Abstract
OBJECTIVES:
To describe the epidemiology and management of gastroesophageal reflux (GER) medications started in the first year of life for premature infants.
METHODS:
Retrospective review of a cohort of infants ≤35 weeks’ gestation presenting for care by 168 days of age to a 30-site network between 2005 and 2009 (n = 2217) and followed to 3 years of age. Medication frequency, types, and duration of use were assessed. Logistic regression identified factors associated with treatment.
RESULTS:
Thirty-seven percent (812) were prescribed GER medications with 77% begun after NICU discharge. Ninety percent (727) received histamine-2 receptor antagonists, 33% (269) proton pump inhibitors, 22% (182) prokinetics; 40% (325) received >1 medication. Outpatient medication was initiated at 95 ± 69 days of life for total of 294 ± 249 days (interquartile ratio: 117–359). Feeding issues (adjusted odds ratio [aOR] 2.05, 95% confidence interval [CI]: 1.24–3.39) were associated with outpatient initiation. Forty-three percent (322) of infants started before 6 months were still on at 1 year of age associated with gestational age <32 weeks (aOR 1.76, 95% CI: 1.16–2.67), chronic lung disease (aOR 2.59, 95% CI: 1.29–5.22), and reactive airways disease (aOR 1.67, 95% CI: 1.05–2.65).
CONCLUSIONS:
Of the 37% of the cohort on GER medications, 77% were started after NICU discharge with prolonged use of medications. Feeding difficulties were associated with starting medication and markers of chronic lung disease with continuation of treatment. With uncertain evidence of efficacy, use of these medications in a high-risk population should be carefully evaluated.
What’s Known on This Subject:
Premature infants are frequently diagnosed with gastroesophageal reflux. Efficacy and safety concerns have resulted in more judicious use of reflux medications in the NICU, although practice variation exists. Once started, many continue treatment at discharge.
What This Study Adds:
How these medications are managed after discharge is unknown. The majority of discharged premature infants receiving reflux medications were started on these in the ambulatory setting. Prolonged and concurrent use of medications was found.
Gastroesophageal reflux (GER) disease is a common diagnosis for premature infants in NICUs. Overall incidence of the disease has been reported to be ∼11.2%, but differences in rates of diagnosis have been reported.1–3 Consensus-based guidelines for the management of GER were developed in 1997 by the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) working group4 and in 2001 by the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN).5 In 2009, ESPGHAN and NASPGHAN combined to publish guidelines6 that were endorsed by the American Academy of Pediatrics in 2013.7 According to the guidelines, the management of infants differs from that of older children in regard to the judicious use of medications, in part due to difficulties in diagnosis. The diagnosis of GER disease is often made clinically; regurgitation is common in infants, and symptoms such as irritability, distress, vomiting, and even weight loss are not specific to GER disease. These issues coupled with a lack of evidence for acid-related disorders in many infants alter the approach to a symptomatic infant.
Infants started on reflux medications in the NICU are frequently discharged from the hospital on these medications.3,8,9 Wade et al10 reported that 13% of the medications refilled for premature infants in the first year of life were reflux medications. However, concerns about the efficacy of these medications in infancy,6,7,11 which include a lack of symptomatic response to acid suppression in controlled studies12,13 and potentially serious complications such as necrotizing enterocolitis and bacteremia/sepsis,14,15 have led to more judicious use of GER medications in the NICU setting.16 Even with these guidelines and lack of efficacy, there remains wide variation in the use of medications to manage GER in NICUs among different health systems,2,3 within the same health system,17 between smaller and larger NICUs, and among different specialists involved in an infant’s care.18
There were no studies found regarding post-NICU management of reflux medication including duration of use, initiation of medication postdischarge, and factors associated with treatment. Thus, the purpose of this study was to describe the epidemiology and management of GER medications started during the first year of life for premature infants as documented in a primary care setting.
Methods
Setting and Study Population
A retrospective cohort design evaluated care received by preterm infants (defined by a gestational age of ≥22 and ≤35 weeks) in the primary care network at the Children’s Hospital of Philadelphia. Those born between January 1, 2005, and January 1, 2009, who presented for primary care by 168 days of life and seen at furthest to 3 years of life (1095 days) were included (N = 2316). The network included 30 urban and suburban sites in Pennsylvania and New Jersey. Infants with syndromes, congenital anomalies, and disorders of the gastrointestinal tract and perinatal asphyxia were excluded (n = 99; see Supplemental Table 4). The remaining 2217 (95.7%) met eligibility criteria.
This study was approved by the Children’s Hospital of Philadelphia Institutional Review Board. Patient information was documented by providers during ambulatory health care encounters in the electronic record using the EPIC Hyperspace system (Verona, WI).
Determination of GER Medication Use
The electronic record was searched for all infants with the diagnosis of GER using International Classification of Diseases, Ninth Revision, codes 530.11, 530.10, 530.12, 530.81, and 787.1. The ambulatory medication record was searched for reflux medications using both generic and trade names regardless of GER diagnosis. In addition, a text search of all ambulatory notes in the first year of life was performed to capture prescription of medications by subspecialists that were not included in the ambulatory medication record. Searched medications included histamine-2 receptor antagonists (H2RAs): ranitidine (Zantac); nizatidine (Axid), famotidine (Pepcid); proton pump inhibitors (PPIs): esomeprazole (Nexium), omeprazole (Prilosec), lansoprazole (Prevacid), pantoprazole (Protonix); prokinetic: metoclopramide (Reglan); and cholinergic: bethanechol (Urecholine).
Medication stops and starts were hand-coded and reconciled with the medication file. If a discrepancy was found, the information hand-coded from provider notes was used because of concerns about the accuracy of medication file reconciliation. If start or stop dates were unclear, the dates were interpolated to the midpoint between visits when the medication was last noted to be used and first noted to be discontinued (median 19 days after previous visit, interquartile ratio [IQR] 9–35 days). Duration of treatment was assessed until 3 years (1095 days) of age.
Because the first notation in the ambulatory medication record might reflect a refill of NICU medication, records from the initial presentation to the primary care site, along with any NICU discharge information provided to the outpatient provider, were reviewed manually to ensure this was a preexisting medication and not one that was started initially at the first visit.
Confounding Variable Definitions
Demographic factors included gestational age, birth weight, ethnicity, race, sex, and multiple gestation, as well as site of primary care. Insurance type was divided into 3 exclusive categories: any office visit without insurance; any use of federal Medicaid insurance without ever being uninsured; and sole use of private insurance during the study period. Potential confounding medical factors as noted in the ambulatory setting during the first year of life included chronic lung disease, reactive airways disease, failure to thrive, aspiration, airway malacia, feeding difficulties, dysphagia, fundoplication, acute life-threating event (ALTE)/apnea, the need for tube feedings, and supplemental oxygen (see Supplemental Table 5).
Data Analysis
Descriptive analysis included cohort demographics, frequencies of medications, chronological and adjusted age at medication initiation, duration of use, use of >1 reflux medication, and simultaneous use of >1 medication. Univariate analysis included χ2 analysis and binary logistic regression identifying factors associated with medication initiation in the outpatient setting, duration of use, and the use of >1 medication. A multivariate logistic regression model quantified the association of the marginal impact of these factors on the likelihood that a medication was ever prescribed; multiple medications were prescribed; and the likelihood that a child received medications at 6 months and 1 year chronological age. As a secondary analysis, the model was repeated using 6 months and 1 year of age after adjusting for gestational age at birth. These models used random effects for outpatient clinical site to control for clustering of outcomes and use by site. Because few infants in this cohort had a fundoplication, this variable was not included in the regression models. A Cox proportional hazards model assessed factors associated with duration of treatment using fixed practice effects to control for differences in duration of treatment by outpatient site.
Results
Univariate Analysis
Overall, 37% (812) of the infants in the cohort were on GER medications during the first year of life. Of these 185 (23%) were started in the NICU, with the remainder (627; 77%), started after presentation to the ambulatory network (Table 1). Infants who were treated tended to be of lower gestational age (P < .0001), white (P < .0001), privately insured (P = .003), be of multiple gestation (P < .0001), and had more medical complications (Table 1). Nearly all received H2RAs, 90% (727), followed by PPIs 33% (269), prokinetics 22% (182), and cholinergics 2% (18).
TABLE 1.
Patient Characteristics per GER Medication Use
| No GER Medication | GER Medication | P | NICU Initiation | Outpatient Initiation | P | |
|---|---|---|---|---|---|---|
| Total (n = 2217) | 1405 (63%) | 812 (37%) | 185 (23%) | 627 (77%) | ||
| Gestational age category (wk) | <.0001 | <.0001 | ||||
| <28 | 79 (6%) | 103 (13%) | 46 (25%) | 57 (9%) | ||
| 28–<32 | 211 (15%) | 226 (28%) | 82 (44%) | 144 (23%) | ||
| 32–<34 | 311 (22%) | 174 (21%) | 27 (15%) | 147 (23%) | ||
| 34–35 | 804 (57%) | 309 (38%) | 30 (16%) | 279 (45%) | ||
| Race/ethnicity | <.0001 | <.0001 | ||||
| White | 522 (37%) | 432 (53%) | 83 (45%) | 349 (56%) | ||
| Black | 677 (48%) | 276 (34%) | 91 (49%) | 185 (30%) | ||
| Asian | 39 (3%) | 12 (1%) | 1 (<1%) | 11 (2%) | ||
| Other/unknown | 167 (12%) | 92 (11%) | 10 (5%) | 82 (13%) | ||
| Hispanic | 77 (5%) | 29 (4%) | .10 | 4 (2%) | 25 (4%) | .15 |
| Male | 705 (50%) | 422 (52%) | .42 | 106 (57%) | 316 (50%) | .10 |
| Multiple gestation | 373 (27%) | 325 (40%) | <.0001 | 68 (37%) | 257 (41%) | .30 |
| Insurance group | .003 | <.0001 | ||||
| Private | 568 (40%) | 388 (48%) | 66 (36%) | 322 (51%) | ||
| Any Medicaid | 557 (40%) | 274 (34%) | 90 (49%) | 184 (29%) | ||
| Any self-pay | 280 (20%) | 150 (18%) | 29 (16%) | 121 (19%) | ||
| Medical factors | ||||||
| Chronic lung disease | 26 (2%) | 75 (9%) | <.0001 | 36 (19%) | 39 (6%) | <.0001 |
| Failure to thrive | 35 (2%) | 65 (8%) | <.0001 | 20 (11%) | 45 (7%) | .12 |
| Aspiration | 8 (<1%) | 20 (2%) | <.0001 | 11 (6%) | 9 (1%) | .002 |
| Airway malacia | 10 (<1%) | 39 (5%) | <.0001 | 13 (7%) | 26 (4%) | .12 |
| Feeding difficulties | 338 (24%) | 212 (26%) | .28 | 31 (17%) | 181 (29%) | .0001 |
| Dysphagia | 0 | 10 (1%) | <.0001 | 8 (4%) | 2 (<1%) | .0002 |
| ALTE/apnea | 134 (10%) | 202 (25%) | <.0001 | 84 (45%) | 118 (19%) | <.0001 |
| Tube feeding | 0 | 21 (3%) | <.0001 | 14 (8%) | 7 (1%) | <.0001 |
| Supplemental oxygen | 10 (<1%) | 34 (4%) | <.0001 | 17 (9%) | 17 (3%) | .0005 |
| Fundoplication | 1 (<1%) | 5 (<1%) | .03 | 4 (2%) | 1 (<1%) | .01 |
| Reactive airway disease | 115 (8%) | 141 (17%) | <.0001 | 42 (23%) | 99 (16%) | .03 |
Start and stop dates were available for 66% of the cohort and interpolated for the remainder. Ambulatory GER medication was started at mean chronological age 95 ± 69 days (median 73; IQR 46–124); and mean adjusted age of 44 +/−62 days (median 21; IQR 0-56), adjusting for prematurity.
Multiplicity of Medications
More than one-third of the treated infants, 40% (325), were on >1 GER medication during the first year of life. Of those, the majority, 73% (238), were on those medications simultaneously, with 61% (197) on 2, 11% (37) on 3, and 1% (4) on 4 medications at the same time. Simultaneous treatment with a H2RA and PPI was found in 30% (99) of those treated with >1 medication with simultaneous use averaging 118 ± 146 days (median 61; IQR 20–182). Simultaneous use of 3 acid-blocking medications was found in 3% (11) of infants averaging 87 ± 93 days (median 32; IQR 11–136).
Multivariate Analysis
Feeding issues (adjusted odds ratio [aOR] 2.05, 95% confidence interval [CI] 1.24–3.39) were associated with the start of medication in the outpatient setting (Table 2). Dysphagia, ALTE/apnea, and lower gestational age were associated with a decreased likelihood of being started on GER medication in the outpatient setting. Practice site accounted for 6.2% (95% CI: 1.6–20.6) of the residual variation in the likelihood of receiving a medication as an outpatient after adjusting for the covariates shown in Table 2.
TABLE 2.
Predictors to Starting GER Medication as an Outpatient and Receiving >1 Medication (n = 812)
| Starting GER Medication as an Outpatient | Receiving >1 GER Medication | |||
|---|---|---|---|---|
| aOR (95%) | P | aOR (95%) | P | |
| Gestational age (wk) | ||||
| <28 | 0.25 (0.13–0.49) | .0000 | 1.95 (1.1–3.46) | .02 |
| 28–<32 | 0.27 (0.16–0.45) | .0000 | 2.36 (1.59–3.52) | .000 |
| 32–<34 | 0.74 (0.41–1.35) | .33 | 1.31 (0.86–1.99) | .20 |
| 34–35 | Reference | Reference | ||
| Race/ethnicity | ||||
| White | Reference | Reference | ||
| Black | 0.71 (0.41–1.24) | .23 | 0.74 (0.48–1.14) | .18 |
| Asian | 3.47 (0.43–32.34) | .23 | 0.69 (0.19–2.5) | .58 |
| Other/ Unknown | 1.56 (0.72–3.39) | .26 | 0.77 (0.46–1.28) | .31 |
| Hispanic | 1.40 (0.43–4.57) | .58 | 0.94 (0.41–2.14) | .88 |
| Male | 0.69 (0.47–1.02) | .07 | 1.22 (0.9–1.65) | .21 |
| Multiple gestation | 1.08 (0.72–1.63) | .70 | 1.09 (0.8–1.5) | .58 |
| Insurance group | ||||
| Private | Reference | Reference | ||
| Any Medicaid | 0.62 (0.37–1.05) | .07 | 0.92 (0.6–1.41) | .70 |
| Any self-pay | 0.83 (0.46–1.49) | .52 | 1.09 (0.71–1.68) | .69 |
| Medical factors | ||||
| Chronic lung disease | 0.78 (0.4–1.5) | .45 | 1.81 (0.98–3.35) | .06 |
| Failure to thrive | 0.90 (0.43–1.88) | .77 | 1.38 (0.75–2.51) | .30 |
| Aspiration | 0.83 (0.27–2.59) | .75 | 3.17 (0.91–10.97) | .07 |
| Airway malacia | 1.18 (0.48–2.87) | .72 | 1.18 (0.56–2.49) | .66 |
| Feeding difficulties | 2.05 (1.24–3.39) | .005 | 1.46 (1.03–2.06) | .03 |
| Dysphagia | 0.09 (0.01–0.59) | .01 | 0.55 (0.09–3.38) | .52 |
| ALTE/apnea | 0.37 (0.25–0.57) | .000 | 1.07 (0.74–1.55) | .73 |
| Tube feeding | 0.31 (0.08–1.19) | .09 | 4.56 (1.09–19.1) | .04 |
| Supplemental oxygen | 0.81 (0.32–2.01) | .65 | 2.63 (1.07–6.44) | .03 |
| Reactive airway disease | 1.11 (0.66–1.85) | .70 | 1.64 (1.08–2.49) | .02 |
We found a strong association with lower gestational age and how GER medications were managed in the ambulatory setting. Factors associated with receipt of >1 GER medication included gestational age <32 weeks (aOR 2.36, 95% CI: 1.59–3.52); feeding difficulties (aOR 1.46, 95% CI: 1.03–2.06), tube feeding (aOR 4.56, 95% CI: 1.09–19.1), need for supplemental oxygen (aOR 2.63, 95% CI: 1.07–6.44), and asthma (aOR 1.64, 95% CI: 1.08–2.49) (Table 2).
Duration of Use
We found prolonged use of medication. For those on medication at NICU discharge, mean duration of use was 375 ± 292 days (median 284, IQR 165–515). For those started after NICU discharge, mean duration of use was 294 ± 249 days (median 225, IQR 117–359). For infants started on GER medication before 6 months of age (743 infants), gestational age <32 weeks (aOR 2.16, 95% CI 1.26–3.71) and reactive airways disease (aOR 2.17, 95% CI: 1.09-4.3) were associated with continued medication use at 6 months’ chronological age (Table 3). There were 69 infants who started initial treatment after 6 months of age.
TABLE 3.
Predictors to Continued Use of Medications for Infants Started Before 6 Months of Age (n = 743)
| Continue Use at 6 Months | Continued Use at 1 Year | Time to Discontinue GER Medicationsa | ||||
|---|---|---|---|---|---|---|
| (n = 607) | (n = 322) | (n = 812) | ||||
| aOR (95% CI) | P | aOR (95% CI) | P | HR (95% CI) | P | |
| Gestational age category (wk) | ||||||
| <28 | 6.91 (2.18–21.86) | .001 | 3.11 (1.65–5.85) | .000 | 0.50 (0.38–0.66) | .000 |
| 28–<32 | 2.16 (1.26–3.71) | .005 | 1.76 (1.16–2.67) | .008 | 0.69 (0.57–0.84) | .000 |
| 32–<34 | 1.23 (0.75–2.02) | .42 | 1.07 (0.67–1.71) | .77 | 0.84 (0.69–1.03) | .09 |
| 34–35 | Reference | Reference | Reference | |||
| Race/ethnicity | ||||||
| White | Reference | Reference | Reference | |||
| Black | 0.59 (0.34–1.03) | .06 | 0.7 (0.43–1.13) | .14 | 1.17 (0.94–1.45) | .16 |
| Asian | 0.36 (0.09–1.36) | .13 | 0.72 (0.19–2.68) | .62 | 1.88 (1.04–3.43) | .04 |
| Other/ Unknown | 0.92 (0.47–1.79) | .81 | 0.57 (0.33–1.00) | .05 | 1.39 (1.09–1.77) | .007 |
| Hispanic | 1.15 (0.36–3.62) | .82 | 1.56 (0.66–3.69) | .31 | 092 (0.62–1.36) | .67 |
| Male | 1.19 (0.8–1.76) | .39 | 0.86 (0.63–1.19) | .38 | 0.93 (0.81–1.08) | .35 |
| Multiple gestation | 1.30 (0.86–1.97) | .22 | 0.73 (0.52–1.02) | .07 | 1.1 (0.94–1.27) | .23 |
| Insurance group | ||||||
| Private | Reference | Reference | Reference | |||
| Any Medicaid | 1.08 (0.62–1.87) | .79 | 0.62 (0.39–0.97) | .04 | 1.19 (0.96–1.46) | .11 |
| Any self-pay | 0.89 (0.51–1.54) | .67 | 1.01 (0.64–1.6) | .97 | 0.96 (0.78–1.17) | .67 |
| Medical factors | ||||||
| Chronic lung disease | 1.70 (0.52–5.55) | .38 | 2.59 (1.29–5.22) | .008 | 0.83 (0.62–1.12) | .22 |
| Failure to thrive | 1.77 (0.65–4.83) | .26 | 1.87 (0.97–3.62) | .06 | 0.62 (0.47–0.82) | .001 |
| Airway malacia | 2.07 (0.58–7.41) | .26 | 2.79 (1.24–6.3) | .01 | 0.75 (0.53–1.05) | .09 |
| Feeding difficulties | 1.03 (0.66–1.61) | .89 | 1.3 (0.9–1.88) | .16 | 0.87 (0.74–1.03) | .10 |
| Dysphagia | 0.57 (0.05–6.55) | .65 | 0.62 (0.09–4.08) | .62 | 1.06 (0.53–2.1) | .87 |
| ALTE/apnea | 0.77 (0.47–1.25) | .29 | 0.95 (0.64–1.4) | .80 | 1.03 (0.86–1.22) | .78 |
| Tube feeding | 1.67 (0.17–16.26) | .66 | 2.44 (0.55–10.8) | .24 | 0.55 (0.34–0.89) | .02 |
| Supplemental oxygen | 0.53 (0.15–1.86) | .32 | 1.58 (0.53–4.33) | .38 | 0.76 (0.52–1.11) | .16 |
| Reactive airway disease | 2.17 (1.09–4.3) | .03 | 1.67 (1.05–2.65) | .03 | 0.73 (0.6–0.89) | .002 |
HR, hazard ratio.
Time to discontinue all medications used a Cox proportional hazards model, where ratios <1 indicated longer use of medications, and ratios >1 indicated shorter use of medications compared with the reference category.
By chronological age of 1 year, 43% (322) of the infants started on medication before 6 months were still being treated. Factors associated with a higher likelihood of medication at chronological age of 1 year included gestational age <32 weeks (aOR 1.76, 95% CI: 1.16–2.67); chronic lung disease (aOR 2.59, 95% CI: 1.29–5.22), airway malacia (aOR 2.79, 95% CI: 1.24–6.3), and reactive airways disease (aOR 1.67, 95% CI: 1.05–2.65) (Table 3). Those with any use of Medicaid insurance were less likely to be on medication at 1 year (aOR 0.62, 95% CI: 0.39–0.97). As a sensitivity analysis, we reran the models looking at continued use at 6 months and adjusted age of 1 year. Overall, the adjusted age models confirmed the chronological age models with the lowest gestational age infants and those with markers of lung disease continuing to receive treatment at 1 year. In addition, failure to thrive was a predictor for continued use at 1 year of adjusted age. Reactive airways disease was not a significant predictor using adjusted age, and multiple-gestation infants were less likely to be on medication at 1 year of adjusted age (see Supplemental Table 6).
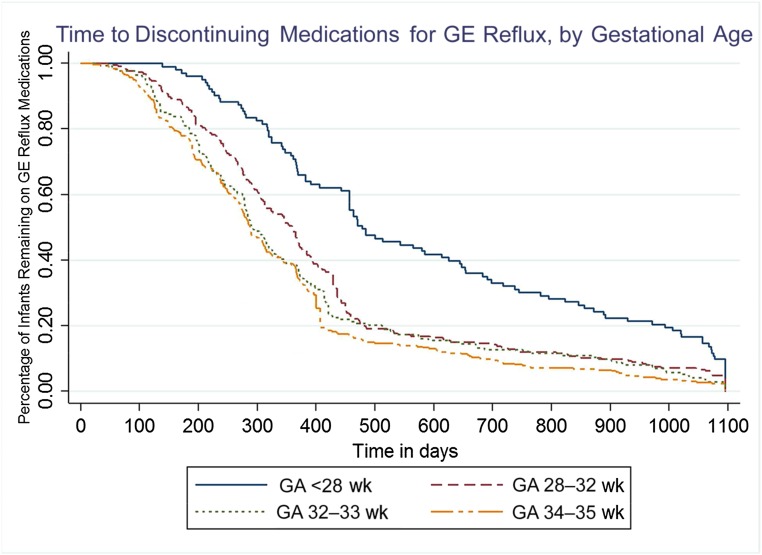
Gestational age <32 weeks was associated with a 31% longer use of GER medications (hazard ratio 0.69; 95% CI 0.57–0.84), and a gestational age of <28 weeks was associated with a 50% longer use (hazard ratio 0.50; 95% CI 0.38–0.66) compared with a gestational age of 34 to 35 weeks (Table 3). Of the treated infants, 92 were lost to follow-up between 18 and 36 months. By 3 years of age, 4% (32) of the children were still being treated (Fig 1).
FIGURE 1.
Kaplan-Meier survival estimates by gestational age (GA). Lower GA was associated with longer receipt of medication.
Discussion
Most literature regarding the management of GER for premature infants has focused on the NICU setting. To our knowledge, this is the first study to explore the outpatient management of GER medications for premature infants who were started during the first year of life. We found that close to 40% of our cohort received reflux medications. Previous reports of the prevalence of GER disease in premature infants have been derived from the NICU setting.1–3 The inclusion of what happens after discharge reveals a greater scope of this issue for premature infants. We found that more than three-quarters of the premature infants on reflux medications in the ambulatory setting were not on these medications at NICU discharge but instead were started on medication after discharge with modest variation between outpatient sites. Although there is increasing awareness of the need for judicious use of these medications in the NICU setting,16 we found frequent use of these medications for premature infants in the outpatient setting.
The efficacy of reflux medications in the management of GER disease in infants has been questioned.6,7,11,19 Although there remains a role for these medications in documented reflux disease, empirical treatment of infants is not recommended.6,7 In addition, adverse outcomes such as community-acquired pneumonia,20–22 gastroenteritis,21,23 lower respiratory tract infections,12 Clostridium difficile infection,24,25 alterations in lung microflora,26 and fractures27,28 have been reported with the use of acid-blocking medications.
H2RAs were the most commonly prescribed medication in our study. A recent systematic review found that, because of the low quality of available evidence, conclusions regarding the safety and efficacy of H2RAs for infants could not be determined, and H2RAs should be used cautiously and only with acid-confirmed reflux disease.29 Rapid tachyphylaxis with H2RAs has been reported starting as early as second day/second dose.30 The associated costs and value of using these medications with questionable efficacy have significant health services implications.
PPIs have not been shown to be effective for those <1 year of age in the management of GER disease symptoms.12,13,31–33 However, we found frequent use of PPIs in our cohort with 22% (180) of those treated with a reflux medication receiving a PPI. These findings are similar to information from US retail pharmacies, which in 2010 found that PPIs were a top drug dispensed to children from US retail pharmacies.34 Although guidelines suggest consideration of a short-term time-limited trial of medication if other measures fail,6,7 once these medications were started, we found that infants tended to stay on medication for prolonged periods of time, possibly influenced by lung disease and reactive airways, which were predictors for prolonged use of medication. We also found that 40% of the treated infants received >1 reflux medication, with the majority of those infants concurrently receiving ≥2 medications, along with prolonged concurrent use of H2RAs and PPIs. Because premature infants are a medically fragile group, the need for 1 acid-suppression medication, let alone ≥2 in combination, should be given careful consideration. The potential impact of acid suppression on community-acquired illnesses has yet to be explored for this vulnerable population.
In our study, infants were started on medication at a mean chronological age of 3 ± 2 months of age. We found feeding issues to be a predictor for outpatient initiation of medication. Physiologic reflux symptoms are reported to peak at 4 months of age.35 Feeding issues are also common for premature infants.36 Whether this combination of issues is influencing the decision to start treatment, as opposed to actual GER disease, is an important distinction for providers to consider before starting medication. There is a lack of controlled data confirming reflux as the cause of these issues.6 Those with dysphagia, ALTE/apnea, and lower gestational age were less likely to initiate treatment in the outpatient setting, which most likely reflects these infants being started on treatment in the NICU setting.
There were small differences in the likelihood of receiving a GER medication between outpatient providers after controlling for patient-level factors. This degree of variation, however, was not large compared with other medications without strong indications for prescription. An issue that may affect management is that premature infants are frequently followed by specialists in addition to their primary care provider. This multiplicity of providers potentially complicates management and may influence the duration of treatment as ownership of reflux medication management may be unclear.
Although the NASPGHAN/ESPGHAN management guidelines have existed for many years, European studies have found that the majority of surveyed general pediatricians had limited awareness of the guidelines that promote conservative treatment.37,38 Conservative management has been shown to be an effective means to avoid medication for some infants.6,39–41 In 1 study, conservative therapy measures taught in a primary care setting, including feeding modifications, positioning, and tobacco smoke avoidance, resulted in symptom improvement in 78% of the infants with resolution of symptoms in 24%.40 The introduction of a training for European primary care physicians was found to increase compliance to guidelines and significantly decreased prescription of medication.37 Guidelines published by the American Academy of Pediatrics provide decision trees regarding the approach to infants with GER symptoms. Conservative management, parental education, assurance, and the avoidance of medication and additional testing for infants with uncomplicated regurgitation is recommended. However, symptoms of reflux accompanied by weight loss are indicators that further investigation and alteration in clinical management is warranted.7
Limitations for our study included actual stop and start dates not being clearly documented for approximately a third of the dates, thus necessitating interpolation. Because the midpoint between known dates off and on medication was used and the median interpolation was 19 days, this should not have significantly affected our results. The duration of treatment may be an underestimate because a few children were still receiving medication at the conclusion of the 3-year study period. These children may not reflect the experience of the vast majority of infants who stopped medications by 3 years of age. Information regarding diagnostic workup for GER disease was not retrievable for the entire cohort, limiting the ability to determine if treatment was initiated based on clinical opinion or diagnostic evaluation.
Conclusions
A lack of evidence supporting the efficacy and safety of reflux medications has led to changes in neonatal practice. Until now, how these medications were being managed in the ambulatory setting after NICU discharge was unknown. Of the 37% of the premature infants in our cohort on GER medications, 77% were started after NICU discharge with a median duration of 294 days. Feeding difficulties were associated with starting medication and markers of chronic lung with continuation of treatment. With uncertain evidence of efficacy, the rationale for using these medications in this high-risk population should be carefully evaluated.
Glossary
- ALTE
acute life-threatening event
- aOR
adjusted odds ratio
- CI
confidence interval
- ESPGHAN
The European Society for Pediatric Gastroenterology Hepatology and Nutrition
- GER
gastroesophageal reflux
- H2RAs
histamine-2 receptor antagonists
- IQR
interquartile ratio
- NASPGHAN
North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition
- PPI
proton-pump inhibitor
Footnotes
Dr D’Agostino conceptualized and designed the study and drafted the initial manuscript; Ms Passarella carried out the initial analyses and critically reviewed the manuscript; Ms Martin acquired the data and critically reviewed the manuscript; Dr Lorch conceptualized and designed the study and critically revised the initial manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: All phases of this study were supported by National Institutes of Health grant R01 HD057168. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2016-2849.
References
- 1.Jadcherla SR, Slaughter JL, Stenger MR, Klebanoff M, Kelleher K, Gardner W. Practice variance, prevalence, and economic burden of premature infants diagnosed with GERD. Hosp Pediatr. 2013;3(4):335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhillon AS, Ewer AK. Diagnosis and management of gastro-oesophageal reflux in preterm infants in neonatal intensive care units. Acta Paediatr. 2004;93(1):88–93 [PubMed] [Google Scholar]
- 3.Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Neonatal histamine-2 receptor antagonist and proton pump inhibitor treatment at United States children’s hospitals. J Pediatr. 2016;174:63–70.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenplas Y, Belli D, Benhamou P, et al. A critical appraisal of current management practices for infant regurgitation—recommendations of a working party. Eur J Pediatr. 1997;156(5):343–357 [DOI] [PubMed] [Google Scholar]
- 5.Rudolph CD, Mazur LJ, Liptak GS, et al. ; North American Society for Pediatric Gastroenterology and Nutrition . Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32(suppl 2):S1–S31 [DOI] [PubMed] [Google Scholar]
- 6.Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. ; North American Society for Pediatric Gastroenterology Hepatology and Nutrition; European Society for Pediatric Gastroenterology Hepatology and Nutrition . Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498–547 [DOI] [PubMed] [Google Scholar]
- 7.Lightdale JR, Gremse DA; Section on Gastroenterology, Hepatology, and Nutrition . Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1684 [DOI] [PubMed] [Google Scholar]
- 8.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–1987 [DOI] [PubMed] [Google Scholar]
- 9.Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM; National Institute of Child Health and Human Development Neonatal Research Network . Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics. 2008;121(1):22–27 [DOI] [PubMed] [Google Scholar]
- 10.Wade KC, Lorch SA, Bakewell-Sachs S, Medoff-Cooper B, Silber JH, Escobar GJ. Pediatric care for preterm infants after NICU discharge: high number of office visits and prescription medications. J Perinatol. 2008;28(10):696–701 [DOI] [PubMed] [Google Scholar]
- 11.Malcolm WF, Cotten CM. Metoclopramide, H2 blockers, and proton pump inhibitors: pharmacotherapy for gastroesophageal reflux in neonates. Clin Perinatol. 2012;39(1):99–109 [DOI] [PubMed] [Google Scholar]
- 12.Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154(4):514–520.e514 [DOI] [PubMed] [Google Scholar]
- 13.Davidson G, Wenzl TG, Thomson M, et al. Efficacy and safety of once-daily esomeprazole for the treatment of gastroesophageal reflux disease in neonatal patients. J Pediatr. 2013;163(3):692–698.e1–2 [DOI] [PubMed] [Google Scholar]
- 14.Bianconi S, Gudavalli M, Sutija VG, Lopez AL, Barillas-Arias L, Ron N. Ranitidine and late-onset sepsis in the neonatal intensive care unit. J Perinat Med. 2007;35(2):147–150 [DOI] [PubMed] [Google Scholar]
- 15.Terrin G, Passariello A, De Curtis M, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/129/1/40 [DOI] [PubMed] [Google Scholar]
- 16.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr, Smith PB; Best Pharmaceuticals for Children Act—Pediatric Trials Network . Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31(9):811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barney CK, Baer VL, Scoffield SH, Lambert DK, Cook M, Christensen RD. Lansoprazole, ranitidine, and metoclopramide: comparison of practice patterns at 4 level III NICUs within one healthcare system. Adv Neonatal Care. 2009;9(3):129–131 [DOI] [PubMed] [Google Scholar]
- 18.Golski CA, Rome ES, Martin RJ, et al. Pediatric specialists’ beliefs about gastroesophageal reflux disease in premature infants. Pediatrics. 2010;125(1):96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tighe M, Afzal NA, Bevan A, Hayen A, Munro A, Beattie RM. Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database Syst Rev. 2014;11(11):CD008550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955–1960 [DOI] [PubMed] [Google Scholar]
- 21.Canani RB, Cirillo P, Roggero P, et al. ; Working Group on Intestinal Infections of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP) . Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117(5). Available at: www.pediatrics.org/cgi/content/full/117/5/e817 [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology. 2009;20(6):800–806 [DOI] [PubMed] [Google Scholar]
- 23.Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol. 2009;104(suppl 2):S10–S16 [DOI] [PubMed] [Google Scholar]
- 24.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011–1019 [DOI] [PubMed] [Google Scholar]
- 25.Nylund CM, Eide M, Gorman GH. Association of Clostridium difficile infections with acid suppression medications in children. J Pediatr. 2014;165(5):979–984.e971 [DOI] [PubMed] [Google Scholar]
- 26.Rosen R, Amirault J, Liu H, et al. Changes in gastric and lung microflora with acid suppression: acid suppression and bacterial growth. JAMA Pediatr. 2014;168(10):932–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok CS, Yeong JK, Loke YK. Meta-analysis: risk of fractures with acid-suppressing medication. Bone. 2011;48(4):768–776 [DOI] [PubMed] [Google Scholar]
- 28.Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Pol R, Langendam M, Benninga M, van Wijk M, Tabbers M. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatr. 2014;168(10):947–954 [DOI] [PubMed] [Google Scholar]
- 30.McRorie JW, Kirby JA, Miner PB. Histamine2-receptor antagonists: rapid development of tachyphylaxis with repeat dosing. World J Gastrointest Pharmacol Ther. 2014;5(2):57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services Proton pump inhibitors: use in pediatric patients. August 2013. Available at: https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-pediatric-factsheet.pdf. Accessed July 31, 2015
- 32.van der Pol RJ, Smits MJ, van Wijk MP, Omari TI, Tabbers MM, Benninga MA. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127(5):925–935 [DOI] [PubMed] [Google Scholar]
- 33.Chen IL, Gao WY, Johnson AP, et al. Proton pump inhibitor use in infants: FDA reviewer experience. J Pediatr Gastroenterol Nutr. 2012;54(1):8–14 [DOI] [PubMed] [Google Scholar]
- 34.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130(1):23–31 [DOI] [PubMed] [Google Scholar]
- 35.Nelson SP, Chen EH, Syniar GM, Christoffel KK; Pediatric Practice Research Group . Prevalence of symptoms of gastroesophageal reflux during infancy. A pediatric practice-based survey. Arch Pediatr Adolesc Med. 1997;151(6):569–572 [DOI] [PubMed] [Google Scholar]
- 36.DeMauro SB, Patel PR, Medoff-Cooper B, Posencheg M, Abbasi S. Postdischarge feeding patterns in early- and late-preterm infants. Clin Pediatr (Phila). 2011;50(10):957–962 [DOI] [PubMed] [Google Scholar]
- 37.Quitadamo P, Urbonas V, Papadopoulou A, et al. Do pediatricians apply the 2009 NASPGHAN-ESPGHAN guidelines for the diagnosis and management of gastroesophageal reflux after being trained? J Pediatr Gastroenterol Nutr. 2014;59(3):356–359 [DOI] [PubMed] [Google Scholar]
- 38.Quitadamo P, Papadopoulou A, Wenzl T, et al. European pediatricians’ approach to children with GER symptoms: survey of the implementation of 2009 NASPGHAN-ESPGHAN guidelines. J Pediatr Gastroenterol Nutr. 2014;58(4):505–509 [DOI] [PubMed] [Google Scholar]
- 39.Shalaby TM, Orenstein SR. Efficacy of telephone teaching of conservative therapy for infants with symptomatic gastroesophageal reflux referred by pediatricians to pediatric gastroenterologists. J Pediatr. 2003;142(1):57–61 [DOI] [PubMed] [Google Scholar]
- 40.Orenstein SR, McGowan JD. Efficacy of conservative therapy as taught in the primary care setting for symptoms suggesting infant gastroesophageal reflux. J Pediatr. 2008;152(3):310–314 [DOI] [PubMed] [Google Scholar]
- 41.Orenstein SR. Infant GERD: symptoms, reflux episodes & reflux disease, acid & non-acid reflux—implications for treatment with PPIs. Curr Gastroenterol Rep. 2013;15(11):353. [DOI] [PubMed] [Google Scholar]