Abstract
Ochratoxin A (OTA) contamination has been established as a world-wide problem. In this study, the strains with the ability of OTA production were screened by analyzing the green fluorescence of the isolates colonies from the grapes in Zhenjiang with 365 nm UV light and confirmed by HPLC with fluorescent detection (HPLC-FLD). The results showed that seven isolates acquired the characteristic of the fluorescence, of which only five showed the ability of OTA production as confirmed by HPLC-FLD analysis. The five OTA-producing strains were identified based on comparative sequence analysis of three conserved genes (ITS, BenA and RPB2) of the strains, and they are Talaromyces rugulosus (O1 and Q3), Penicillium commune (V5-1), Penicillium rubens (MQ-5) and Aspergillus aculeatus (MB1-1). There are two Penicillium species of the five OTA-producing strains and our study is the first to report that P. rubens, T. rugulosus and A. aculeatus can produce OTA. This work would contribute to comprehensively understanding the fungi with an OTA-producing ability in grapes before harvest and then take effective measures to prevent OTA production.
Keywords: screening, identification, ochratoxin A (OTA), fungi, grapes
1. Introduction
Many fungi produce mycotoxins that can cause acute or chronic intoxication and damage to humans and animals after ingestion of contaminated food and feed [1]. Among the mycotoxins, aflatoxins and ochratoxin A (OTA) occupy special places due to their high occurrence, widespread distribution and acute toxicity. As one of the first group of secondary metabolites of fungi that are toxic to animals, OTA, like aflatoxins started the distinctive science of mycotoxicology in the 1960s [2,3]. OTA is the most toxic member of the Ochratoxin group that includes OTA, its methyl ester, its ethyl ester (OTC), 4-hydroxyochratoxin A (4-OH OTA), ochratoxin B (OTB) and its methyl and ethyl esters and ochratoxin α (OTα). OTA is nephrotoxic, hepatotoxic, teratogenic and immunotoxic to animals and has been associated with fatal endemic human nephropathies [4,5]. Toxicity studies have revealed that OTA mainly affects the kidney and liver, after absorption from the gut and circulation via the portal vein, inducing hepatotoxicity in rats and hepatocellular carcinomas in mice [6]. The European Commission has established maximum allowable limits for this toxin in some food products. A tolerable daily intake (TDI) of no more than 5 ng OTA/kg bw/day is recommended [7].
The occurrence of OTA was mainly attributed to contamination by toxigenic molds with the ability of toxin production before or after harvesting. OTA is a ubiquitous mycotoxin produced by Aspergillus and Penicillium species that can be detected in a wide variety of agricultural commodities, livestock products and processed food [7]. OTA was firstly described as a secondary metabolite of Aspergillus ochraceus from laboratory experiments [2]. In addition, there have been reports about the production of OTA by other strains, mainly Aspergillus carbonarius and a small percentage of isolates of the closely related species Aspergillus niger [8,9,10,11,12,13,14]. Soon after the isolation of OTA from A. ochraceus, the formation of OTA by Penicillium viridicatum was reported [3]. The species was later correctly identified as Penicillium verrucosum [15,16,17]. In fact, the natural occurrence and practical importance of OTA was firstly linked to Penicillium species [18,19]. Afterwards, some other OTA-producing strains of Penicillium genus were isolated and all the OTA-producing strains were separated into two large groups: P. verrucosum and Penicillium nordicum [17,20]. In summary, A. ochraceus and P. verrucosum were the main OTA producing species. However, A. carbonarius, as another major source of OTA, has also been identified mainly in grapes [21].
Although cereals are known to be particularly susceptible to OTA contamination [22,23], there is an increasing occurrence of OTA in a large variety of foods, such as beer [24,25] and milk [26]. OTA has also been recently reported in grapes and grape juices [27,28,29,30]. The occurrence of OTA in wine is linked to the presence of molds on grapes. Previous studies have shown that the occurrence of OTA is likely due to environmental conditions (climate) and badly controlled harvesting procedures, poor drying and inadequate storage conditions [31,32].
The objectives of this study were to screen the natural OTA-producing fungi related to preharvest decay and OTA contamination of grapes and test their OTA production capacity in vitro with special attention to those not reported in literature.
2. Results
2.1. Preliminary Screening of OTA-Producing Fungi by 365 nm Ultraviolet Light
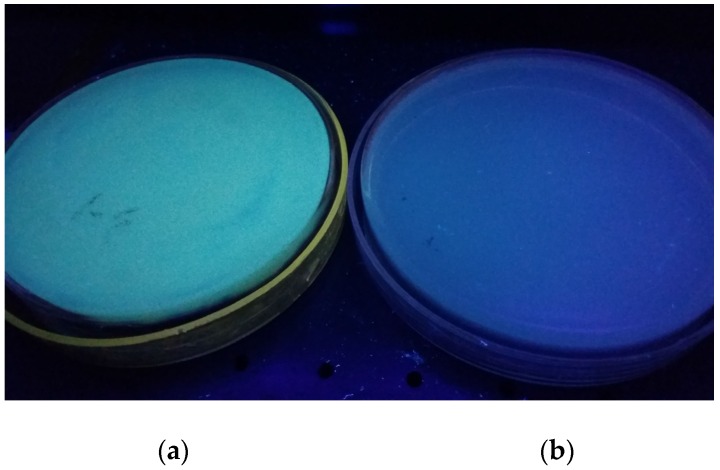
One hundred and thirty-nine molds were isolated by macroscopic characters (colony growth and colony morphology) and microscopic characters. The isolates were cultured for 7 or 14 days to accumulate metabolites in the solid culture medium. In 365 nm ultraviolet light, there was one suspected Aspergillus isolate and six other isolates that produced green fluorescence in culture medium. After fumigating with 25%–28% ammonia, all seven of the isolates took on enhanced fluorescence intensity and were recorded as positive strains. The green fluorescence of O1, one of the positive strains, in 365 nm ultraviolet light was showed in Figure 1a, but there was no fluorescence observed in the negative strain (Figure 1b).
Figure 1.
Green fluorescence (a) on the reverse of the colony of O1 (an Ochratoxin A (OTA)-producing strain) grown on Czapek’s Agar medium (CA) when was exposed to long wavelength UV light (365 nm). No fluorescence (b) was displayed by a non-OTA-producing strain in the same conditions.
2.2. Confirmation of Positive Strains by HPLC-FLD
The OTA-producing ability of the above positive strains was confirmed by HPLC-FLD. The HPLC results confirmed that five of the seven positive strains could produce OTA (Table 1). Therefore, two false positive strains analyzed by HPLC-FLD were screened out and the isolates O1, Q3, V5-1, MQ-5 and MB1-1 have been confirmed as OTA-producing strains.
Table 1.
Ochratoxin A (OTA) concentration produced by the five positive strains analyzed by high-pressure liquid chromatography with a fluorescence detector (HPLC-FLD). Each value is the mean of three experiments.
| Isolates | OTA Content (ng/g) |
|---|---|
| O1 | 97.5 ± 2.8 |
| Q3 | 50.11 ± 2.1 |
| V5-1 | 43.94 ± 1.8 |
| MQ-5 | 96.86 ± 3.2 |
| MB1-1 | 25.9 ± 1.9 |
The OTA produced by the toxicogenic strains cultured at 25 °C for seven (Aspergillus isolate) or 14 days (other isolates) was respectively higher than that produced by corresponding strains cultured in other conditions (data not shown). The maximum and minimum concentrations of OTA production were 97.5 ng/g by O1 and 25.9 ng/g by MB1-1, respectively. The OTA produced by the five strains (Table 1) were all significantly higher (p < 0.05) than the minimum level determined by the HPLC-FLD method established for OTA production in grapes.
2.3. Identification of OTA-Producing Fungi
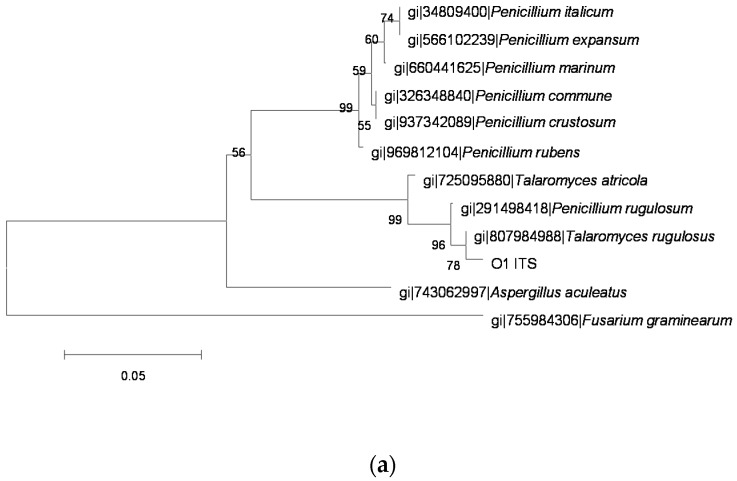
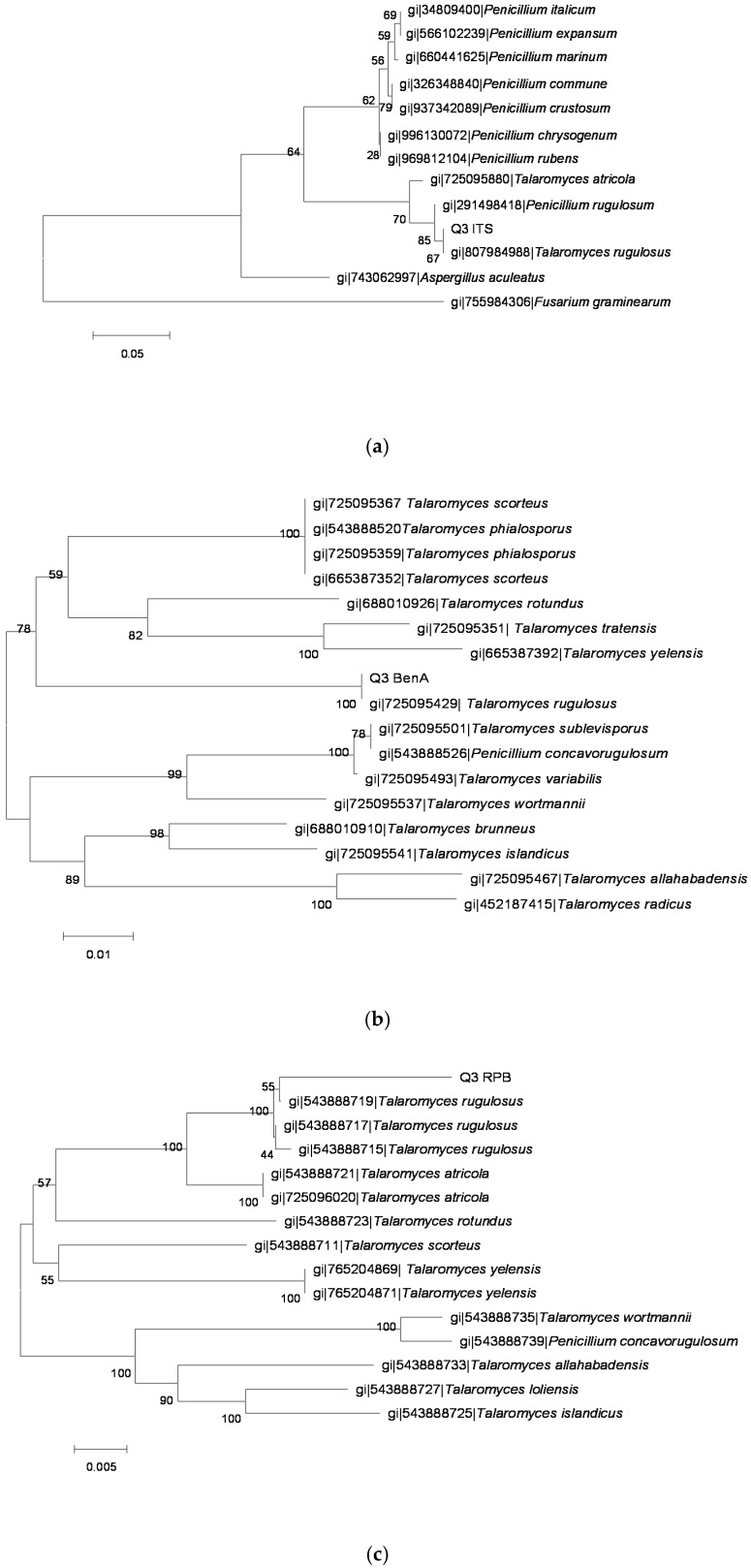
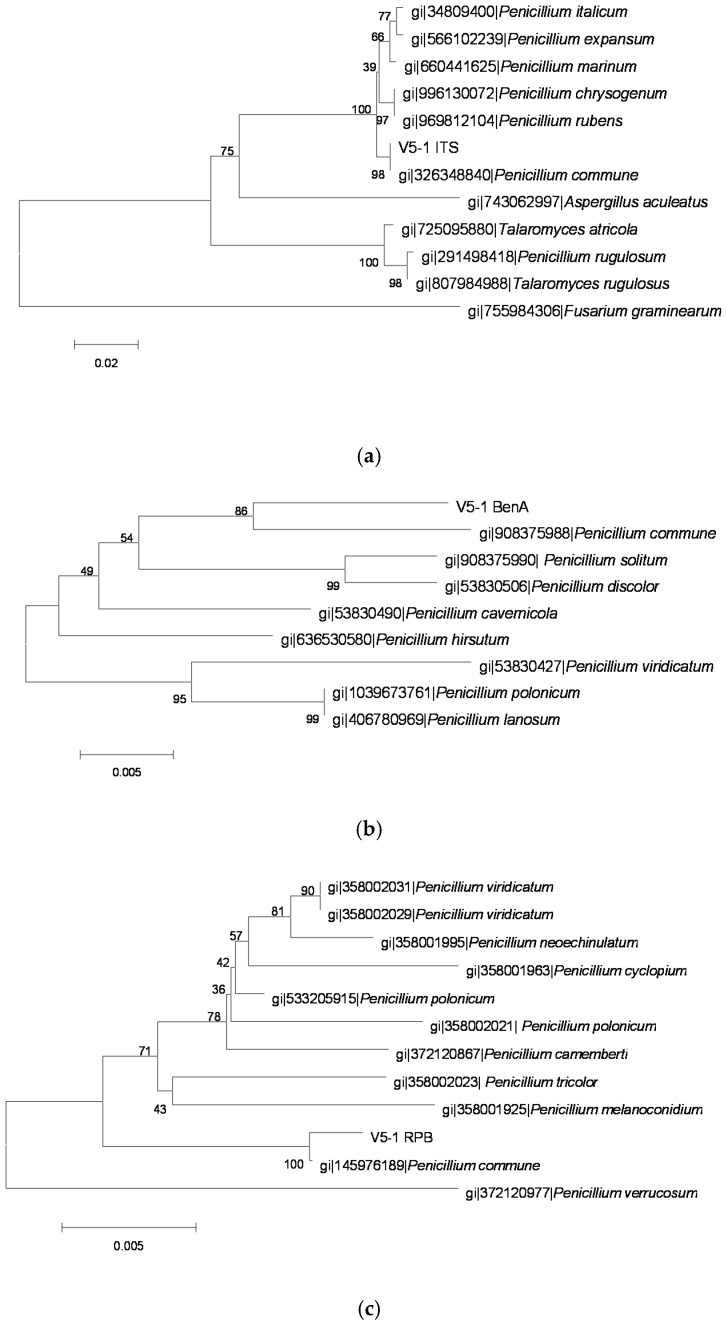
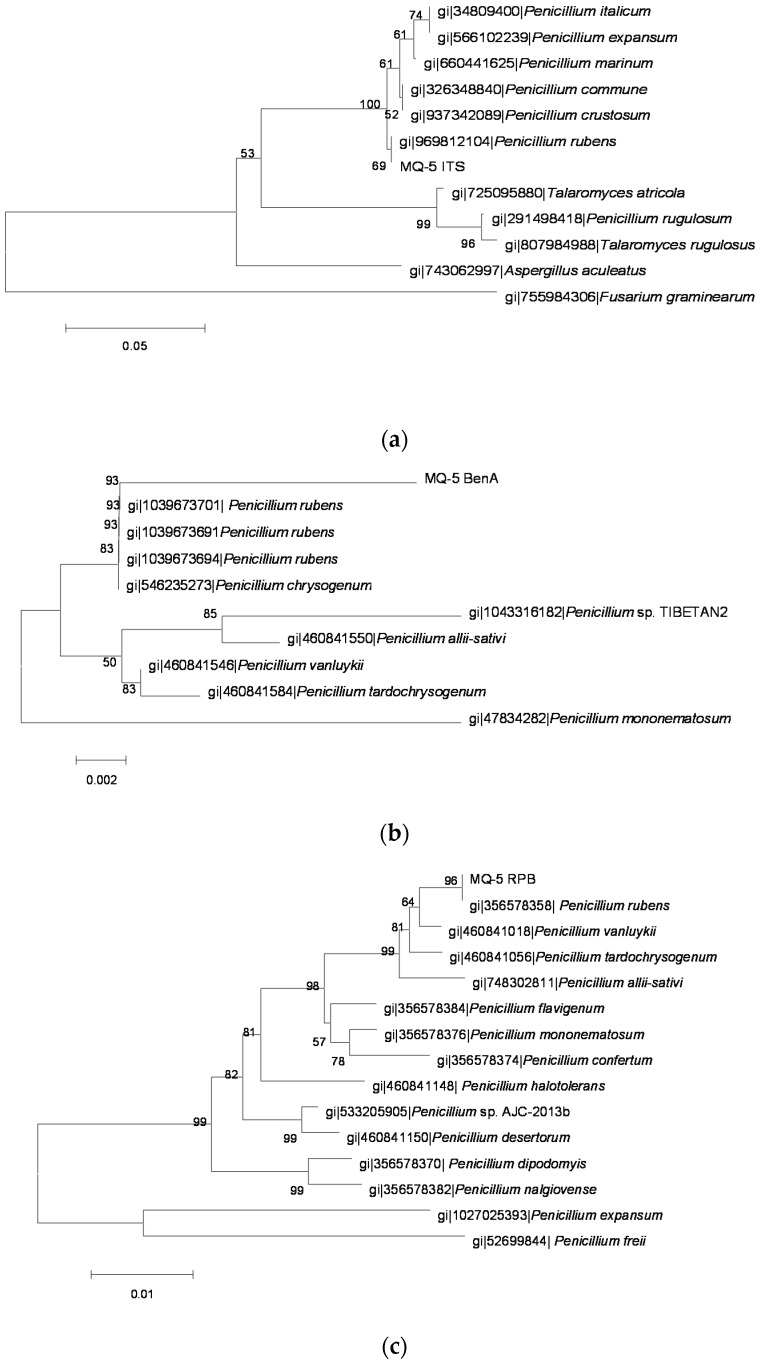
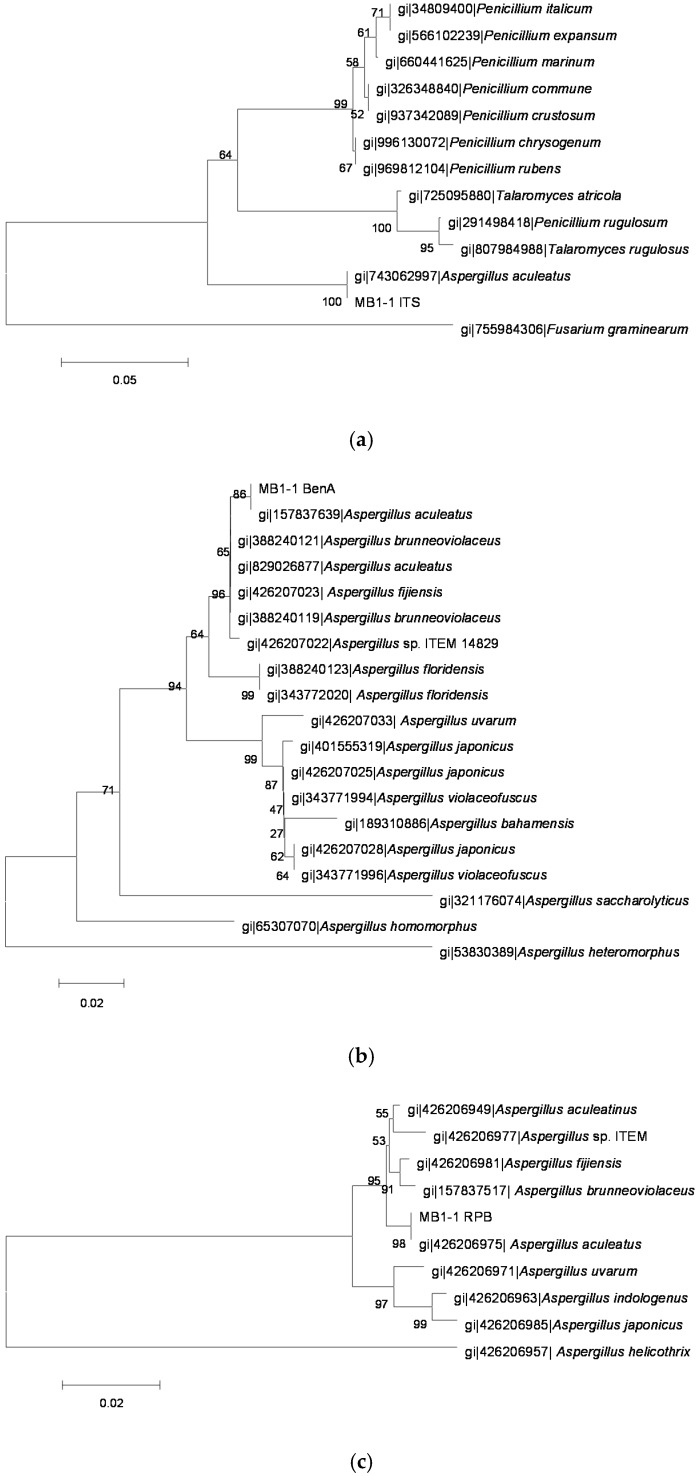
The sequences of PCR amplification products were analyzed and molecular phylogenetic trees (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6) were constructed based on comparative sequence analysis of ITS, BenA and RPB2 of the five strains. As shown in Figure 2 and Figure 3, the ITS, BenA and RPB2 of the strain O1 and Q3 have high homology with Talaromyces rugulosus. The strain V5-1 is Penicillium commune because of the ITS, BenA and RPB are all homology with Penicillium commune (Figure 4). Similarly, the MQ-5 is Penicillium rubens (Figure 5) and MB1-1 is Aspergillus aculeatus (Figure 6).
Figure 2.
The phylogenetic tree of the O1 strain by the Neighbor-Joining method based on analysis of ITS, BenA and RPB2 sequences. (a) the phylogenetic tree based on analysis of ITS; (b) the phylogenetic tree based on analysis of BenA; and (c) the phylogenetic tree based on analysis of RPB2.
Figure 3.
The phylogenetic tree of the Q3 strain by the Neighbor-Joining method based on analysis of ITS, BenA and RPB2 sequences. (a) the phylogenetic tree based on analysis of ITS; (b) the phylogenetic tree based on analysis of BenA; and (c) the phylogenetic tree based on analysis of RPB2.
Figure 4.
The phylogenetic tree of the V5-1 strain by the Neighbor-Joining method based on analysis of ITS, BenA. and RPB2 sequences. (a) The phylogenetic tree based on analysis of ITS; (b) the phylogenetic tree based on analysis of BenA; and (c) the phylogenetic tree based on analysis of RPB2.
Figure 5.
The phylogenetic tree of the MQ5 strain by the Neighbor-Joining method based on analysis of ITS, BenA and RPB2 sequences. (a) the phylogenetic tree based on analysis of ITS; (b) the phylogenetic tree based on analysis of BenA; and (c) the phylogenetic tree based on analysis of RPB2.
Figure 6.
The phylogenetic tree of the MB1-1 strain by the Neighbor-Joining method based on analysis of ITS, BenA and RPB2 sequences. (a) The phylogenetic tree based on analysis of ITS; (b) the phylogenetic tree based on analysis of BenA; and (c) the phylogenetic tree based on analysis of RPB2.
3. Discussion
Of the five toxicogenic strains, there are two Penicillium species (V5-1 and MQ-5), two Talaromyces species (O1 and Q3) and one Aspergillus species (MB1-1). In tropical regions of the world, Aspergillus species, such as A. ochraceus and A. carbonarius, are generally the OTA-producing species. However, in temperate regions, OTA is produced by Penicillium species, mainly by P. verrucosum [20,33]. Zhenjiang is situated in the region where there is a transitional climate from temperate to subtropical climate. Among the five strains isolated from the grapes in the vineyards of Zhenjiang, Penicillium species and Talaromyces rugulosus (synonyms or basionym of Penicillium tardum, Penicillium chrysitis and Penicillium rugulosum) were the dominant OTA-producing strains. This result is in accordance with the distribution characteristics of OTA-producing strains in different climates reported by Stander et al [33].
P. Commune, one of the five OTA-producing strains, is the most frequently occurring spoilage fungus on cheese [34] and some other foods. Many years ago, it was reported that this mold could produce mycotoxins such as OTA [35] and cyclopiazonic acid [36] in contaminated foods.
P. rubens (formerly known as Penicillium chrysogenum) is frequently found in indoor environments and foods. It has been confirmed as Fleming’s original penicillin producing strain [37] and has gained much attention for its ability to produce penicillin, but there is no report about OTA production. For the first time in this study, it was found that P. rubens has the ability to produce OTA.
As Peterson et al [38] reported, P. tardum and P. chrysitis were synonyms of T. rugulosus and P. rugulosum is basionym of T. rugulosus. The reports about the four strains are very few. Tatsuno et al. [39] confirmed that the cytotoxic metabolic substances of P. tardum were endocrocin and emodin, and the known toxic metabolites, rugulosin and skyrin were also isolated. However, the studies about OTA produced by the four strains mentioned above have not been reported so far.
A. aculeatus is one member of the Trichocomaceae family [40] and usually found in foods. Two toxic metabolites previously isolated from Penicillium oralicum, secalonic acid D and F, were separated from the extrolite profiles of A. aculeatus [41,42]. The OTA production ability of 189 strains of black aspergilli on grapes from Europe and Israel were studied by DNA-based molecular methods. The results showed that A. aculeatus was unable to produce OTA [43]. It was also found that none of the isolates belonging to A. niger aggregate and A. japonicus var. aculeatus (obligative or homotypic synonyms format of A. aculeatus) from Spanish wine grapes were able to produce OTA [44].
In conclusion, O1 and Q3 (Talaromyces rugulosus), V5-1 (Penicillium commune), MQ-5 (Penicillium rubens) and MB1-1 (Aspergillus aculeatus ) were OTA-producing molds from grapes before harvest. Up to the period of this study, there has not been any reports on OTA produced by T. rugulosus (or P. tardum, P. chrysitis, P. rugulosus), P. Rubens and A. aculeatus. This is the first study to report that T. rugulosus, P. rubens and A. aculeatus strains with relatively strong abilities of OTA production were isolated from grapes.
Furthermore, these results showed that OTA production is a latent risk if the grapes contaminated with these OTA-producing species are not handled properly. Therefore, it is essential to take measures to avoid contamination by fungi and OTA production in grapes in both growth and post-harvest stages; for example, avoiding exposition to high temperature, minimizing grape accumulation in high relative humidity during harvest and wine making processes and biological controls with antagonistic yeast [45]. The initial discovery of the three OTA-producing strains (P. rubens, T. rugulosus and A. Aculeatus) in grapes is very important. Consequently, this work would contribute to the in-depth understanding of the fungi with OTA-producing ability in grapes. Lastly, it is necessary for researchers to broaden the scope of study and take effective measures to ensure the safety of grapes and its products.
4. Conclusions
In conclusion, five molds were screened out from grapes before harvest and identified as Talaromyces rugulosus (O1 and Q3), Penicillium commune (V5-1), Penicillium rubens (MQ-5) and Aspergillus aculeatus (MB1-1). This is the first report that T. rugulosus, P. rubens and A. aculeatus strains isolated from grapes can produce OTA.
5. Materials and Methods
5.1. Samples
Fifteen bunches of grapes infected with diseases (downy mildew, gray mold and white rot) were collected randomly from each one of 3 organic vineyards in Zhenjiang, China. The samples were immediately put in sterilized paper bags and stored at 4 °C, and then were transferred to the laboratory where they were processed.
5.2. Culture Medium
Potato Dextrose Agar media (PDA, g/L): potato 200, glucose 20 and agar 20.
Czapek’s Agar medium (CA, g/L): sucrose 30, NaNO3 3, KCl 0.5, MgSO4∙7H2O 0.5, FeSO4·7H2O 0.01, K2HPO4 1, agar 20.
Coconut Cream Agar medium (CCA, g/L): coconut milk 50%, distilled water 50%, agar 20 g/L.
Rose Bengal medium (g/L): peptone 5, glucose 10, KH2PO4 1, MgSO4∙7H2O 0.5, Rose Bengal 0.0333, chloramphenicol 0.1, agar 20.
All the culture media were prepared with distilled water and were autoclaved at 121 °C for 20 min.
5.3. Isolation of Fungi from Decay Grapes
The disease positions of moldy berries were plated onto Rose Bengal medium in Petri dishes by sterilized tweezers. All plates were incubated at 25 °C until colonies were formed followed by repeatedly streaking inoculation of each colony on Rose Bengal medium until single mold colonies were obtained. The three-point inoculation method was employed to foster the purification of fungal strains, which were used for the subsequent experiment.
5.4. Preliminary Screening of Potential OTA-Producing Isolates by Ultraviolet Light
Aspergillus isolates were incubated on CCA at 25 °C for 7 days, and other isolates were cultured on CA at 25 °C for 14 days. Then, the reverse of CCA or CA with colonies was exposed to long wavelength UV light (365 nm) in the dark environment [8,46,47]. OTA-producing isolates would take on characteristic green fluorescence. Isolates producing fluorescence were recorded as potential OTA-producing strains. Then, the suspected strains were fumigated with 25%–28% ammonia in dark. The strains that showed enhanced fluorescence intensity under the 365 nm UV light were recorded as positive strains.
5.5. Confirmation of OTA-Producing Strains by High-Pressure Liquid Chromatography with a Fluorescence Detector (HPLC-FLD)
OTA produced by the positive strains was extracted by a variation of a method described by Cabañes et al. [48]. All the strains were cultured as above. Three agar plugs (the diameter was 6 mm) were removed from the inner, middle and outer area of each colony of screened positive strains and extracted with 500 μL methanol for 1 hour in darkness. The extracts were vortexed and filtered with 0.22 um filter and frozen (−18 °C) until analysis by HPLC-FLD.
OTA production was detected with an Agilent Technologies 1100 (Agilent, Santa Clara, CA, USA) series liquid chromatographic system equipped with a scanning fluorescence detector (λexc 330 nm; λem 460 nm). A reversed phase analytical column (Agilent ZORBAX SB -C18, 5 μm, 2 4.6 × 250 mm, Agilent, Santa Clara, CA, USA) was used with a mobile phase of acetonitrile (HPLC grade)—1% acetic acid (60:40, v/v) at a flow rate of 1 mL/min. The volume of injection was 20 μL and detection temperature was 30 °C [49,50]. The detection limit of the OTA analysis was 2 ng/mL, based on a signal-to-noise ratio of 3:1.
The OTA standard (Pribolab Pte. Ltd, Singapore) solution was prepared in methanol (HPLC grade) and confirmed by HPLC-FLD as above. The calibration curve (25 ng/mL to 2 µg/mL) was drawn for quantifying the OTA in extracts.
5.6. Identification of OTA-Producing Fungi
5.6.1. Genomic DNA Extraction
Fungal strains were cultured in PDB at 25 °C and 180 rpm for 4 days before DNA extraction. The mycelia were collected and freeze-dried, and then ground to powder with liquid nitrogen. The mycelia powder (200 mg) added with 700 μL of lysis buffer (2% CTAB, 1.4 M NaCl, 20 mM ethylene diamine tetraacetic acid (EDTA), 100 mM Tris-HCl pH 8) were incubated at 65 °C for 1 h and then cooled on ice for 1 h. The subsequent procedures of genomic DNA extraction were in accordance with the method described by Tannous et al [51]. The DNA concentration was measured using a Nanodrop2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA).
5.6.2. PCR Amplification and Sequencing
The conserved genes used to identify the fungi were ITS, beta-tublin gene (BenA) and RNA polymerase II second largest subunit (RPB2) [52,53,54,55]. The primers of the three genes were as follows. ITS: ITS1:GGTGAACCTGCGG; ITS4: TCCTCCGCTTATTGATATGC. BenA: Bt2a (Forward) GGTAACCAAATCGGTGCTGCTTTC; Bt2b (Reverse) ACCCTCAGTGTAGTGACCCTTGGC. RPB2: 5F-Eur (Forward): GAYGAYCGKGAYCAYTTCGG; 7CR-Eur (Reverse): CCCATRGCYTGYTTRCCCAT. The PCR reaction (25 µL) mixture contained 2 μL DNA template, 2.5 μL PCR buffer (Mg2+ plus), 2.5 μL (2.5 μm) dNTP, 2.5 μL primer, 0.2 μL Taq DNA Polymerase and H2O up to 25 μL. The amplification protocol is showed in Table 2.
Table 2.
PCR amplification protocol.
| Gene | Profile Type | Initial Denaturing | Cycles | Denaturing Annealing Elongation | Final Elongation | Rest Period |
|---|---|---|---|---|---|---|
| ITS, BenA | standard | 94 °C, 10 min | 35 | 94 °C, 45 s; 55 °C, 45 s; 72 °C, 60 s | 72 °C, 10 min | 4 °C, ∞ |
| RPB | touch-up | 94 °C, 5 min | 5 | 94 °C, 45 s; 50 °C, 45 s; 72 °C, 60 s | - | - |
| - | - | - | 5 | 94 °C, 45 s; 52 °C, 45 s; 72 °C, 60 s | - | - |
| - | - | - | 30 | 94 °C, 45 s; 55 °C, 45 s; 72 °C, 60 s | 72 °C, 10 min | 4 °C, ∞ |
All of the PCR amplification products were analyzed by gel electrophoresis and then sent to Shanghai Sangon Biological Engineering Technology and Services Co., Ltd, China for sequencing. The sequences were blast on National Center for Biotechnology Information (NCBI). Part of the genes homologous with ITS, BenA and RPB was acquired. These homologous genes were clustered by the clustalW software 2.0 (University college Dublin, Dublin, Ireland), and phylogenetic trees based on these sequences were constructed with the neighbor-joining algorithm by MEGA5.0 (University college Dublin, Dublin, Ireland).
Acknowledgments
This research was financially supported by the National Key Research and Development Program of China (2016YFD0400902), the National Natural Science Foundation of China (NNSFC-31271967), the Technology Support Plan of Jiangsu Province (BE2014372), the Agricultural Science and Technology Support Program of Zhenjiang City (NY2013004), the Open Foundation of Hubei Key Laboratory of Edible Wild Plants Conservation and Utilization (EWPL201608), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author Contributions
X.Z. analyzed the results, and wrote the paper. Y.L revised the manuscript. The two authors contributed equally to this work and shared first author duties. H.W. conducted most of the experiments and wrote part of the paper. X.Z. conducted experiments on the identification of the fungi. X.G., Y.W., J.D. And Y.P. constructed the phylogenetic trees of the fungi. H.Z. conceived the idea for the project and provided the funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gonzalez G., Hinojo M.J., Mateo R., Medina A., Jimenez M. Occurrence of mycotoxin producing fungi in bee pollen. Int. J. Food Microbiol. 2005;105:1–9. doi: 10.1016/j.ijfoodmicro.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Van der Merwe K.J., Stein P.S., Fourie L. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 3.Van Walbeek W., Scott P.M., Harwig J., Lawrence J.W. Penicillium viridicatum Westling: A new source of ochratoxin A. Can. J. Microbiol. 1969;15:1281–1285. doi: 10.1139/m69-232. [DOI] [PubMed] [Google Scholar]
- 4.Covarelli L., Beccari G., Marini A., Tosi L. A review on the occurrence and control of ochratoxigenic fungal species and ochratoxin A in dehydrated grapes, non-fortified dessert wines and dried vine fruit in the Mediterranean area. Food Control. 2012;26:347–356. doi: 10.1016/j.foodcont.2012.01.044. [DOI] [Google Scholar]
- 5.Suarez-Quiroz M., Gonzalez-Rios O., Barel M., Guyot B., Schorr-Galindo S., Guiraud J.P. Effect of the post-harvest processing procedure on OTA occurrence in artificially contaminated coffee. Int. J. Food Microbiol. 2005;103:339–345. doi: 10.1016/j.ijfoodmicro.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Ramyaa P., Krishnaswamy R., Padma V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells-up regulation of Nrf2 expression and down regulation of NF-kappaB and COX-2. Biochim. Biophys. Acta. 2014;1840:681–692. doi: 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Abrunhosa L., Venâncio A., Teixeira J.A. Optimization of process parameters for the production of an OTA-hydrolyzing enzyme from Aspergillus niger under solid-state fermentation. J. Biosci. Bioeng. 2011;112:351–355. doi: 10.1016/j.jbiosc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Heenan C.N., Shaw K.J., Pitt J.I. Ochratoxin A production by Aspergillus carbonarius and A. niger isolates and detection using coconut cream agar. J. Food Mycol. 1998;1:67–72. [Google Scholar]
- 9.Horie Y. Productivity of ochratoxin A of Aspergillus carbonarius in Aspergillus section Nigri. Nippon Kingakkai Kaiho. 1995;36:73–76. [Google Scholar]
- 10.Nakajima M., Tsubouchi H., Miyabe M., Ueno Y. Survey of aflatoxin B1 and ochratoxin A in commercial green coffee beans by high-performance liquid chromatography linked with immunoaffinity chromatography. Food Agric. Immunol. 1997;9:77–83. doi: 10.1080/09540109709354938. [DOI] [Google Scholar]
- 11.Ono H., Kataoka A., Koakutsu M., Tanaka K., Kawasugi S., Wakazawa M., Ueno Y., Manabe M. Ochratoxin A producibility by strains of Aspergillus niger group stored in IFO culture collection. Mycotoxins. 1995;41:47–51. doi: 10.2520/myco1975.1995.47. [DOI] [Google Scholar]
- 12.Perrone G., Mulè G., Susca A., Battilani P., Pietri A., Logrieco A. Ochratoxin A production and AFLP analysis of Aspergillus carbonarius, Aspergillus tubingensis, and Aspergillus niger strains isolated from grapes in Italy. Appl. Environ. Microbiol. 2006;72:680–685. doi: 10.1128/AEM.72.1.680-685.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Téren J., Varga J., Hamari Z., Rinyu E., Kevei F. Immunochemical detection of ochratoxin A in black Aspergillus strains. Mycopathologia. 1996;134:171–176. doi: 10.1007/BF00436726. [DOI] [PubMed] [Google Scholar]
- 14.Varga J., Kocsubé S., Tóth B., Frisvad J.C., Perrone G., Susca A., Merger M., Samson R.A. Aspergillus brasiliensis sp. nov. a biseriate black Aspergillus species with worldwide distribution. Int. J. Syst. Evol. Microbiol. 2007;57:1925–1932. doi: 10.1099/ijs.0.65021-0. [DOI] [PubMed] [Google Scholar]
- 15.Frisvad J.C. The connection between the Penicillia and Aspergilli and mycotoxins with special emphasis on misidentified isolates. Arch. Environ. Contam. Toxicol. 1989;18:452–467. doi: 10.1007/BF01062373. [DOI] [PubMed] [Google Scholar]
- 16.Frisvad J.C., Filtenborg O. Terverticillate Penicillia: Chemotaxonomy and mycotoxin production. Mycologia. 1989;81:837–861. doi: 10.2307/3760103. [DOI] [Google Scholar]
- 17.Pitt J.I. Penicillium viridicatum, Penicillium verrucosum and production of ochratoxin A. Appl. Environ. Microbiol. 1987;53:535–539. doi: 10.1128/aem.53.2.266-269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciegler A., Fennell D.J., Mintzlaff H.J., Leistner L. Ochratoxin synthesis by Penicillium species. Sci. Nat. 1972;59:365–366. doi: 10.1007/BF00617916. [DOI] [PubMed] [Google Scholar]
- 19.Krogh P., Hald B., Pedersen E.J. Occurrence of ochratoxin A and citrinin incereals associated with mycotoxic porcine nephropathy. Acta. Pathol. Microbiol. Scand. 1973;81:689–695. doi: 10.1111/j.1699-0463.1973.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 20.Larsen T.O., Svendsen A., Smedsgaard J. Biochemical characerization of ochratoxin A-producing strains of the genus Penicillium. Appl. Environ. Microbiol. 2001;67:3630–3635. doi: 10.1128/AEM.67.8.3630-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellí N., Pardo E., Marín S., Farré G., Ramos A.J., Sanchis V. Occurrence of ochratoxin A and toxigenic potential of fungal isolates from Spanish grapes. Appl. Environ. Microbiol. 2004;84:541–546. doi: 10.1002/jsfa.1658. [DOI] [Google Scholar]
- 22.Duarte S.C., Pena A., Lino C.M. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Control. 2010;27:187–198. doi: 10.1016/j.fm.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Kuiper-Goodman T., Scott P.M. Risk assessment of the mycotoxin ochratoxin A. Biomed. Environ. Sci. 1989;2:179–248. [PubMed] [Google Scholar]
- 24.Jφrgensen K. Survey of pork, poultry, coffee, beer and pulses for ochratoxin A. Food Addit. Contam. 1998;15:550–554. doi: 10.1080/02652039809374680. [DOI] [PubMed] [Google Scholar]
- 25.Ueno Y. Residue and risk of ochratoxin A in human plasma and beverages in Japan. Mycotoxins. 1998;47:25–32. doi: 10.2520/myco1975.1998.47_25. [DOI] [Google Scholar]
- 26.Skaug M.A. Analysis of Norwegian milk and infant formulas for ochratoxin A. Food Addit. Contam. 1999;16:75–78. doi: 10.1080/026520399284235. [DOI] [PubMed] [Google Scholar]
- 27.Burdaspal P.A., Legarda T.M. Ocratoxina A in wines and grape products originated from Spain and other European countries. Alimentaria. 1999;36:107–112. [Google Scholar]
- 28.Covarelli L., Tosi L., Beccari G. Risks related to the presence of fungal species and mycotoxins in grapes, wines and other derived products in the mediterranean area. In: Preddy V.R., Watson R.R., editors. The Mediterranean Diet: An. Evidence-Based Approach. Elsevier; Amsterdam, The Netherlands: 2015. pp. 563–575. [Google Scholar]
- 29.Pardo E., Marín S., Sanchis V., Ramos A.J. Impact of relative humidity and temperature on visible fungal growth and OTA production of ochratoxigenic Aspergillus ochraceus isolates on grapes. Food Microbiol. 2005;22:383–389. doi: 10.1016/j.fm.2004.11.004. [DOI] [Google Scholar]
- 30.Zimmerli B., Dick R. Ochratoxin A in table wines and grape-juice: Occurrence and risk assessment. Food Addit. Contam. 1996;13:655–668. doi: 10.1080/02652039609374451. [DOI] [PubMed] [Google Scholar]
- 31.Romani S., Sachetti G., López C.C., Pinnavia G.G., Rosa M.D. Screening on the occurrence of chratoxin A in green coffee beans of different origins types. J. Agric. Food Chem. 2000;48:3616–3619. doi: 10.1021/jf990783b. [DOI] [PubMed] [Google Scholar]
- 32.Taniwaki M.H., Pitt J.I., Teixeira A.A., Iamanaka B.T. The source of ochratoxin A in Brazilian coffee and its formation in relation to process methods. Int. J. Food Microbiol. 2003;82:173–179. doi: 10.1016/S0168-1605(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 33.Stander M.A., Steyn P.S. Survey of ochratoxin A in South African wines. S. Afr. Jenol. Vitic. 2002;23:9–13. [Google Scholar]
- 34.Lund F., Filtenborg O., Frisvad J.C. Associated mycoflora of cheese. Food Microbiol. 1995;12:173–180. doi: 10.1016/S0740-0020(95)80094-8. [DOI] [Google Scholar]
- 35.Ciegler A. Bioproduction of ochratoxin A and penicillic acid by membersof the Aspergillus ochraceus group. Can. J. Microbiol. 1972;18:631–636. doi: 10.1139/m72-100. [DOI] [PubMed] [Google Scholar]
- 36.Pitt J.I., Cruickshank R.H., Leistner L. Penicillium Commune, P. camembertii, the origin of white cheese moulds, and the production of cyclopiazonic acid. Food Microbiol. 1986;3:363–371. doi: 10.1016/0740-0020(86)90022-5. [DOI] [Google Scholar]
- 37.Houbraken J., Frisvad J.C., Samson R.A. Fleming’s penicillin producing strain is not Penicillium Chrysogenum but P. Rubens. IMA Fungus. 2011;2:87–95. doi: 10.5598/imafungus.2011.02.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson S.W., Jurjević Ž. Talaromyces columbinus sp. nov., and Genealogical Concordance Analysis in Talaromyces Clade 2a. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0078084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsuno T., Kobayashi N., Okubo K., Tsunoda H. Recherches Toxicologiques sur les Substances Toxiques de Penicillium tardum I. Isolement et Identification des Substances. Chem. Pharm. Bull. 1975;23:351–354. doi: 10.1248/cpb.23.351. [DOI] [PubMed] [Google Scholar]
- 40.Iizuka Aspergillus aculeatus. J. Agric. Food Chem. Soc. 1953;27:807. [Google Scholar]
- 41.Andersen R., Buchi G., Kobbe B., Demain A.L. Secalonic acids D and F are toxic metabolites of Aspergillus aculeatus. J. Org. Chem. 1977;42:352–353. doi: 10.1021/jo00422a042. [DOI] [PubMed] [Google Scholar]
- 42.Perrone G., Varga J., Susca A. Aspergillus uvarum sp. nov., an uniseriate black Aspergillus species isolated from grapes in Europe. Int. J. Syst. Evol. Microbiol. 2008;58:1032–1039. doi: 10.1099/ijs.0.65463-0. [DOI] [PubMed] [Google Scholar]
- 43.Bau M., Castellá G., Bragulat M.R., Cabañes F.J. DNA-based characterization of ochratoxin-A-producing and non-producing Aspergillus carbonarius strains from grapes. Res. Microbiol. 2005;156:375–381. doi: 10.1016/j.resmic.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Bau M., Bragulata M.R., Abarca M.L., Minguezb S., Cabañes F.J. Ochratoxigenic species from Spanish wine grapes. Int. J. Food Microbiol. 2005;98:125–130. doi: 10.1016/j.ijfoodmicro.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Zhu C.Y., Sh J.L., Jiang C.M., Liu Y.L. Inhibition of the growth and ochratoxin A production by Aspergillus carbonarius and Aspergillus ochraceus in vitro and in vivo through antagonistic yeasts. Food Control. 2015;50:125–132. doi: 10.1016/j.foodcont.2014.08.042. [DOI] [Google Scholar]
- 46.Dyer S.K., McCammon S. Detection of aflatoxigenic isolates of Aspergillus flavusand related species on coconut cream agar. J. Appl. Bacteriol. 1994;76:75–78. doi: 10.1111/j.1365-2672.1994.tb04418.x. [DOI] [PubMed] [Google Scholar]
- 47.Kizis D., Natskoulis P., Nychas G.J.E., Panagou E.Z. Biodiversity and ITS-RFLP characterization of Aspergillus. Section Nigri isolates in grapes from four traditional grape-producing areas in Greece. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0093923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabañes F.J., Bragulat M.R., Castella G. Characterization of nonochratoxigenic strains of Aspergillus carbonarius from grapes. Food Microbiol. 2013;36:135–141. doi: 10.1016/j.fm.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Abarca M.L., Bragulat M.R., Cabañes F.J. A new in vitro method to detect growth and ochratoxin A-producing ability of multiple fungal species commonly found in food commodities. Food Microbiol. 2014;44:243–248. doi: 10.1016/j.fm.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Chiotta M.L., Ponsone M.L., Sosa D.M., Combina M., Chulze S.N. Biodiversity of Aspergillus section Nigri populations in Argentinian vineyards and ochratoxin A contamination. Food Microbiol. 2013;36:182–190. doi: 10.1016/j.fm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Tannous J., Atoui A., El Khoury A., Kantar S., Chdid N., Oswald I.P., Puel O., Lteif R. Development of a real-time PCR assay for Penicillium expansum quantification and patulin estimation in apples. Food Microbiol. 2015;50:28–37. doi: 10.1016/j.fm.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Samson R.A., Visagie C.M., Houbraken J., Hong S.B., Hubka V., Klaassen C.H.W., Perrone G., Seifert K.A., Susca A., Tanney J.B., et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Visagie C.M., Houbraken J., Frisvad J.C., Hong S.B., Klaassen C.H.W., Perrone G., Seifert K.A., Varga J., Yaguch T., Samson R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sultan Y., Magan N. Mycotoxigenic fungi in peanuts from different geographic regions of Egypt. Mycotox. Res. 2010;26:133–140. doi: 10.1007/s12550-010-0048-5. [DOI] [PubMed] [Google Scholar]
- 55.Noonim P., Mahakarnchanakul W., Varga J., Frisvad J.C., Samson R.A. Two novel species of Aspergillus section Nigri from Thai coffee beans. Int. J. Syst. Evol. Microbiol. 2008;58:1727–1734. doi: 10.1099/ijs.0.65694-0. [DOI] [PubMed] [Google Scholar]