Abstract
Desminopathy represents a subgroup of myofibrillar myopathies caused by mutations in the desmin gene. Three novel disease-associated mutations in the desmin gene were identified in unrelated Spanish families affected by cardioskeletal myopathy. A selective pattern of muscle involvement, which differed from that observed in myofibrillar myopathy resulting from mutations in the myotilin gene, was observed in each of the three families with novel mutations and each of three desminopathy patients with known desmin mutations. Prominent joint retractions at the ankles and characteristic nasal speech were observed early in the course of illness. These findings suggest that muscle imaging in combination with routine clinical and pathological examination may be helpful in distinguishing desminopathy from other forms of myofibrillar myopathy and ordering appropriate molecular investigations.
Keywords: Myofibrillar myopathy, Desminopathy, DES mutations, Phenotype, Muscle imaging
1. Introduction
Desminopathies are a subgroup of myofibrillar myopathies (MFM) caused by mutations in the desmin gene. The disease usually presents with distal muscle weakness in the lower extremities eventually spreading to other muscle groups, including facial, bulbar, neck and respiratory muscles. Cardiomyopathy manifested with conduction blocks, arrhythmias and hypertrophy or dilatation of heart chambers occur in a high proportion of patients. A major histological hallmark of the disease is the intracytoplasmic accumulation of desmin and many other proteins in muscle cells [1].
Desmin is the major intermediate filament of muscle. It is highly expressed in skeletal, cardiac and smooth muscles. In striated muscles, desmin localizes at the Z-lines where it plays a pivotal role in maintaining the structural integrity of the myofibrils by connecting adjacent myofibrils laterally and linking peripheral myofibrils to the sarcolemma and nuclei [2]. Desmin interacts with other intermediate filament proteins to form an intracytoplasmic network that maintains spatial relationships between the contractile apparatus and other structural elements of the cell [2]. Desmin is encoded by a single copy gene located in chromosome 2q35 band; it encompasses nine exons and codes for 476 amino acids [3]. Desmin molecule contains a highly conserved central alpha-helical “rod” domain flanked by globular N- (“head”) and C-terminal (“tail”) domains. The alpha-helical rod domain consists of four segments, 1A, 1B, 2A and 2B [4]. Since the first identification of mutations in the desmin gene in 1998, more than 25 disease-associated mutations have been reported to date [1]. Most of these mutations are located in the 2B region of the alpha-helical rod domain, the integrity of which is critically important for desmin filament assembly [5]. In this report, we provide a detailed description of phenotypic manifestations associated with three novel mutations in the desmin gene. Several recognizable phenotypic features elicited in these patients, including the pattern of muscle involvement, may be helpful in the diagnostic process and providing direction in molecular investigations. We also reviewed our previous desminopathy cases to validate these new findings.
2. Case reports
2.1. Family one
A 49-year-old woman presented at the age of 22 with distal weakness in the lower extremities and toe walking. Over the next several years, weakness spread to distal muscles of the upper extremities and proximal muscles of all four limbs. Difficulty swallowing and nasal voice were present from the onset of the disease. At the age of 46 she suffered from an episode of acute heart failure. Echocardiography showed mild enlargement of the left atria and normal morphology and function of both ventricles. 2D-Doppler examination revealed marked shortened deceleration time of E wave (90 ms) and annular velocity Em reduced to 10 cm/s indicating restrictive cardiomyopathy. Two years later she experienced dizziness and syncopal episodes due to heart block; a permanent pacemaker was inserted. On examination, deep tendon reflexes were hypoactive and absent at the ankles. She had marked nasal speech, and muscle weakness and atrophy in all limbs, more pronounced in the anterior compartment of legs, the wrist, finger extensors and hand muscles. Facial, neck and trunk muscles were not involved. The patient had prominent contractures at the ankles and at the long finger flexors (Fig. 1). CK levels were twice the normal value, and EMG examination showed a myopathic pattern with spontaneous activity at rest. Nerve conduction studies were normal.
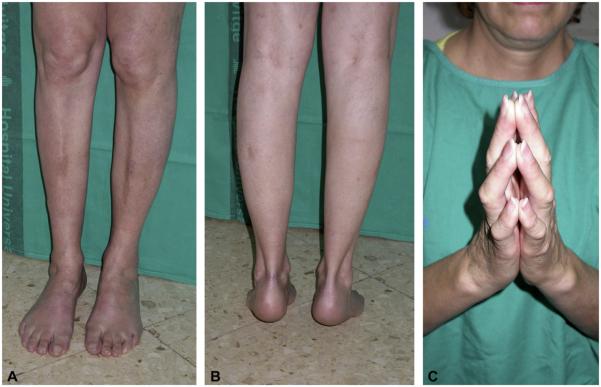
Fig. 1.
Clinical findings in the index case of family one (patient 1-1), showing weakness and wasting in the lower extremites (A), and contractures at the Achilles tendons (B) and long finger flexors (C).
The patient’s parents, brother and daughter were unaffected. Her 25-year-old son complained of toe walking and swallowing difficulties from the age of 22 years. At the age of 24, he was diagnosed with hypertrophic cardiomyopathy. On examination, he had marked nasal speech, bilateral heel cord contractures, and mild rigidity of the spine, as well as mild distal muscle weakness in each limb and trunk muscles. CK levels were four times normal.
2.2. Family two
A 46-year-old woman presented around the age of 25 years with swallowing difficulties and nasal voice. Over the next 2 years she developed progressive lower limb weakness with difficulty climbing stairs, foot drop and ankle contractures. Weakness progressed to involve proximal and distal muscles of the upper and lower limbs. At 41 she was diagnosed with hypertrophic cardiomyopathy. Three years later, she developed restrictive respiratory insufficiency requiring nocturnal ventilator support. By the age of 43 the patient was wheelchair-bound. On examination, she had prominent nasal speech, and generalized muscle weakness involving limb, face, neck and trunk muscles. In the upper extremities, biceps, triceps and wrist flexors were relatively preserved, whereas in the lower extremities each muscle was severely involved leading to almost complete absence of muscle contraction. She had prominent bilateral ankle contractures. Deep tendon reflexes were hypoactive. Sensation was intact. Slit lamp examination revealed bilateral subcapsular posterior lens opacities. CK level was twice normal level, and EMG examination showed a myogenic pattern with prominent spontaneous activity at rest. Nerve conduction studies were normal. Twenty-four-hour Holter monitoring and echocardiogram revealed non-sustained cardiac conduction defects and concentric hypertrophy of the left ventricle. The patient’s grandfather, mother, brother and sister died from cardiac failure at the age of 50, 43, 33 and 50 years, respectively. All suffered from progressive muscular weakness, marked nasal speech and dysphagia that started in each case around 25–35 years. According to clinical and Echocardiography reports they also had restrictive cardiomyopathy and cardiac conduction block requiring pacemaker implantation. Additionally, the affected sister had lens opacities, and the affected brother suffered from severe respiratory insufficiency.
2.3. Family three
A 27-year-old man presented at the age of 25 years with gait abnormality and nasal voice. A few months prior to admission he has been diagnosed with complete AV block and a pacemaker was implanted. Echocardiography revealed hypertrophic cardiomyopathy. On examination, he had marked nasal speech, bilateral heel cord contractures, and muscle weakness in distal muscles of the legs. CK levels were four times normal. Several members of the family, including the patient’s grandfather and father, died from cardiac failure around the age of 50. All of them had suffered from progressive muscular weakness, cardiac conduction block and restrictive cardiomyopathy.
3. Methods
3.1. DES gene analyses
3.1.1. Mutation analysis
Genomic DNA was isolated from peripheral lymphocytes and coding exons of the DES gene amplified using polymerase chain reaction (PCR) with intronic primers. PCR products were sequenced using an ABI PRISM 3100 DNA analyzer (Applied Biosystems, Foster City, CA). Sequences were compared to the human genomic DES database sequence (NM_001927) and analysed using the BCM Search Launcher utilities (http://www.searchlauncher.bcm.tmc.edu/), In addition, PCR products were digested overnight at 37 °C with restriction endonucleases MnlI, XcmI and BsrDI and electrophoresed on a 10% polyacrylamide gel.
3.2. Muscle biopsy
An open biopsy was performed in the index cases of each of the three families. Samples were obtained from the right gastrocnemius muscle in families one and three, and the biceps brachii muscle in family two. The samples were immediately frozen in liquid nitrogen chilled iso-pentane and processed by routine histological and histo-chemical techniques and for desmin (Dako), dystrophin (Novocastra), αB-crystallin (Novocastra), myotilin (Novocastra) and ubiquitin (Dako) immunohistochemistry as described [6]. In addition, γ-filamin immunohistochemistry was performed using a monoclonal anti-filamin C antibody RR90, kindly provided by van der Ven [7]. Amyloid-like deposits were visualized with congo Red and Thioflavine-T stains. A sample of biopsy tissue was processed for ultrastructural examination using standard methods.
3.3. Muscle CT scan studies
Muscle CT scan studies were performed in members of the three families and in three additional desminopathy patients. Axial images were obtained from the mid-tight and mid-calf level in each patient using a helical CT scanner (HiSpeed NX/i PRO, GE Medical Systems Milwaukee, Wis). In each patient, the following muscles were examined:
Tight level: rectus femoris, vastus lateralis and intermedius, sartorius, gracilis, adductor group, semimembranosus, semitendinosus and biceps femoris. Mid-calf level: medial and lateral gastrocnemius, soleus, anterior tibialis and peroneal group.
A summary of clinical and genetic data of all the patients in whom CT scan studies were performed is provided in Table 1. Cases 5 and 6 have been reported elsewhere [8,9].
Table 1.
Phenotypic characteristics of patients included in the present study
| Family and patient identification | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Patients with novel desmin mutations | Patients with previously reported destin mutations | ||||||
|
|
|
||||||
| 1-1 | 1-2 | 3 | 4 | 5 | 6 | 7 | |
| Age/Gender | 49/F | 25/M | 46/F | 27/M | 28/F | 24/M | 56/F |
| Age at onset | 25 | 22 | 25 | 25 | 15 | 14 | 36 |
| Distal weakness | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Proximal weakness | Mild | No | Yes | No | Yes | Yes | Yes |
| Nasal voice | Yes | Yes | Yes | No | Yes | Yes | No |
| Ankle contracture | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| AV block | Yes | No | Yes | Yes | Yes | Yes | No |
| Cardiomyopathy | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Respiratory insufficiency | No | No | Yes | No | Yes | Yes | Yes |
| Inheritance | AD | AD | AD | AD | Sporadic | AD | AD |
| Mutation | Pro419Ser | Pro419Ser | Leu392Pro | Ile367Phe | Arg406Trp | Arg406Trp | N366del |
| Other relevant findings | Sudden death at 28 |
Heart transplantation at 15 |
Sudden death at 56 |
||||
4. Results
4.1. Genetic studies
Analysis of the desmin gene in family one revealed a heterozygous T-to-C transition at codon 419 of exon 7 in the affected mother (patient 1-1) and son (patient 1-2) but not in the unaffected daughter. This mutation resulted in a replacement of proline with serine (Pro419-Ser). This mutation generates a new cleavage site for MnlI. Using restriction analysis, the Pro419Ser substitution was confirmed in two affected family members and excluded in the unaffected member and control samples. This analysis provides strong evidence that Pro419Ser is the mutation causing the disease and not a coincidental polymorphism.
In patient 2, a T-to-C transition at codon 392 of DES exon 6 generated a leucine to proline amino acid change (Leu392Pro). This mutation creates a new XcmI restriction site. Restriction analysis confirmed the presence of this mutation in the patient and its absence in 100 unrelated control individuals. DNA samples from other family members were unavailable.
Analysis of desmin gene sequences in the patient of family three led to the identification of a heterozygous A-to-T missense mutation at codon 367. The nucleotide substitution resulted in codon sequence change from ATT to TTT and replacement of isoleucine with phenyl-alanine (Ile367Phe). Restriction analysis using the BsrDI endonuclease confirmed the presence of this mutation in the patient and its absence in 100 unrelated spanish control individuals.
4.2. Muscle biopsy
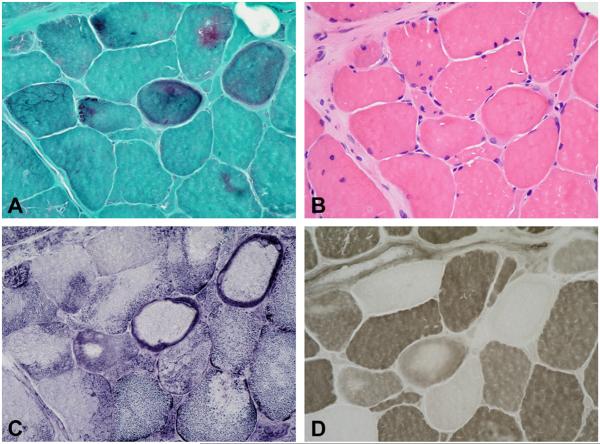
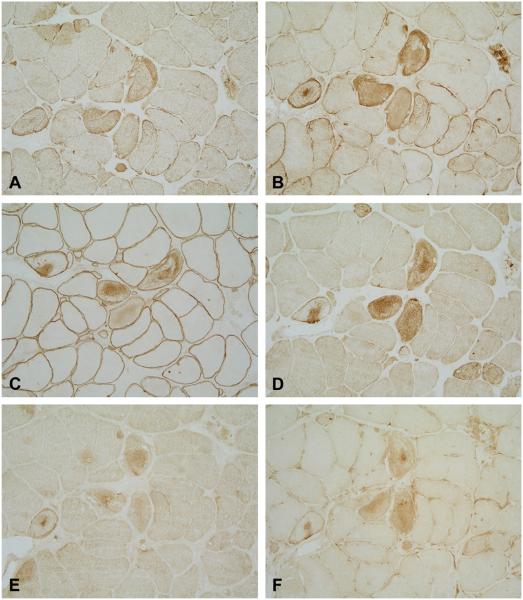
Muscle pathology was similar in the three families and showed a wide variation in the size of fibres and increased numbers of internal nuclei. Several fibres contained patches of non-hyaline inclusions located under the sarcolemma or within the cytoplasm, which were best identified on modified Gomori stain. These inclusions were devoid of ATPase and oxidative activity (Fig. 2). Rimmed vacuoles were present in a few fibers in patients 1 and 2, but were abundant in patient 3. Necrotic fibres or inflammatory cells were not observed. Congophilia was never observed in any of the three cases. Immunocytochemical studies revealed subsarcolemmal or cytoplasmic aggregates strongly immunoreactive for desmin, dystrophin, αB-crystallin and γ-filamin. Myotilin and ubiquitin were weakly expressed, or absent (Fig. 3). Ultrastructural examination revealed a matrix of granulofilamentous material lying at the Z-line level in the three index patients (not shown).
Fig. 2.
Cryostat consecutive sections stained with modified Trichrome stain (A), H&E (B), NADH (C), and ATPase at pH 4.65 (D). Several fibers contain dark-bluish cytoplasmic inclusions which are devoid of oxidative and ATPase activity.
Fig. 3.
Serial consecutive sections showing strong desmin (A), αB-crystallin (B), dystrophin (C) and γ-filamin (D) immunoreactivity, and weak myotilin (E), and ubiquitin (F) immunoreactivity.
4.3. CT scan studies
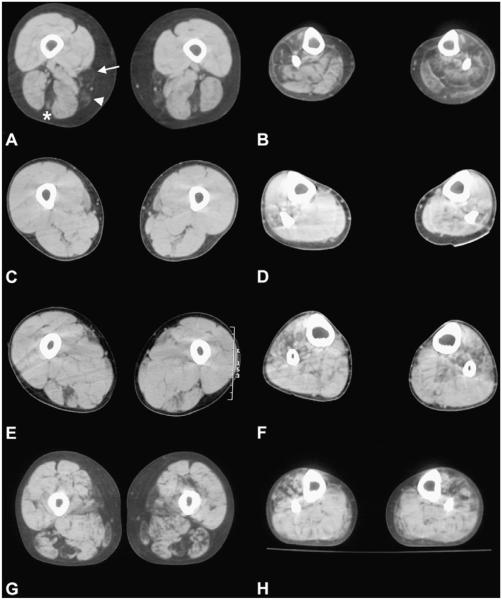
The earliest abnormalities were observed at the mid-thigh level: first in semitendinosus and sartorius, and later in the gracilis muscle. As the disease progressed, areas of decreased attenuation were also seen in the hip adductors. In advanced illness, the quadriceps femoris becomes affected. At the mid-calf level, initial changes were seen in the peroneal group, followed by the anterior tibialis and the posterior group (Fig. 4). In the later stages of illness all muscles, except for soleus, were completely replaced by fatty tissue.
Fig. 4.
Muscle CT scan images of four desminopathy patients at mid-thigh (A, C, E, G) and mid-lower-leg (B, D, F, H) levels. At the mid-tight level involvement of the semitendinosus (asterisk), sartorius (arrow) and gracilis (arrowhead) are seen in patient 1-1 (A), and in patient 6 (G). At the very early stages of illness initial changes are already seen in the same muscles (C and E, patients 1-2 and 4, respectively). At the mid-calf level (B, D, F and H) areas of decreased attenuation are patchily distributed in the peroneal group, anterior tibialis and later in the posterior group. (A and B: patient 1-1, 20 years after the disease onset; C and D: patient 1-2, 3 years after the disease onset; E and F: patient 4, 2 years after the disease onset; G and H: patient 6, 10 years after the disease onset).
5. Discussion
We have identified three novel missense mutations in the desmin gene – Pro419Ser, Leu392Pro and Ile367Phe – in unrelated Spanish families suffering from autosomal dominant cardiac and skeletal myopathy. These patients presented in early adulthood with progressive distal and proximal myopathy associated with restrictive or hypertrophic cardiomyopathy and conduction blocks. Although dilated cardiomyopathy has been considered the most common form of heart involvement in patients with desmin mutations [10–12], recent studies, including the present observations have demonstrated the presence of restrictive cardiomyopathy in several desminopathy patients [13–16]. The occurrence of restrictive cardiomyopathy does not seem to be mutation-specific since it has been found associated with mutations along the desmin molecule. Taken together these observations suggest that restrictive cardiomyopathy in desminopathy patients is more frequent than initially thought. Bulbar muscle involvement causing difficulties with swallowing and nasal voice was evident early in the course of illness, before the limb muscles became involved. Prominent joint retraction at the ankles leading to toe walking were observed in each family at the disease onset. Furthermore, long finger flexor contractures, which are classically associated with Bethlem myopathy [17] and some cases of Emery Dreifuss muscular dystrophy [18], were seen in the proband of family one, and rigidity of the spine was observed in her son. These findings expand the phenotypic manifestations of desminopathies. Restrictive respiratory insufficiency requiring nocturnal ventilator support and lens opacities were present in members of family two. The association of skeletal myopathy, pharyngeal weakness, hypertrophic cardiomyopathy, respiratory disturbances, lens opacities and a muscle biopsy showing desmin aggregates and granulofilamentous material at the ultrastructural level strongly suggest a αB-crystallinopathy [19,20]. However, DNA sequence analysis ruled out mutations in the αB-crystallin gene [21].
CT scan studies identified a recognizable pattern of distinct muscle groups being affected and potentially could be a tool for detecting subclinical evidence for involvement of specific muscles. Thus, in the early stages of illness, a selective involvement of the semitendinosus, followed by involvement of the gracilis and sartorius muscles was observed at the thigh level. At the mid-calf level, areas of decreased attenuation were observed predominantly in the anterior tibialis and peroneal groups and in more advanced disease in the posterior group. Similar findings were observed in two unrelated patients with a previously reported Arg406Trp desmin mutation and in the patient with a single amino acid deletion at position 366. This particular pattern of muscle involvement clearly differed from that observed in myofibrillar myopathies resulting from mutations in myotilin gene [6]. Taken together, these observations demonstrate that muscle imaging in combination with clinical and pathological examination is a useful tool for distinguishing between various forms of MFM. The Pro419Ser mutation newly identified in family one is located in the tail domain of the desmin molecule, where at least seven other pathogenic desmin mutations have been identified [10,22–25]. Interestingly, patients exhibiting mutations in the tail domain predominantly develop isolated cardiomyopathy or cardiomyopathy followed by skeletal involvement [25], and only in two reported patients the skeletal myopathy was the first sign of the disease [22] as family one of the present series. One of the tail’s major functions is interaction with other cytoskeletal proteins to establish a cytoplasmic intermediate filament network [26]. Expression studies in transfected cells with most of the desmin tail mutations did not prevent normal desmin assembly and network formation. However, severe disturbances of filament formation and filament– filament interactions have been observed recently [25]. By contrast, the Leu392Pro and the Ile367Phe mutations found in families two and three, respectively, are located in the 2B region of the alpha-helical rod domain, which has been shown to be a hot-spot for mutations [1]. Similarly to several other mutations previously found in this region, the Leu392Pro mutation introduces proline, which is known to be a potent helix breaker. Experimental studies demonstrated that mutant desmin is assembly incompetent and able to disrupt a pre-existing filamentous network, resulting in the accumulation of abnormal desmin aggregates [27–29]. The severity of illness caused by the mutations located in the 2B alpha-helical domain may be explained by structural relationships these mutations potentially disrupt [22]. In accordance with these previous observations, a much more disabling disease was observed in the patient with the Leu392Pro mutation than in the patient with the Pro419Ser mutation.
In conclusion, this report adds to the definition of desminopathy phenotype by establishing a selective pattern of muscle involvement, the presence of prominent joint retraction at the ankles, and characteristic nasal speech early in the course of illness. Muscle imaging may be helpful in distinguishing desminopathy from other forms of MFM.
Acknowledgements
Supported by FIS Grant PI051213. We thank Dolores Moreno for her excellent technical assistance. We also thank Tom Yohanann for editorial advice.
References
- [1].Goldfarb L, Vicart P, Goebel HH, Dalakas MC. Desmin myopathy. Brain. 2004;127:723–34. doi: 10.1093/brain/awh033. [DOI] [PubMed] [Google Scholar]
- [2].Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980;283:249–56. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- [3].Li ZL, Colucci-Guyon E, Pincon-Raymond M, et al. Human desmin-coding gene: complete nucleotide sequence, characterization and regulation of expression during myogenesis and development. Gene. 1989;78:243–54. doi: 10.1016/0378-1119(89)90227-8. [DOI] [PubMed] [Google Scholar]
- [4].Fuchs E, Weber K, Cleveland DW. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–82. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- [5].Hermann H, Strelkov SV, Feja B, et al. The intermediate filament protein consensus motif of helix 2B: its atomic structure and contribution to assembly. J Mol Biol. 2000;298:817–32. doi: 10.1006/jmbi.2000.3719. [DOI] [PubMed] [Google Scholar]
- [6].Olivé M, Goldfarb L, Shatunov A, Fischer D, Ferrer I. Myotilinopathy: refining the clinical and myopathological phenotype. Brain. 2005;128:2315–26. doi: 10.1093/brain/awh576. [DOI] [PubMed] [Google Scholar]
- [7].van der Ven PFM, Obermann WMJ, Lemke B, Gautel M, Weber K, Fürtst DO. The characterization of mouse filamin isoforms suggest a possible role of gamma-filamin/ABP-L in sarcomeric Z-disc formation. Cell Motil Cytoskeleton. 2000;45:149–62. doi: 10.1002/(SICI)1097-0169(200002)45:2<149::AID-CM6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [8].Dagvadorj A, Olive M, Urtizberea JA, et al. A series of West European patients with severe cardiac and skeletal myopathy associated with a de novo R406W mutation in desmin. J Neurol. 2004;251:143–9. doi: 10.1007/s00415-004-0289-3. [DOI] [PubMed] [Google Scholar]
- [9].Kaminska A, Strelkov SV, Goudeau B, et al. Small deletions disturb desmin architecture leading to breakdown of muscle cells and development of skeletal or cardioskeletal myopathy. Hum Genet. 2004;114:306–13. doi: 10.1007/s00439-003-1057-7. [DOI] [PubMed] [Google Scholar]
- [10].Li D, Tapscoft T, Gonzalez O, et al. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100:461–4. doi: 10.1161/01.cir.100.5.461. [DOI] [PubMed] [Google Scholar]
- [11].Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail Rev. 2005;10:225–35. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- [12].Miyamoto Y, Akita H, Shiga N, et al. Frequency and clinical characteristics of dilated cardiomyopathy caused by desmin gene mutation in a Japanese population. Eur Heart J. 2001;22:2284–9. doi: 10.1053/euhj.2001.2836. [DOI] [PubMed] [Google Scholar]
- [13].Luethje LG, Boennemann C, Goldfarb L, Goebel HH, Halle M. Prophylactic implantable cardioverter defibrillator placement in a sporadic desmin related myopathy and cardiomyopathy. Pacing Clin Electrophysiol. 2004;27:559–60. doi: 10.1111/j.1540-8159.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- [14].Vrabie A, Goldfarb LG, Shatunov A, et al. The enlarging spectrum of desminopathies: new morphological findings, eastward geographic spread, novel exon 3 desmin mutation. Acta Neuropathol. 2005;109:411–7. doi: 10.1007/s00401-005-0980-1. [DOI] [PubMed] [Google Scholar]
- [15].Arbustini E, Pasotti M, Pilotto A, et al. Desmin accumulation restrictive cardiomyopathy and atrioventricular block associated with desmin gene defects. Eur J Heart Fail. 2006;8:477–83. doi: 10.1016/j.ejheart.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [16].Pruszczyk P, Kostera-Pruszczyk A, Shatunov A, et al. Restrictive cardiomyopathy with atrioventricular conduction block resulting from a desmin mutation. Int J Cardiol. 2006 Aug 2; doi: 10.1016/j.ijcard.2006.05.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [17].Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42:673–85. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lampe AK, Hoogendijk JE, Eagle M, et al. Bethlem myopathy, autosomal dominant and X-linked Emery-Dreifuss muscular dystrophy - comparison of contractural phenotypes. Neuromuscul Disord. 2005;15:729. [abstract] [Google Scholar]
- [19].Fardeau M, Godet-Guillain J, Tomé FMS, et al. Une nouvelle affection musculaire familiale, définie par l’accumulation intrasarcoplasmique d’un matériel granulofilamentaire dense en microscopie électronique. Rev Neurol. 1978;134:411–25. [PubMed] [Google Scholar]
- [20].Fardeau M, Vicart P, Caron A, et al. Myopathie familiale avec surcharge en desmine, sous forme de matériel granulo filamentaire dense en microscopie électronique, avec mutation dans le géne de l’αB-cristalline. Rev Neurol (Paris) 2000;156:497–504. [PubMed] [Google Scholar]
- [21].Pou A, Lloreta J, Corominas JM, Guicheney P. Miopatía familiar con sobrecarga de desmina, bajo forma de material gránulo-filamentoso denso en microscopía electrónica, sin mutación en el gen de la αB-cristalina. Neurología. 2001;16:195–203. [PubMed] [Google Scholar]
- [22].Dalakas MC, Dagvadorj A, Goudeau B, et al. Progressive skeletal myopathy, a phenotypic variant of desmin myopathy associated with desmin mutations. Neuromuscul Disord. 2003;13:252–8. doi: 10.1016/s0960-8966(02)00271-7. [DOI] [PubMed] [Google Scholar]
- [23].Selcen D, Ohno K, Engel AG. Myofibrillar myopathy: clinical, morphological and genetic studies in 63 patients. Brain. 2004;127:439–51. doi: 10.1093/brain/awh052. [DOI] [PubMed] [Google Scholar]
- [24].Muntoni F, Bonne G, Goldfarb LG, et al. Disease severity in dominant Emery Dreifuss is increased by mutations in both emerin and desmin proteins. Brain. 2006;129:1260–8. doi: 10.1093/brain/awl062. [DOI] [PubMed] [Google Scholar]
- [25].Bar H, Goudeau B, Walde S, et al. Conspicuous involvement of desmin tail mutations in diverse cardiac and skeletal myopathies. Hum Mutat. 2007 Jan 12; doi: 10.1002/humu.20459. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [26].Rogers KR, Eckelt A, Nimmrich V, et al. Truncation mutagenesis of the non-alpha-helical carboxyterminal tail domain of vimentin reveals contributions to cellular localization but not to filament assembly. Eur J Cell Biol. 1995;66:136–50. [PubMed] [Google Scholar]
- [27].Muñoz-Mármol AM, Strasser G, Isamat M, et al. A dysfunctional desmin mutation in a patient with severe generalised myopathy. Proc Natl Acad Sci USA. 1998;95:11312–7. doi: 10.1073/pnas.95.19.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sjöberg G, Saavedra-Matiz CA, Rosen DR, et al. A missense mutation in the desmin rod domain is associated with autosomal dominant distal myopathy, and exerts a dominant negative effect on filament formation. Hum Mol Genet. 1999;8:2191–8. doi: 10.1093/hmg/8.12.2191. [DOI] [PubMed] [Google Scholar]
- [29].Goudeau B, Rodrigues-Lima F, Fischer D, et al. Variable pathogenic potentials of mutations located in the desmin alpha-helical domain. Hum Mutat. 2006;27:906–13. doi: 10.1002/humu.20351. [DOI] [PubMed] [Google Scholar]