Abstract
BACKGROUND AND OBJECTIVES:
Neonatal hypoglycemia has been associated with abnormalities on brain imaging and a spectrum of developmental delays, although historical and recent studies show conflicting results. We compared the cognitive, academic, and behavioral outcomes of preterm infants with neonatal hypoglycemia with those of normoglycemic controls at 3 to 18 years of age.
METHODS:
A secondary analysis of data from the Infant Health and Development Program, a national, multisite, randomized controlled longitudinal intervention study of long-term health and developmental outcomes in preterm infants. Of the 985 infants enrolled in the Infant Health and Development Program, 745 infants had glucose levels recorded. Infants were stratified into 4 groups by glucose level. By using standardized cognitive, academic, and behavioral assessments performed at 3, 8, and 18 years of age, we compared groups after adjusting for intervention status, birth weight, gestational age, sex, severity of neonatal course, race, maternal education, and maternal preconception weight.
RESULTS:
No significant differences were observed in cognitive or academic skills between the control and effected groups at any age. Participants with more severe neonatal hypoglycemia reported fewer problem behaviors at age 18 than those without hypoglycemia.
CONCLUSIONS:
No significant differences in intellectual or academic achievement were found between preterm infants with and without hypoglycemia. A statistical difference was found in behavior at age 18, with hypoglycemic children showing fewer problematic behaviors than normoglycemic children. This difference was not clinically meaningful. Using extended outcomes, our results are consistent with previous studies that found no significant neurodevelopmental outcomes associated with neonatal hypoglycemia in preterm-born children.
What’s Known on This Subject:
Neonatal hypoglycemia is common and has been associated with neurodevelopmental impairment. Controversy exists about the definition of hypoglycemia and the long-term developmental outcomes in prolonged or recurrent neonatal hypoglycemia.
What This Study Adds:
We found no differences in cognitive or academic achievement in preterm-born infants stratified by glucose level. An unexpected finding was significantly fewer problematic behaviors at 18 years in preterm-born children with the most severe hypoglycemia in comparison with normoglycemic children.
Hypoglycemia is one of the most common metabolic problems in neonatal medicine and has been recognized since 1911.1 Negative effects of prolonged hypoglycemia on long-term outcomes of preterm2–5 and term-born6–8 children previously reported include both transient and permanent structural abnormalities on brain imaging and adverse neurodevelopmental outcomes. More recent studies show conflicting data on long-term developmental outcomes in both term and preterm-born children with neonatal hypoglycemia.9,10
Several studies have examined the actual level and/or duration of hypoglycemia that is harmful to the infant brain, with inconclusive findings. However, existing reviews generally conclude that well-designed studies are scarce and more research is needed.11 There is no currently agreed on clinical definition for hypoglycemia.12–14 Leading experts comment “the definition of clinically significant hypoglycemia remains one of the most confused and contentious issues in contemporary neonatology”15 with insufficient evidence to identify a specific plasma glucose concentration range to describe hypoglycemia, given the person-to-person variability of glucose homeostasis and hypoglycemic symptoms.16 Despite this, most clinicians define hypoglycemia as a plasma glucose level of ≤40 to 45 mg/dL,7,9,15,17 although some studies have used higher glucose cutoffs.2,4,10 The conflicting results of developmental outcomes may be partially due to this inconsistency in the definition of hypoglycemia.
Secondary analyses of a large, longitudinal data set allowed us to further examine the question of negative developmental outcomes in association with neonatal hypoglycemia. The objective of our study was to test the association of neonatal hypoglycemia at various levels of severity with academic, cognitive, and/or behavioral outcomes from middle childhood to young adulthood in a cohort of individuals born low birth weight and preterm.
Methods
Subject Population
Study participants were from the Infant Health and Development Program (IHDP).18–22 IHDP was a national collaborative, multisite, randomized controlled longitudinal intervention study initiated in 1985 and designed to evaluate early educational intervention efficacy on long-term mental and physical health in preterm infants. The study managed patients from birth until 3 years and follow-up through 18 years of age. Infants were ≤2500 g and ≤37 weeks’ gestation. Details of recruitment, methods, and intervention are described elsewhere.18–22
Briefly, 985 low birth weight, preterm infants were randomly assigned to early-intervention (n = 377) or a follow-up group (n = 608) by using a 2:1 adaptive randomization scheme. After the clinical trial was completed, subjects were assessed at 5, 8, and 18 years with a broad array of developmental and behavioral assessments.
Identification of Infants With Hypoglycemia
The IHDP protocol called for the highest and lowest glucose levels obtained by Dextrostix and/or plasma sample during hospitalization to be recorded. Timing and duration of hypoglycemia were not reported. Glucose was obtained per hospital protocol and/or medical need per physician decision. There was no standardized protocol in obtaining glucose levels.
Current expert opinion recommends a plasma concentration or whole blood glucose level at which clinicians should consider intervention (>2.5 mmol/L = >45 mg/dL) that would be applicable to any term or preterm infant.15 Given this, we chose the cutoff glucose value of ≤45 mg/dL as definition of hypoglycemia for our study.
Outcomes
Per IHDP protocol, the children were assessed throughout the study by trained assessors masked to the child’s treatment group and history.18 Outcomes included in the examination of hypoglycemia are cognitive, academic, and behavioral assessments at ages 3 years, 8 years, and 18 years (see Table 1).
TABLE 1.
Standardized Measures of Assessment of Cognitive, Behavioral, and Academic Achievement by Age Group
| Measure | Domain | Respondent | Age Tested, y |
|---|---|---|---|
| Stanford-Binet Intelligence Scale, Form L-M, scale mean 100 ± 15 | Cognitive: verbal IQ, performance IQ, total IQ | Child | 3 |
| PPVT, scale mean 100 ± 15 | Cognitive: receptive vocabulary | Child | 3 |
| CBCL, ages 2–3, T score >70 = significant problem | Behavior | Mother | 3 |
| WISC-III, scale mean 100 ± 15 | Cognitive: verbal IQ, performance IQ, total IQ | Child | 8 |
| PPVT-R, scale mean 100 ± 15 | Cognitive: receptive vocabulary | Child | 8 |
| CBCL | Behavior | Mother | 8, 18 |
| WJ Test of Achievement-Revised, scale mean 100 ± 15 | Academic achievement: reading and math subsets | Child | 8, 18 |
| WASI, scale mean 100 ± 15 | Cognitive: verbal IQ, performance IQ, total IQ | Child | 18 |
| PPVT-III, scale mean 100 ± 15 | Cognitive: receptive vocabulary | Child | 18 |
| YRBSS | Behavior | Child | 18 |
Cognitive Assessment
Cognitive skills were directly assessed by using the Stanford-Binet Intelligence scales forms L-M23 and The Peabody Picture Vocabulary Test-Revised (PPVT-R),24 a measure of nonverbal intelligence, at 3 years. The Wechsler Intelligence Scale for Children (WISC-III)25 and PPVT-R assessed intelligence at 8 years. Given the subcomponents of the WISC-III, full-scale IQ, verbal IQ, and performance IQ can be generated.
The PPVT-version III (PPVT-III)26 and the Wechsler Abbreviated Scale of Intelligence (WASI)27 assessed intelligence at 18 years. Similar to the WISC-III, the WASI has 4 subtests and can produce a verbal IQ, performance IQ, and full-scale IQ measurement.
Academic Achievement
Academic achievement was measured by using the Woodcock-Johnson (WJ) Tests of Achievement-Revised28 at ages 8 and 18 years. This assessment measures letter-word identification, passage comprehension, mathematic calculation, and applied problems.
All assessments are standardized with mean 100 and SD ±15.
Behavioral Assessment
The child’s behavior was measured by using parent report on the Child Behavior Checklist (CBCL) for ages 2 to 3 for the 3-year evaluation29 and the CBCL ages 3 to 18 for the 8-year evaluation.30 Results are interpreted based on a T score, with a T score of >70 being clinically significant. At age 18, subscales of the CBCL and the Youth Report Behavior Surveillance System (YRBSS)22 were completed by the child. The CBCL subscales were scored by using standardized raw scores per problem per age group. The YRBSS was developed by the Centers for Disease Control and Prevention in 1990 to monitor risk behaviors that “contribute to morbidity, mortality, and social problems in adolescents,” and is known to have excellent test-retest reliability.31,32 A detailed analysis33 resulted in YRBSS summary score ranging from 0 (low risk) to 18 (high risk). It consists of 3 subdomains each looking at problem behaviors and substance use (antisocial behavior, suicidal ideation/attempt, smoking, alcohol, marijuana usage, and sexual behavior), scaled to a 3-level scoring approach ranging from 0 (low risk) to 3 (high risk).
Statistical Analysis
Statistical analysis was aimed at comparing cognitive, academic achievement, and behavioral outcomes among the following 4 groups based on degree of severity of hypoglycemia: severe hypoglycemia (≤35 mg/dL), moderate hypoglycemia (36–40 mg/dL), mild hypoglycemia (41–45 mg/dL), and normoglycemia (46–180 mg/dL). Power analysis was performed to determine if the data were sufficiently powered to detect reasonable effect sizes seen among the groups. By using analysis of covariance (ANCOVA), the sample sizes seen in the 4 groups in this study had at least 97% power to detect a very small effect size of 0.15 in outcomes among the groups and 100% power to detect a medium effect size of 0.40 among groups, suggesting the study was adequately powered. Sample size calculation was done by using the software PASS 14 (NCSS Statistical Software, LLC, Kaysville, UT). We summarized data by using frequency and percentages for categorical variables and mean and SD for continuous variables. Baseline demographic and confounding variables were summarized and compared among the 4 groups of patients. The χ2 test of association was used to compare categorical variables among the 4 groups, and a 1-way analysis of variance method was used for comparing means among the 4 groups for continuous variables.
Cognitive and behavioral outcomes, such as standardized IQ scores, test scores, and CBCL were compared among the 4 groups at 3, 8, and 18 years by using an ANCOVA method. The following confounding variables were included in the ANCOVA model: intervention, site, Home Observation for Measurement of the Environment inventory total environment score (a measure of the nurturing and stimulating qualities of the home environment) maternal preconception weight, maternal education, maternal race, infant sex, gestational age, and neonatal health index score. The neonatal health index score was computed as a composite severity score by taking the ratio of birth weight and neonatal length of stay.34 As a sensitivity analysis, we also compared outcomes among patients in the hypoglycemic (≤45 mg/dL) and normoglycemic groups (>45 mg/dL). Effects from the ANCOVA model were summarized as differences in least square means (estimated from the model) and associated 95% confidence intervals.
To examine selection bias at age 18 years, we examined whether drop out at 18 years was related to demographic variables or outcomes by comparing demographic variables between those who dropped out at 18 years and those who remained. Further, we compared outcomes at 3 and 8 years between the hypoglycemic and normoglycemic groups among those who dropped out of the study at 18 years. We used Bonferroni correction to adjust P values for multiple testing wherever appropriate. Statistical analyses were performed by using the SAS/STAT software, Version 9.4 (SAS Institute, Inc, Cary, NC). All tests were 2-sided assuming a significance level of 5%.
Results
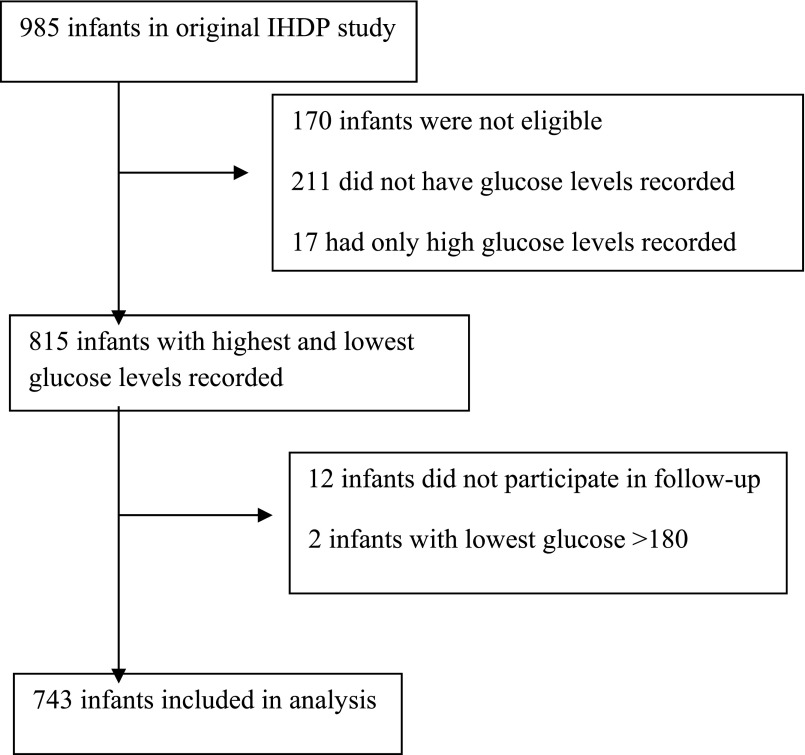
Of the 985 infants, 745 infants had glucose levels recorded. Two children were excluded for hyperglycemia defined as lowest glucose >180 mg/dL. Most infants (n = 461) were considered hypoglycemic with a glucose ≤45mg/dL (Fig 1). These were then further subdivided by glucose ranges into degree of severity, with most severe defined as glucose ≤35 mg/dL (n = 153), moderate defined as glucose level of 36 to 40 mg/dL (n = 126), and mild defined as glucose level of 41 to 45 (n = 182.) The remaining (n = 284) were considered normoglycemic, with glucose levels between 46 and 180 mg/dL and served as the control group (Table 2).
FIGURE 1.
Flowchart showing selection of eligible infants for secondary analysis.
TABLE 2.
Frequency of Patients in Each Glucose Category
| Glucose Group | Frequency | Percent |
|---|---|---|
| ≤35 mg/dL | 153 | 20.6 |
| 36–40 mg/dL | 126 | 17.0 |
| 41–45 mg/dL | 182 | 24.5 |
| 46–180 mg/dL | 282 | 38.0 |
Table 3 summarizes baseline demographic and confounding variables among the patients with varying degrees of glucose concentrations. The distribution of site (P < .0001), maternal education (.0001), maternal race (.007), gestational age (.008), and birth weight (.007) was different among the 4 groups. There were no differences among the 4 groups with the other baseline variables, including treatment group status. However, when comparing outcomes among the 4 groups, we adjusted for all demographic and confounding variables listed in Table 3.
TABLE 3.
Summary Statistics and Analysis of Variance and χ2 Comparison of Baseline Demographics by Glucose Category
| Demographics and Confounding Variables | Glucose Groups | P | |||
|---|---|---|---|---|---|
| ≤35, n = 153 | 36–40, n = 126 | 41–45, n = 182 | 46–180, n = 282 | ||
| Site, n (%) | <.0001 | ||||
| Little Rock, Arkansas | 22 (14.4) | 14 (11.1) | 23 (12.6) | 59 (20.9) | |
| Bronx, New York | 26 (17.0) | 9 (7.1) | 9 (4.9) | 63 (22.3) | |
| Boston, Massachusetts | 29 (19.0) | 8 (6.3) | 36 (19.8) | 31 (11.0) | |
| Miami, Florida | 8 (5.2) | 3 (2.4) | 38 (20.9) | 31 (11.0) | |
| Philadelphia, Pennsylvania | 13 (8.5) | 8 (6.3) | 6 (3.3) | 25 (8.9) | |
| Dallas, Texas | 23 (15) | 80 (63.5) | 8 (4.4) | 19 (6.7) | |
| Seattle, Washington | 2 (1.3) | 1 (0.8) | 3 (1.6) | 43 (15.2) | |
| New Haven, Connecticut | 30 (19.6) | 3 (2.4) | 59 (32.4) | 11 (3.9) | |
| Home total score at 12 mo, mean (SD) | 33.2 (6.0) | 32.5 (5.1) | 34.0 (7.0) | 33.4 (5.9) | .29 |
| Maternal preconception weight, mean (SD) | 133.1 (28.7) | 131.2 (32.9) | 133.0 (28.8) | 128.8 (29.4) | .38 |
| Treatment group, n (%) | .50 | ||||
| Follow-up | 95 (62.1) | 77 (61.1) | 120 (65.9) | 166 (58.9) | |
| Intervention | 58 (37.9) | 49 (38.9) | 62 (34.1) | 116 (41.1) | |
| Birth weight group, n (%) | .22 | ||||
| High | 37 (24.2) | 37 (29.4) | 61 (33.5) | 74 (26.2) | |
| Low | 116 (75.8) | 89 (70.6) | 121 (66.5) | 208 (73.8) | |
| Maternal education, n (%) | <.0001 | ||||
| <9th grade | 7 (4.6) | 10 (7.9) | 6 (3.3) | 11 (3.9) | |
| 9th–12th grade | 49 (32.0) | 62 (49.2) | 51 (28.0) | 112 (39.7) | |
| High school graduate | 45 (29.4) | 38 (30.2) | 44 (24.2) | 84 (29.8) | |
| Completed some college | 35 (22.9) | 10 (7.9) | 45 (24.7) | 48 (17.0) | |
| College degree or more | 17 (11.1) | 6 (4.8) | 36 (19.8) | 27 (9.6) | |
| Maternal race, n (%) | .007 | ||||
| White | 52 (34.0) | 23 (18.3) | 66 (36.3) | 94 (33.3) | |
| Black | 78 (51.0) | 88 (69.8) | 95 (52.2) | 141 (50.0) | |
| Hispanic | 19 (12.4) | 15 (11.9) | 15 (8.2) | 37 (13.1) | |
| Others/Unknown | 4 (2.6) | 0 | 6 (3.3) | 10 (3.5) | |
| Infant sex, n (%) | .75 | ||||
| Boys | 79 (51.6) | 57 (45.2) | 89 (48.9) | 141 (50.0) | |
| Girls | 74 (48.4) | 69 (54.8) | 93 (51.1) | 141 (50.0) | |
| Gestational age, wk, mean (SD) | 32.1 (2.9) | 32.8 (2.8) | 33.1 (2.5) | 32.5 (2.7) | .008 |
| Birth weight, kg, mean (SD) | 1.62 (0.5) | 1.73 (0.4) | 1.79 (0.4) | 1.7 (0.5) | .007 |
| Neonatal Health Index, mean (SD) | 97.5 (16.7) | 98.8 (16.0) | 100.2 (16.6) | 98.6 (16.0) | .50 |
P values correspond to χ2 test for categorical variables and 1-way analysis of variance for continuous variables.
Outcomes
All major outcomes by degree of hypoglycemia are depicted in Table 4. No differences were found among the 4 groups of preterm-born infants in cognitive or academic assessment measures at 3, 8, or 18 years after adjusting for demographics and confounding variables. At 3 years, mean cognitive scores were low average to average. Stanford-Binet scores were not significantly different among the glucose categories (P = .46). At 18 years, mean cognitive scores were also found to be in the average ranges (P = .33). No difference in academic achievement was found at ages 8 or 18, with all scores on the WJ within average ranges in reading (P = .42; P = .14) and math (P = .31; P = .73). Behavior assessment was not significantly different among the 4 groups at 8 years with mean total CBCL scores ranging from 32.0 to 34.2 among the 4 groups, respectively (P = .80). Although raw scores are all within the average range of problematic behaviors, total CBCL scores at 18 years were significantly different among at least 1 pair of groups, with the severe hypoglycemic children showing lower problematic behaviors than other groups (P = .001). Mean (SE) CBCL scores among the severe hypoglycemia group was 6 (1.3), whereas it was 10.7 (1.0) in the normoglycemic group. The YRBSS scores were also lower in the more severe hypoglycemic infants compared with the normoglycemic infants (P = .06), although this difference was not statistically significant (Table 4). There was no linear trend in the association between the level of hypoglycemia and the problem behavior scores.
TABLE 4.
Adjusted Means From ANCOVA of Outcomes at 3, 8, and 18 Years by Glucose Category After Controlling for Demographics and Risk Factors
| Outcomes | Glucose Groups | P | |||
|---|---|---|---|---|---|
| ≤35 | 36–40 | 41–45 | 46–180 | ||
| 3-y | |||||
| Achenbach total | 43.3 (2.3) | 50.4 (2.9) | 43.7 (2.3) | 45.8 (2.0) | .09 |
| PPVT-R | 88.3 (1.6) | 86.7 (1.9) | 89.4 (1.5) | 86.2 (1.4) | .19 |
| Stanford-Binet IQ, corrected age | 87.9 (1.7) | 90.5 (2.1) | 89.8 (1.7) | 88.2 (1.5) | .46 |
| 8-y | |||||
| CBCL, total | 32.0 (2.4) | 32.4 (3.0) | 33.0 (2.4) | 34.2 (2.1) | .80 |
| PPVT-R, standard | 84.9 (2.2) | 91.6 (2.7) | 87.5 (2.2) | 86.5 (1.9) | .10 |
| WJ broad reading standard | 100.3 (2.3) | 102.1 (2.9) | 100.7 (2.3) | 98.1 (2.0) | .42 |
| WJ broad math standard | 95.7 (2.6) | 101.1 (3.2) | 98.3 (2.6) | 96.3 (2.2) | .31 |
| WISC-III verbal IQ | 95 (1.8) | 96.8 (2.2) | 97.5 (1.8) | 93.7 (1.5) | .11 |
| WISC-III performance IQ | 92.8 (1.8) | 94.9 (2.2) | 94.5 (1.8) | 91.6 (1.5) | .23 |
| WISC-III total IQ | 93.2 (1.8) | 95.4 (2.2) | 95.6 (1.7) | 91.9 (1.5) | .11 |
| 18-y | |||||
| CBCL, total | 6.0 (1.3) | 10.2 (1.5) | 8.6 (1.1) | 10.7 (1.0) | .001 |
| Youth risk behavior | 1.98 (0.4) | 1.70 (0.5) | 1.98 (0.4) | 2.65 (0.3) | .06 |
| PPVT-III, standard | 100.5 (2.2) | 100.7 (2.7) | 99.4 (2) | 99.1 (1.8) | .87 |
| WJ broad reading standard | 99.9 (2.9) | 106.7 (3.6) | 101.6 (2.7) | 102 (2.4) | .32 |
| WJ broad math standard | 93.6 (2.3) | 97.5 (2.9) | 93.6 (2.2) | 92.3 (1.9) | .30 |
| WASI, verbal, IQ | 96.2 (1.9) | 98.3 (2.4) | 96.5 (1.9) | 97.2 (1.6) | .84 |
| WASI, performance IQ | 96.3 (2) | 94.6 (2.5) | 95 (1.9) | 95 (1.6) | .87 |
| WASI, full-scale IQ | 96 (1.9) | 95.8 (2.4) | 95.4 (1.8) | 96 (1.6) | .99 |
P values <.0028 are considered to be statistically significant after correcting for multiple testing by using Bonferroni adjustment as 18 separate analyses were conducted. Means and P values were from an ANCOVA model after adjusting for intervention, site, home total environment score at 12 months, maternal preconception weight, maternal education, maternal race, infant gender, gestational age (weeks), birth weight, and neonatal health index score.
Sensitivity Analysis
A separate analysis was performed comparing outcomes at 3, 8, and 18 years between infants classified into hypoglycemic and normoglycemic groups. None of the outcomes were significantly different between the 2 groups after adjusting for confounding variables and for multiple testing using the Bonferroni correction (results not shown).
Missing Data Due to Dropout
Few subjects had missing information on all outcomes at 3 years (44 missing) and 8 years (28 missing). Of the 745 subjects, 182 (34%) were lost to follow-up at age 18. Baseline demographics were not significantly different between participants who were evaluated and those not evaluated at 18 years. In addition, among those who dropped out at 18 years, none of the outcomes at 3 and 8 years were significantly different between the hypoglycemic and normoglycemic groups (Table 5), suggesting that the dropout mechanism was at random and not likely to be related to outcomes.
TABLE 5.
Three-Year and 8-Year Outcomes Among Subjects Who Were Lost to Follow-up at 18 Years
| Outcome | Normoglycemia | Hypoglycemia (≤45) | P | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| 3-y | |||||
| CBCL, total | 67 | 47.4 (20.4) | 128 | 49.0 (20.0) | .59 |
| PPVT-R | 57 | 82.3 (17.0) | 122 | 85.7 (17.0) | .22 |
| Stanford-Binet IQ, corrected age | 68 | 81.3 (20.7) | 137 | 83.7 (18.7) | .41 |
| 8-y | |||||
| CBCL, total | 57 | 35.3 (22.9) | 123 | 33.8 (19.8) | .65 |
| PPVT-R, standard | 57 | 83.1 (22.6) | 124 | 81.5 (20.9) | .65 |
| WJ broad reading standard | 58 | 90.5 (22.3) | 120 | 95.6 (21.2) | .14 |
| WJ broad math standard | 57 | 90.2 (23.1) | 124 | 91.5 (23.7) | .73 |
| WISC-III verbal IQ | 58 | 87.3 (16.3) | 124 | 89.8 (16.5) | .33 |
| WISC-III performance IQ | 58 | 85.7 (17.4) | 124 | 88.2 (17.1) | .37 |
| WISC-III total IQ | 58 | 85.3 (17.1) | 124 | 88.0 (17.1) | .33 |
P values are from 2-sample t test.
Discussion
We hypothesized that we would find adverse long-term outcomes in preterm-born children with neonatal hypoglycemia. This was not the case, with infants with hypoglycemia having similar academic and cognitive outcomes and less problem behaviors as those without hypoglycemia. This finding is in contrast to the historic report by Lucas et al2 and a recent report by Kaiser et al.9 Our results are similar to those of Tin et al5 and McKinlay et al,10 who found no significant differences in neurodevelopment of infants with neonatal hypoglycemia.
Lucas et al2 found that moderate recurrent hypoglycemia (glucose level ≤47 mg/dL) significantly affected developmental scores at 18 months in a preterm (mean 30.5 weeks’ gestation) population. Similar results were found on follow-up at 7 to 8 years of age.35 Lucas et al assessed cognitive skills by using a standardized assessment tool (Bayley Motor and Mental Development Scales), but unlike the assessments used in our study, this tool has poor predictive validity of future cognitive skills.36 Our study also examined additional aspects of neurodevelopment that were not assessed by Lucas et al,2 including behavior and academic achievement. The standardized assessments used in our study have been validated and normed to specific age groups and were considered the gold standards for academic, cognitive, and behavioral assessment in the United States at time of direct assessment. Unlike the Lucas et al study,2 we do not have data regarding duration or recurrence of hypoglycemia.
Tin et al5 attempted to replicate the Lucas et al study2 with more stringent guidelines and protocols for glucose sampling and treatment. Infants born <32 weeks obtained daily glucose measurements for the first 10 days of life, with hypoglycemia defined as a glucose concentration of ≤2.5 mmol/L (≤45 mg/dL). A small percentage of these children had recurrent hypoglycemia, defined as glucose concentration ≤2.5 mmol/L on ≥3 consecutive days. No association was found at ages 2 and 15 years between hypoglycemia and normoglycemia on academic, cognitive, adaptive, and behavioral outcomes. Our findings support the findings of Tin et al,5 although given the nature of our dataset, we were not able to ascertain duration or recurrence of hypoglycemia.
More recent studies continue to show conflicting outcomes in both preterm-born and term-born children with hypoglycemia. Kaiser et al9 reported an association between early transient newborn hypoglycemia and lower achievement test scores, determined by the Benchmark examinations in a single state, at age 10 years. This study was a retrospective, population-based cohort study of 1395 term and preterm (23–42 weeks’ gestation) infants born at a university hospital with educational data matched from social security number, name, and date of birth. It controlled for maternal variables and socioeconomic status, but did not include individually administered cognitive or behavioral assessments. The academic assessment used was a state-based academic proficiency test. In contrast, our study looks not only at individually administered academic achievement, but also individually administered cognitive and behavioral measures by using assessments standardized, normed, and validated to children throughout the United States.
In another recent report, McKinlay et al10 found no association between neonatal hypoglycemia and adverse neurodevelopmental outcomes at age 2 years. This study was a prospective cohort of late preterm and term infants (≥35 weeks’ gestation) at risk for hypoglycemia, defined as glucose concentration <47 mg/dL, that assessed the relation between the duration, frequency, and severity of low glucose concentrations in the neonatal period and neurodevelopmental outcomes at 2 years. They found that the lowest blood glucose concentration, number of hypoglycemic episodes, and negative interstitial increment did not predict neurodevelopmental outcome.
Our finding of lower problematic behaviors in children with the most severe hypoglycemia in comparison with normoglycemic peers and less severe hypoglycemia was unexpected. Although behavior scores were within the average ranges for all degrees of hypoglycemia and normoglycemia, statistical differences were found among the hypoglycemia continuum. We can only speculate about this finding. This may in fact be a spurious finding, although the second behavior measure, the YRBSS, approached statistical significance as well. We speculate that some normoglycemic infants might have had artificial inflation of glucose due to increased stressors related to birth that were not controlled for. This might have given a false sense of normoglycemia or mild hypoglycemia and resulted in less frequent monitoring of glucose and therefore less aggressive treatment in the groups considered mild hypoglycemia or normoglycemia. Again, due to differing protocols per hospital, we are able only to speculate on this.
This study has several limitations. The study is a secondary analysis of the IHDP and was not designed to monitor hypoglycemia. There was no standard protocol in obtaining blood glucose or in treating hypoglycemia once recognized. Instead, these medical decisions were made based on hospital protocol and physician judgment. As a result, duration of hypoglycemia was not recorded. It is also important to note that some glucose concentrations were obtained via Dextrostix, whereas others were obtained via plasma sample, depending on hospital protocol. For those infants with glucose obtained via Dextrostix, the glucose level was reported as a range (eg, 45–90) with instructions to record the lowest number in the range. Therefore, there is a possibility of having normoglycemic children in the 41- to 45-mg/dL glucose category. Another limitation is what we consider the crux of the question of hypoglycemia, the actual definition of hypoglycemia. Experts in the field admit that using a numerical definition for a “cutoff” of hypoglycemia ignores some important aspects, such as person-to-person variability in glucose homeostasis and the spectrum and variability of biological problems seen in hypoglycemia.15 They recommend considering the low blood glucose in addition to symptoms of hypoglycemia. Unfortunately, we do not know how many of the hypoglycemic children in our study were also symptomatic.
Strengths of this study include extended follow-up of subjects and the broad array of information gleaned from multiple standardized assessments over different critical developmental periods. Other strengths include the vast amount of demographic data and neonatal outcome data obtained by trained personnel. The initial study also examined in detail the nurturing and stimulating quality of the home environment of each child, along with other demographic variables. Socioeconomic background has been shown to also have adverse association with developmental outcome.37 By obtaining detailed information on home and family life, we were able to control for this important factor. Finally, the retention rate of the primary study participates was impressive at 72%.22
Conclusions
By using extended outcomes, our results are consistent with previous studies that found no significant neurodevelopmental outcomes associated with neonatal hypoglycemia in preterm-born children. To best direct future newborn hypoglycemia treatment guidelines, high-quality longitudinal, prospective studies with large samples are needed.
Glossary
- ANCOVA
analysis of covariance
- CBCL
Child Behavior Checklist
- IHDP
Infant Health and Development Program
- PPVT-R
Peabody Picture Vocabulary Test-Revised
- PPVT-III
Peabody Picture Vocabulary Test-version III
- WASI
Wechsler Abbreviated Scales of Intelligence
- WISC-III
Wechsler Intelligence Scale for Children-third edition
- WJ
Woodcock-Johnson
- YRBSS
Youth Report Behavior Surveillance System
Footnotes
Dr Goode conceptualized the study question, and drafted the initial manuscript; Drs Lyle and Casey designed the study, acted as mentors for the study group, and reviewed and revised the manuscript; Drs Rettiganti and Whiteside-Mansell and Ms Li designed the data analyses, carried out initial analyses, drafted the description of the statistical analyses, and reviewed and revised the manuscript; Ms Barrett facilitated the use of the original data set; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The Infant Health and Development Program was supported, in its first 3 years, by the Robert Wood Johnson Foundation; follow-up evaluations were supported by the Pew Charitable Trust, the National Institute of Child Health and Human Development, and the Maternal Child Health Bureau, with the Robert Wood Johnson Foundation. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2016-2881.
References
- 1.Cornblath M. Neonatal hypoglycemia 30 years later: does it injure the brain? Historical summary and present challenges. Acta Paediatr Jpn. 1997;39(suppl 1):S7–S11 [PubMed] [Google Scholar]
- 2.Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerstjens JM, Bocca-Tjeertes IF, de Winter AF, Reijneveld SA, Bos AF. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics. 2012;130(2). Available at: www.pediatrics.org/cgi/content/full/130/2/e265 [DOI] [PubMed] [Google Scholar]
- 4.Duvanel CB, Fawer CL, Cotting J, Hohlfeld P, Matthieu JM. Long-term effects of neonatal hypoglycemia on brain growth and psychomotor development in small-for-gestational-age preterm infants. J Pediatr. 1999;134(4):492–498 [DOI] [PubMed] [Google Scholar]
- 5.Tin W, Brunskill G, Kelly T, Fritz S. 15-year follow-up of recurrent “hypoglycemia” in preterm infants. Pediatrics. 2012;130(6). Available at: www.pediatrics.org/cgi/content/full/130/6/e1497 [DOI] [PubMed] [Google Scholar]
- 6.Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122(1):65–74 [DOI] [PubMed] [Google Scholar]
- 7.Tam EWY, Haeusslein LA, Bonifacio SL, et al. Hypoglycemia is associated with increased risk for brain injury and adverse neurodevelopmental outcome in neonates at risk for encephalopathy. J Pediatr. 2012;161(1):88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand PL, Molenaar NL, Kaaijk C, Wierenga WS. Neurodevelopmental outcome of hypoglycaemia in healthy, large for gestational age, term newborns. Arch Dis Child. 2005;90(1):78–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser JR, Bai S, Gibson N, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: A population-based study. JAMA Pediatr. 2015;169(10):913–921 [DOI] [PubMed] [Google Scholar]
- 10.McKinlay CJ, Alsweiler JM, Ansell JM, et al. ; CHYLD Study Group . Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med. 2015;373(16):1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boluyt N, van Kempen A, Offringa M. Neurodevelopment after neonatal hypoglycemia: a systematic review and design of an optimal future study. Pediatrics. 2006;117(6):2231–2243 [DOI] [PubMed] [Google Scholar]
- 12.Hawdon JM. Neonatal hypoglycemia: Are evidence-based clinical guidelines achievable? Neoreviews. 2014;15(3):e91–e98 [Google Scholar]
- 13.Rozance PJ, Hay WW Jr. Hypoglycemia in newborn infants: features associated with adverse outcomes. Biol Neonate. 2006;90(2):74–86 [DOI] [PubMed] [Google Scholar]
- 14.Rozance PJ, Hay WW Jr. Neonatal hypoglycemia—answers, but more questions. J Pediatr. 2012;161(5):775–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105(5):1141–1145 [DOI] [PubMed] [Google Scholar]
- 16.Hay WW Jr, Raju TN, Higgins RD, Kalhan SC, Devaskar SU. Knowledge gaps and research needs for understanding and treating neonatal hypoglycemia: workshop report from Eunice Kennedy Shriver National Institute of Child Health and Human Development. J Pediatr. 2009;155(5):612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan JE, Price-Douglas W, Hay WW Jr. Glucose homeostasis. In: Merenstein GB, Gardner SL, eds. Handbook of Neonatal Intensive Care. 6th ed. St. Louis, MO: Mosby Elsevier; 2006:368–390 [Google Scholar]
- 18.The Infant Health and Development Program . Enhancing the outcomes of low-birth-weight, premature infants. A multisite, randomized trial. JAMA. 1990;263(22):3035–3042 [DOI] [PubMed] [Google Scholar]
- 19.Brooks-Gunn J, McCarton CM, Casey PH, et al. Early intervention in low-birth-weight premature infants. Results through age 5 years from the Infant Health and Development Program. JAMA. 1994;272(16):1257–1262 [PubMed] [Google Scholar]
- 20.McCarton CM, Brooks-Gunn J, Wallace IF, et al. Results at age 8 years of early intervention for low-birth-weight premature infants. The Infant Health and Development Program. JAMA. 1997;277(2):126–132 [PubMed] [Google Scholar]
- 21.McCormick MC, McCarton C, Brooks-Gunn J, Belt P, Gross RT. The Infant Health and Development Program: interim summary. J Dev Behav Pediatr. 1998;19(5):359–370 [DOI] [PubMed] [Google Scholar]
- 22.McCormick MC, Brooks-Gunn J, Buka SL, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117(3):771–780 [DOI] [PubMed] [Google Scholar]
- 23.Terman LM, Merrill MA. Stanford-Binet Intelligence Scale: Manual for the Third Revision, Form L-M. Boston, MA: Houghton Mifflin; 1973 [Google Scholar]
- 24.Dunn LM, Dunn LM. Examiner’s Manual for the Peabody Picture Vocabulary Test, Revised edition. Circle Pines, MN: American Guidance Service; 1981 [Google Scholar]
- 25.Wechsler D. Manual for the Wechsler Intelligence Scale for Children-III. San Antonio, TX: Psychological Corp; 1991 [Google Scholar]
- 26.Dunn LM, Dunn LM. Examiner’s Manual for the Peabody Picture Vocabulary Test, 3rd ed. Circle Pines, MN: American Guidance Service; 1997 [Google Scholar]
- 27.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999 [Google Scholar]
- 28.Woodcock RW, Johnson MB. Manual for the Woodcock-Johnson Tests of Achievement-Revised. Allen, TX: RCL Enterprises; 1980 [Google Scholar]
- 29.Achenbach TM, Edelbrock C, Howell CT. Empirically based assessment of the behavioral/emotional problems of 2- and 3- year-old children. J Abnorm Child Psychol. 1987;15(4):629–650 [DOI] [PubMed] [Google Scholar]
- 30.Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, VT: Queen City Printers; 1983 [Google Scholar]
- 31.US Department of Health and Human Services An epidemiological surveillance system to monitor the prevalence of youth behaviors that most affect health. Chronic Disease and Health Promotion. MMWR 1990 Youth Surveillance System Atlanta, GA: Centers for Disease Control and Prevention; 1990. [Google Scholar]
- 32.Rosenbaum JE. Truth or consequences: the intertemporal consistency of adolescent self-report on the Youth Risk Behavior Survey. Am J Epidemiol. 2009;169(11):1388–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods ER, Buka SL, Martin CR, et al. Assessing youth risk behavior in a clinical trial setting: lessons from the infant health and development program. J Adolesc Health. 2010;46(5):429–436 [DOI] [PubMed] [Google Scholar]
- 34.Scott DT, Bauer CR, Kraemer HC, Tyson J. A neonatal health index for preterm infants. Pediatr Res. 1989;24(4):263A [Google Scholar]
- 35.Lucas A, Morley R. Outcome of neonatal hypoglycaemia [letter] BMJ. 1999;318(7177):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bode MM, D’Eugenio DB, Mettelman BB, Gross SJ. Predictive validity of the Bayley, third edition at 2 years for intelligence quotient at 4 years in preterm infants. J Dev Behav Pediatr2014;35(9):570–575 [DOI] [PubMed] [Google Scholar]
- 37.Aber JL, Bennett NG, Conley DC, Li J The effects of poverty on child health and development. Annu Rev Public Health 1997;18:463–483 [DOI] [PubMed] [Google Scholar]