy
| Gender | Diagnosis | Duration of untreated disease (DUD) (mo) | Location of calcification | MSA status | TNF-α-308 | Age at scan 1 (years) | Age at scan 2 (years) | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | F | JDM | 2.00 | Arm | Ro indeterminate, MJ+ | GG | 11.25 | 11.71 |
| Case 2 | M | JDM | 2.99 | Forearm | Ro indeterminate, MJ+ | GG | 13.94 | 14.43 |
| Case 3 | F | JDM | 2.00 | Forearm | Ro indeterminate, p155/140+ | GA | 10.93 | 11.43 |
| Case 4 | M | JDM | 0.36 | Tibia | Mi-2 indeterminate | GA | 16.32 | 16.86 |
| Case 5 | F | JDM | 14.32 | Knee | Negative | GG | 8.89 | 9.48 |
| Case 6 | F | JDM | 16.99 | Arm | Negative | GG | 20.58 | 21.17 |
| Case 7 | F | OVERLAP | 0.00 | Elbow | PMScl+ | GG | 13.72 | 14.35 |
| Case 8 | F | JDM | 11.99 | Knee | Negative | GA | 20.06 | 20.73 |
| Case 9 | F | OVERLAP | 10.05 | Chest | U1RNP indeterminate, Ro indeterminate, MJ+ | GG | 20.27 | 20.93 |
| Case 10 | F | JDM | 20.04 | Arm | Ro+, p155/140+ | GA | 9.46 | 9.88 |
Calcifications and CT scanning data
The calcifications were located as follows: three above the elbow, two in the forearm, one at the elbow, one in the area of the tibia, two around the knee and one in the chest wall. The average CTDI and DLP for the upper extremity (n = 12), lower extremity (n = 6) and chest (n = 1) are presented in Table 2.
Table 2.
Average radiation dose estimates by CT scan location
| Total number of scans | Average CTDI mGy (stdv) | Average DLP mGyacm (stdv) | Average effective dose mSv (stdv) | |
|---|---|---|---|---|
| Upper extremity | 12 | 4.322 (1.656) | 16.133 (4.770) | 0.007 (0.002) |
| Lower extremity | 6 | 4.283 (0.581) | 18.167 (3.488) | 0.010 (0.005) |
| Chesta | 1 | 4.200 | 17.000 | 0.245 |
amissing radiation information from first scan
Table 2. The CTDI and DLP are relatively similar for upper and lower extremities scanned in this study but the chest has a higher effective dose than the extremity studies. It is important to note, for comparison, that the comparable average effective radiation dose for a 15 year old child’s standard chest x-ray is 0.02 mSv [22], compared to the average effective doses above for the extremities of 0.007 and 0.010mSV), where the majority of the calcifications occur. Figure 1a presents an axial CT image of the upper arm showing a large cluster of calcifications medially, while Fig. 1b depicts the same axial CT image showing the calculated calcification volume highlighted in gray.
Fig. 1.
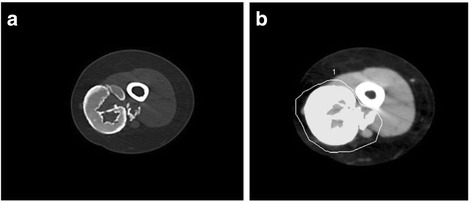
a Axial CT image of the upper arm of a child with JDM, showing a large cluster of calcifications medially. b The same axial CT image showing the calculated calcification volume highlighted in gray
The volume of the calcifications
There was a positive association of the change of the volume of calcification and DUD although this association was not statically significant (correlation 0.27, p = 0.46 Pearson correlation coefficient), (Fig. 2). The average size of calcifications at the first scan was 2.79 ± 1.98 and 2.29 ± 2.25 cm3 at the second scan, for an average decrease in size of 0.51 ± 1.38 cm3 (approximately 18%). However, the 6/10 who were compliant with their medications, did have a decrease in calcification volume from 2.32 ± 1.77 cm3 on the first scan to 1.00 ± 0.92 cm3 on the second scan, which was a measurable average decrease of 1.32 ± 1.14 cm3 (Fig. 3).
Fig. 2.
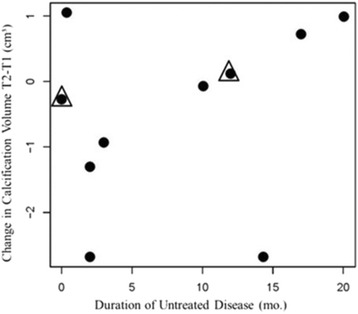
Black dots represent children with JDM. Black dots in a triangle represent two children with overlap syndromes (Pm-Scl and U1RNP)
Fig. 3.
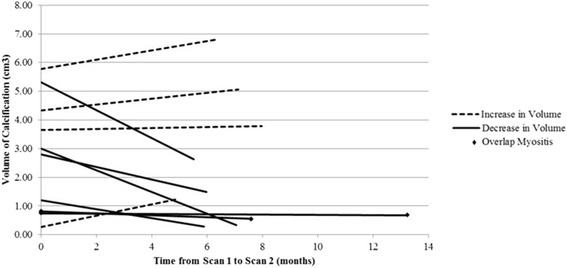
Change in the volume of calcifications: Six of the ten patients, who were compliant, did have a decreased calcification volume from 2.32 ± 1.77 cm3 on the first scan to 1.00 ± 0.92 cm3 on the second scan 6 months later scan with an average decrease of 1.32 cm3. The other four non-compliant patients had variable changes in the volume of their calcifications
Discussion
The present study represents the first pilot study to evaluate objectively the volume of calcifications in patients with JIIM over time. The mechanisms controlling the pathophysiology of calcifications remain poorly understood. Studies of the composition of these calcifications have shown that hydroxyapatite is the main mineral component, as well as calcium carbonate [23, 24]. Other bone matrix proteins such as osteonectin, osteopontin and bone sialoprotein have been documented [24], in addition to members of the integrin family [25]. Reported risk factors for the development of calcinosis include delayed diagnosis and treatment along with inadequate levels and duration of immunosuppressive therapy, [26] suggesting that prolonged inflammation contributes to tissue injury which promotes the calcium deposition. A common feature among all the children in this study was the persistent and chronic course of an active inflammatory process, which may be a major factor in the development/progression of calcifications in children with myositis [20]. Thirty percent of the JIIM group had a substitution of A at the TNFα-308 promoter region, which is similar to the 27–30% frequency in the general population, while 50% of the patients with JDM (4/8) were positive for the A substitution [20].
This study was not designed to test the efficacy of specific agents to elicit change in the volume of the calcifications, but to document that the single slice CT gives sufficient information to evaluate and compare outcomes. Our analysis of 20 CT scans of patients with JIIM and calcifications during a 2 year period while they were given immunosuppressive therapy showed a tendency for the specific calcification to decrease in volume in that time frame. In this group, 6/10 had positive MSAs, of whom 3/8 JDM were positive for one of the more frequently occurring MSAs, anti-MJ, reported to be associated with calcification [27].
We are not aware of any previous study that has used an objective method to measure the volume of calcifications embedded in the soft tissue of children with JIIM. For patients with systemic sclerosis, multidector computed tomography with multiplanar format has been employed successfully [28]. Experimentally, microcomputed tomography was employed to measure the volume of calcification in Abcc6 deficient mice [29] and to monitor the rate of resolution of induced calcification in mice with defective macrophage function [11].
Effective radiation dose in CT is dependent on three primary factors: the amount of radiation necessary to achieve the desired contrast between tissues, the extent of the body that is included in the field-of-view and the types of organs exposed to the radiation. The exceptionally low radiation dose needed to acquire the images in this study is a result of the favorable constitution, size, and location of JIIM calcifications. First, by their very nature –the deposits contain calcium (and are therefore comparatively radiopaque)– the calcifications are a strong contrast when compared to surrounding soft tissue (similar to bone). For this reason, very little radiation dose is needed to achieve excellent subject contrast and subsequent volume delineation; this was reflected in the reported CTDI value, which was low. Second, the physical size of the calcifications was easily captured in a 12 mm z-axis length, therefore limiting the radiation exposure to a very small anatomical region, which is reflected in the low DLP of this study. Finally, the JIIM calcifications in this study were located primarily in the extremities, which are relatively radio-insensitive. The radiosensitivity of cells increases with the reproductive rate and decreases with the level of differentiation, therefore the nerve and muscle tissue in the extremities is the least radiosensitive tissue in the body. The radiosensitivity of the exposed tissue is included in the calculation of effective dose in this report. Due to the low radiosensitivity of extremities to radiation, it would be optimal if future clinical studies were to select calcifications located in a limb as one inclusion criteria for evaluation of therapeutic efficacy using CT [30].
There are a few limitations to this pilot study: first, relatively few patients with calcifications were available at our Center for imaging. Another limitation is the lack of information to provide validation of this method (intra and inter rater reliability, validity, responsiveness). However, in a somewhat similar study, assessment of intrasubject change in lung tissue content over three CT scans was 2.75% +/−2.29% (mean and SD) [31]. In the present report, although their CT scans did not document a significant improvement in the volume of the calcifications in the 7 months between the first and second scan, the data do suggest that a longer interval between scans might be more helpful.
Conclusion
We conclude that limited low dose CT provides a safe, objective measurement of calcifications in JIIM. Each study was found to deliver less radiation than a single chest xray due to the low dose technique, limited field of view, and relative radio-insensitivity of extremities [32]. We speculate that this method may be a useful research tool to monitor progression and regression of calcifications. The four slice CT method offers the possibility of detecting and documenting change in the volume of calcifications in children over time, and may be a useful adjunct in assessing the child’s response to therapy.
Acknowledgements
This study could not have been performed without the support of the CureJM Foundation, for which the authors are very thankful. Expert administative assistance was suppllied by Ms Brittitany Hudanick, and was much appreciated. Finally, the authors valued the reviewer’s expert comments, which served to improve the manuscript.
Funding
Support for this study was provided by the Cure JM Foundation.
Availability of data and materials
All the available data, pertinent to this topic has been presented in this manuscript.
Authors’ contributions
The PI and first author, MI designed and executed this study as her Pediatric Rheumatology Fellowship project. She collected the data, wrote and edited the many drafts of the manuscripts and gave her approval of the final draft of this manuscript for publication. CR the second author, arranged for the CT studies, reviewed the data with MI, selected the figures used in this manuscript and reviewed and approved the final draft of this manuscript for publication. GAM data base manager, entered the data, participated in the creation of the figures, reviewed the many versions of the manuscript and gave her final approval of this submitted version. CLS is the radiation safety officer for the Ann and Robert H. Lurie Children’s Hospital of Chicago. She reviewed the data, verified the dosage calculations and concurred with the conclusions reached by this study. She reviewed the final manuscript and gave her approval for publication. CCH Biostatistics, participated in the study design, reviewed the data, and performed the analysis of the data. He reviewed the final manuscript and gave it his approval to be published. DX participated in the study design, performed the assays for the TNF-α-308 promotor polymorphism, and reviewed the final manuscript, giving his consent to publish. IT performed the immunological assays for the Myositis Specific Antibodies in the sera of the patients included in this study. He reviewed the final manuscript and gave his approval for publication. LMP mentored MI in the design of the study, data collection and analysis and in the preparation of the manuscript. She reviewed the final manuscript and gave her approval for publication.
Authors’ information
M. Ibarra, MD, Associate Professor, Division of Rheumatology, Children’s Mercy Hospital, Kansas City, Missouri, USA.
C. K. Rigsby, MD, Department of Medical Imaging, Ann & Robert H. Lurie Children’s Hospital of Chicago, Professor of Radiology and Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
G.A. Morgan, MA, Database Manager, Stanley Manne Children’s Research Institute, Cure JM Center of Excellence in Juvenile Myositis (JM) Research and Care, Ann & Robert H. Lurie Children’s Hospital of Chicago, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
C.L. Sammet, Ph.D., Research Assistant Professor, Department of Radiology, Northwestern University, Medical Physicist, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois, USA.
I.N. Targoff, MD, Professor, Department of Internal Medicine, The University of Oklahoma College of Medicine, Oklahoma City, Oklahoma; Veterans Affairs Medical Center (INT), University of Oklahoma Health Sciences Center, and Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma, USA.
C.C. Huang, PhD, Associate Professor, Joseph J. Zilber School of Public Health, University of Wisconsin, Milwaukee, Wisconsin, USA.
D. Xu, MD, Research Assistant Professor, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois., Stanley Manne Children’s Research Institute, Cure JM Center of Excellence in Juvenile Myositis (JM) Research and Care.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All the authors have reviewed the final manuscript and given their consent for publication.
Ethics approval and consent to participate
This study was reviewed by the Lurie Children’s IRB board and was approved, (IRB#2008-13316).
Departments and Institutions to which work is attributed
Ann & Robert H. Lurie Children’s Hospital of Chicago, Departments of Radiology and Rheumatology.
Sources of support
Supported by the Cure JM Foundation.
Abbreviations
- CMAS
Childhood myositis assessment scale
- CT
Computed tomography
- CTDI
Computed tomography dose index
- DAS
Disease activity score
- DLP
Dose length product
- JDM
Juvenile dermatomyositis
- JIIM
Juvenile idiopathic inflammatory myopathy
- MSA
Myositis specific antibodies
References
- 1.Faller G, Mistry BJ, Tikly M. Juvenile dermatomyositis in South African children is characterised by frequent dystropic calcification: a cross sectional study. Pediatr Rheumatol Online J. 2014;12:2. doi: 10.1186/1546-0096-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pachman LM, Morgan GA, Curran ML, et al. Decreased frequency of dystrophic calcifications in children with juvenile dermatomyositis: a 10-year study [abstract] Arthritis Rheum. 2012;64(Suppl 10):301. [Google Scholar]
- 3.Hoeltzel MF, Oberle EJ, Robinson AB, et al. The presentation, assessment, pathogenesis, and treatment of calcinosis in juvenile dermatomyositis. Curr Rheumatol Rep. 2014;16(12):467. doi: 10.1007/s11926-014-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahi V, Wetter DA, Howe BM, et al. Plain radiography is effective for the detection of calcinosis cutis occurring in association with autoimmune connective tissue disease. Br J Dermatol. 2014;170(5):1073–9. doi: 10.1111/bjd.12785. [DOI] [PubMed] [Google Scholar]
- 5.Nazir L, Saeed M. The calcium invasion: calciphylaxis in Lupus. J Pak Med Assoc. 2015;65(4):427–8. [PubMed] [Google Scholar]
- 6.Tristano AG, Villarroel JL, Rodriguez MA, et al. Calcinosis cutis universalis in a patient with systemic lupus erythematosus. Clin Rheumatol. 2006;25(1):70–4. doi: 10.1007/s10067-005-1134-5. [DOI] [PubMed] [Google Scholar]
- 7.Blane CE, White SJ, Braunstein EM, et al. Patterns of calcification in childhood dermatomyositis. AJR Am J Roentgenol. 1984;142(2):397–400. doi: 10.2214/ajr.142.2.397. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Sever Z, Mukamel M, Harel L, et al. Scintigraphic evaluation of calcinosis in juvenile dermatomyositis with Tc-99 m MDP. Clin Nucl Med. 2000;25(12):1013–6. doi: 10.1097/00003072-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Sarmiento AH, Alba J, Lanaro AE, et al. Evaluation of soft-tissue calcifications in dermatomyositis with 99mTc-phosphate compounds: case report. J Nucl Med. 1975;16(6):467–8. [PubMed] [Google Scholar]
- 10.Stock S, Ignatiev K, Lee P, et al. Pathological calcification in juvenile dermatomyositis (JDM): microCT and synchrotron x-ray diffraction reveal hydroxyapatite with varied microstructures. Connect Tissue Res. 2004;45(4–5):248–56. doi: 10.1080/03008200490903066. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Urganus AL, Spevak L, et al. Characterization of dystrophic calcification induced in mice by cardiotoxin. Calcif Tissue Int. 2009;85(3):267–75. doi: 10.1007/s00223-009-9271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohan A, Peter JB. Polymyositis and Dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 13.Toonen EJ, Barrera P, Fransen J, et al. Meta-analysis identified the TNFA -308G > A promoter polymorphism as a risk factor for disease severity in patients with rheumatoid arthritis. Arthritis Res Ther. 2012;14(6):R264. doi: 10.1186/ar4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trieu EP, Targoff IN. Immunoprecipitation: Western blot for proteins of low abundance. Methods Mol Biol. 2015;1312:327–42. doi: 10.1007/978-1-4939-2694-7_34. [DOI] [PubMed] [Google Scholar]
- 15.Rider LG, Shah M, Mamyrova G, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 2013;92(4):223–43. doi: 10.1097/MD.0b013e31829d08f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bode RK, Klein-Gitelman MS, Miller ML, et al. Disease activity scores for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49(1):7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 17.Huber AM, Feldman BM, Rennebohm RM, et al. Validation and clinical significance of the Childhood Myositis Assessment Scale for assessment of muscle function in the juvenile idiopathic inflammatory myopathies. Arthritis Rheum. 2004;50(5):1595–603. doi: 10.1002/art.20179. [DOI] [PubMed] [Google Scholar]
- 18.Saltybaeva N, Jafari ME, Hupfer M, et al. Estimates of effective dose for CT scans of the lower extremities. Radiology. 2014;273(1):153–9. doi: 10.1148/radiol.14132903. [DOI] [PubMed] [Google Scholar]
- 19.Deak PD, Smal Y, Kalender WA. Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology. 2010;257(1):158–66. doi: 10.1148/radiol.10100047. [DOI] [PubMed] [Google Scholar]
- 20.Pachman LM, Liotta-Davis MR. Hong DK, et al TNF-alpha-308A allele in Juvenile Dermatomyositis: association with increased production of TNF-alpha, disease duration and pathological calcifications. Arthritis Rheum. 2000;43(10):2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Rouster-Stevens KA, Morgan GA, Wang D, et al. Mycophenlate mofetil: a possible therapeutic agent for children with Juvenile Dermatomyositis. Arthritis Care Res (Hoboken) 2010;62(10):1446–51. doi: 10.1002/acr.20269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas D, Bible JE, Bohan M, et al. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882–9. doi: 10.2106/JBJS.H.01199. [DOI] [PubMed] [Google Scholar]
- 23.Eidelman N, Boyde A, BushbyAJ, et al. Microstructure and mineral composition of dystrophic calcifications associated with the idiopathic inflammatory myopathies. Arthrtis Res Ther. 2009;11(5):R159. doi:10.1186/ar2841 [published Online First: 25 Oct 2009]. [DOI] [PMC free article] [PubMed]
- 24.Pachman LM, Veis A, Stock S, et al. Composition of calcifications in children with juvenile dermatomyositis: association with chronic cutaneous inflammation. Arthritis Rheum. 2006;54(10):3345–50. doi: 10.1002/art.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urganus AL, Zhao YD, Pachman LM. Juvenile dermatomyositis clacifications selectively displayed markers of bone formation. Arthritis Rheum. 2009;61(4):501–8. doi: 10.1002/art.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PachmanLM, Abbott K, Sinacore JM, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148(2):247–53. [DOI] [PubMed]
- 27.Tansley SL, Betteridge ZE, Shaddick G, et al. Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology (Oxford) 2014;53(12):2204–8. doi: 10.1093/rheumatology/keu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freire V, Becce F, Feydy A, et al. MDCT imaging of calcinosis in systemic sclerosis. Clin Radiol. 2013;68(3):302–9. doi: 10.1016/j.crad.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Le Corre Y, Le Saux O, Froeliger F, et al. Quantification of the calcification phenotype of Abcc6-deficient mice with microcomputed tomography. Am J Pathol. 2012;180(6):2208–13. doi: 10.1016/j.ajpath.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed BA, Connolly BL, Shroff P, et al. Cumulative effective doses from radiologic procedures for pediatric oncology patients. Pediatrics. 2010;126(4):e851–8. doi: 10.1542/peds.2009-2675. [DOI] [PubMed] [Google Scholar]
- 31.Hu S, Hoffman EA, Reinhardt JM. Automatic Lung Segmentation for accurate quanitation of volumetric x-ray CT images. IEEE Trans Med Imaging. 2001;20(6):490–8. doi: 10.1109/42.929615. [DOI] [PubMed] [Google Scholar]
- 32.Lin EC. Radiation risk from medical imaging. Mayo Clin Proc. 2010;85(12):1142–6. doi: 10.4065/mcp.2010.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the available data, pertinent to this topic has been presented in this manuscript.


