Abstract
Two important response criteria in systemic mastocytosis (SM) are the elimination or reduction in percentage of bone marrow mast cells (MCs) and the reduction of serum tryptase levels. We investigated the accuracy of a single time point reduction of bone marrow MCs and serum tryptase level as response criteria in 50 patients with SM with available serial assessments. Bone marrow MC percentage varied significantly, with an average coefficient of variation (CV) of 65% (range, 6–173%) and 44% of patients having a CV higher than the average. The average CV for serum tryptase level was 19% (range, 0–96%), with 36% of patients having a CV higher than average. These wide variations in bone marrow MC burden and serum tryptase level were independent of the administration of SM-directed therapy, type of therapy or disease subtype. Furthermore, the achievement of a single time point therapy-induced bone marrow complete response (no histological evidence of malignant bone marrow MCs) did not correlate with tryptase level or symptomatic improvement. In conclusion, the value of single measurements of bone marrow MC percentage and serum tryptase level as response criteria in SM is not supported by clinical data. Incorporation of an assessment of the degree in reduction of MCs and tryptase, and assessment of response durability, would make response criteria more clinically meaningful.
Keywords: Systemic mastocytosis, mast cell burden, tryptase, response criteria
Introduction
Systemic mastocytosis (SM) is characterized by a clonal proliferation of mast cells (MCs) that infiltrate the bone marrow, spleen and/or other extracutaneous organs [1,2]. Both indolent and aggressive variants of the disease have been described [3]. Clinically, SM presents with bone marrow infiltration with or without cutaneous or visceral involvement and/or mediator-related symptoms (skin rash, gastrointestinal symptoms, syncope, anaphylaxis, osteoporosis) [1,2]. ASM is characterized by the presence of signs of organ dysfunction caused by MC infiltration. The 2008 World Health Organization (WHO) classification system subdivided mastocytosis into several categories including cutaneous mastocytosis, extracutaneous mastocytosis, mast cell sarcoma and SM, which is the most frequently diagnosed mast cell disorder in adults [4]. SM can be further categorized as indolent (ISM), which includes two provisional subvariants: isolated bone marrow mastocytosis (IMM) and smoldering SM (SSM), SM associated with another clonal hematological non-mast cell lineage disease (SM-AHNMD), aggressive SM (ASM) and mast cell leukemia.
The diagnosis of SM is based on diagnostic criteria issued by the WHO in 2001 [5], which ultimately rely on the demonstration of neoplastic spindle-shaped CD2 and/or CD25-positive MC tissue infiltration, typically involving the bone marrow in adults [6–8]. Patients with SM frequently exhibit elevated levels of mediators released by MCs such as serum tryptase and urinary histamine, and they commonly carry the KITD816V mutation [4,9]. However, several caveats to the WHO diagnostic criteria must be noted. For instance, an aberrant immunophenotype and atypical mast cell morphology are almost universally present in SM, but they are only considered minor criteria. In addition, a persistently elevated serum tryptase level (≥ 20 ng/mL), which is a minor diagnostic criterion, is present in only 85% of patients, its diagnostic specificity is limited by the presence of high tryptase levels in other myeloid malignancies or in severe allergic reactions [10], its reliability is very poor in patients with SM-AHNMD, and the cut-off of 20 ng/mL has been established based on retrospective studies involving a limited number of patients [11]. Once a diagnosis of SM is made, patients with this malignancy are further categorized according to the presence of “B- and C-findings,” which are surrogates of disease burden and aggressiveness, respectively. Patients with SM with no findings are categorized as ISM, those with B-findings are considered as having SSM, a subtype of ISM with a more aggressive clinical course, and those with C-findings are best classified as ASM and are candidates for immediate therapeutic intervention.
Current therapy for patients with ASM includes cytoreductive agents such as hydroxyurea, cladribine and interferon-α as well as targeted agents such as imatinib, dasatinib or midostaurin. However, the prognosis of patients with SM remains poor [12]. Several criteria are used to assess response to ASM-directed therapy, based on a consensus statement set forth by a panel of experts [13,14]. Two important criteria are the elimination or reduction in percentage of bone marrow MCs, and the reduction of serum tryptase levels. Tryptase is a neutral serine protease concentrated in MCs [15,16], whose levels have been advocated as a reliable marker of MC activation [17]. Current response criteria do not include an assessment of the degree of reduction in these two markers, nor the durability of a response, but a single time point assessment of the elimination or “reduction” in these two parameters. We contend that these two metrics, as currently used, may not be accurate markers of response.
Design and methods
We studied 50 patients with SM diagnosed and treated at M. D. Anderson Cancer Center from May 2001 to January 2009 for whom at least two sequential good-quality bone marrow biopsies (n = 48) and at least two serum tryptase level determinations (n = 50) were available. Serum tryptase levels were assessed using a sandwich enzyme-linked immunosorbent assay (ELISA), which measures both the alpha and beta tryptase isoforms (detection range, 0–200 ng/mL) [18]. Bone marrow MC burden, expressed as the percentage of MC involvement with respect to the total marrow cellularity, was determined by standard pathological examination of trephine specimens and by immunohistochemical staining for CD25 (soluble interleukin-2 receptor alpha chain) and tryptase. This was done by expert hematopathologists in the Hematopathology Department at M. D. Anderson, as a part of routine clinical practice. The coefficient of variation (CV, i.e. the ratio of the standard deviation to the mean) was used as a normalized measure of dispersion of a probability distribution for the percentage of MCs in bone marrow biopsies and serum tryptase levels. The study was conducted in compliance with an Institutional Review Board-approved chart review protocol.
Results
Patient characteristics
Patient and disease characteristics are presented in Table I. Patients had a diagnosis of ISM (n = 25), ASM (n = 16) or SM-AHNMD (n = 9). All but one patient received SM-directed therapy (median number of therapies 2, range 1–5), including: imatinib (n = 16), dasatinib (n = 23), everolimus (n = 8) or denileukin diftitox (n = 7). No patient received interferon or cladribine. All patients underwent at least two bone marrow biopsies. The median number of bone marrow biopsies available per patient was 4 (range, 2–14), and the median number of tryptase measurements was 6 (range, 2–18); they were obtained both on and off SM-directed therapy. The frequency of the KITD816V mutation was relatively low at 28%, which may reflect the high proportion of patients with < 10% marrow mast cells (88%).
Table I.
Patient characteristics at first presentation to M. D. Anderson Cancer Center.
| Parameter | Number (n = 50) | Percentage | |
|---|---|---|---|
| Age (median, range) | 54 (29–78) | ||
| Sex | Male | 19 | 38 |
| Type of SM | ISM | 25 | 50 |
| ASM | 16 | 32 | |
| SM–AHNMD | 9 | 18 | |
| Hemoglobin (median, range) | 12.95 (9.2–15.6) | ||
| WBC (median, range) | 6.3 (2.3–49) | ||
| Platelets (median, range) | 246 (44–885) | ||
| Tryptase (median, range) | 55 (2–200) | ||
| Albumin (median, range) | 4.4 (2.4–5.2) | ||
| ALT (median, range) | 17.5 (11–82) | ||
| KITD816V mutation | Detected | 14 | 28 |
| Not detected | 14 | 28 | |
| ND | 22 | 44 | |
| B symptoms | Yes | 25 | 50 |
| No | 25 | 50 | |
| Cutaneous symptoms | Yes | 22 | 44 |
| No | 28 | 56 | |
| Splenomegaly | Yes | 5 | 10 |
| No | 44 | 88 | |
| Splenectomy | 1 | 2 | |
| Lymphadenopathy | 2 | 4 | |
| Cytogenetics | Diploid | 40 | 80 |
| Abnormal | 6 | 12 | |
| Insufficient metaphases | 4 | 8 | |
| Bone marrow cellularity | Hypocellular (< 30%) | 9 | 18 |
| Hypercellular (> 70%) | 10 | 20 | |
| Normocellular (30–70%) | 28 | 56 | |
| ND | 3 | 6 | |
| BM mast cell % | < 10 | 44 | 88 |
| 10–30 | 4 | 8 | |
| > 30 | 2 | 4 | |
| BM blast % | < 5 | 50 | 100 |
| 5–10 | 0 | 0 | |
| > 10 | 0 | 0 | |
| BM eosinophil % (median, range) | 3 (0–22) |
SM, systemic mastocytosis; ISM, indolent systemic mastocytosis; ASM, aggressive systemic mastocytosis; SM-AHNMD, SM associated with another clonal hematological non-mast cell lineage disease; WBC, white blood cells; ALT, alanine aminotransferase; ND, not done; BM, bone marrow.
Variability of serum tryptase and bone marrow MC burden
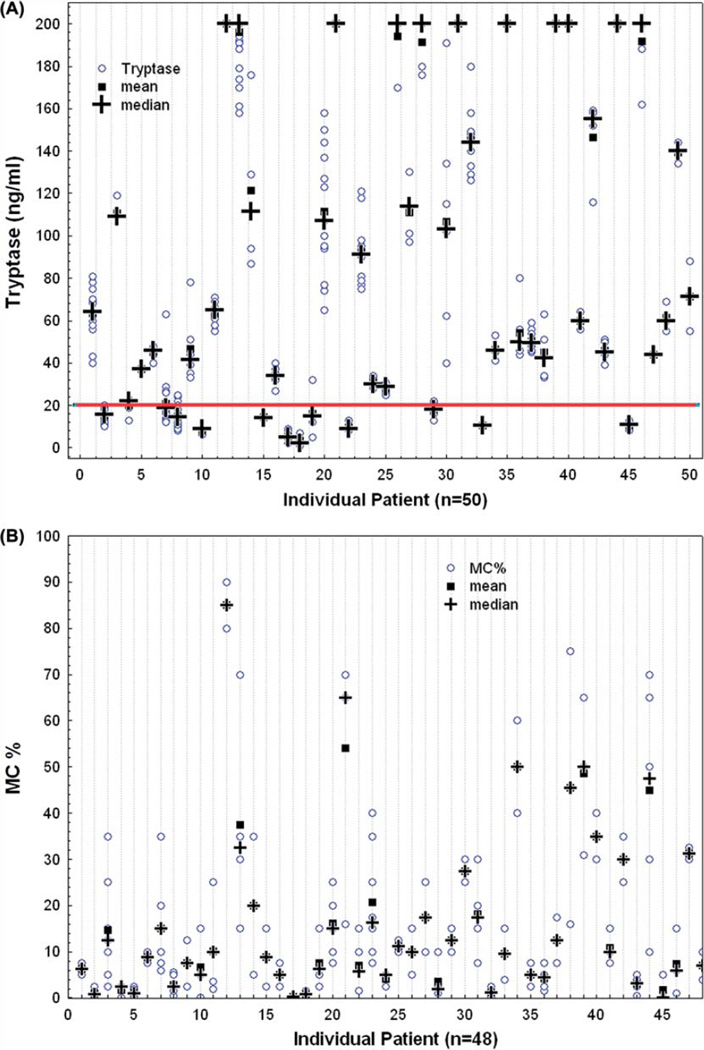
In order to evaluate the variability of serum tryptase levels and bone marrow MC percentage from baseline (first recorded value) during the course of therapy, we used the CV, a normalized measure of dispersion [Figures 1(A) and 1(B)]. Among the 50 patients, the percentage of bone marrow MCs in trephine biopsies varied significantly, with an average CV of 65% (range, 6–173%). Notably, 44% of patients had a CV equal to or higher than the average. Similar results were observed regarding tryptase levels, with an average CV of 19% (range, 0–96%). Thirty-six percent of patients had a CV higher than average. The variations in tryptase levels might have been even broader were it not for the limit of sensitivity of the ELISA test employed, which was established at 200 ng/mL: any value greater than 200 ng/mL was automatically off set at 200 ng/mL. These wide variations in single time point measurements of bone marrow MC burden and serum tryptase levels were observed both in the presence and in the absence of SM-directed therapy, or in the presence of different therapies, indicating that they may vary significantly irrespective of the anti-SM activity of current therapies, as single time point variations were seen both in patients failing to respond to therapy and in a few evaluable responders. Finally, variability in single time point measurements was not influenced by the heterogeneity of disease subtypes.
Figure 1.
Scatter plots illustrating intra-patient variability of serum tryptase levels (A) and bone marrow mast cell burden (B).
Analysis of histological complete responders
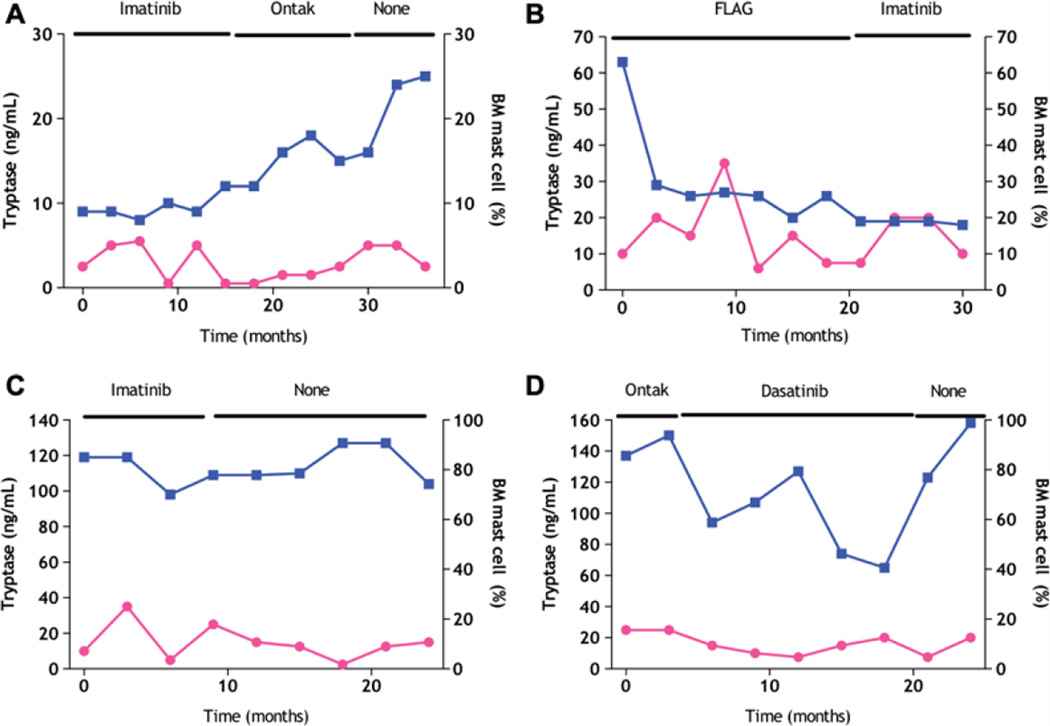
Bone marrow pathological complete responses (CRs; i.e. no evidence of malignant MCs in the bone marrow by immunohistochemistry and flow cytometry) can be observed sporadically in patients with SM receiving therapy, particularly in patients with low bone marrow MC involvement. The clinical relevance of such single time point bone marrow CRs is questionable. For instance, in our cohort, three patients with SM achieved a bone marrow CR. Patient 1 achieved resolution of all SM-related symptoms on dasatinib 70 mg twice daily. Among eight bone marrow biopsies done during the therapy, one showed a bone marrow CR; all others had less than 5% MC involvement. At the time of pathological bone marrow CR, the serum tryptase level only modestly decreased from a baseline level of 56 ng/dL to 46.6 ng/dL. Patient 2 achieved a bone marrow CR on dasatinib 70 mg twice daily (initially MC involvement < 5%). However, this patient never had symptomatic improvement and the baseline cutaneous lesions persisted. Patient 3 had a diagnosis of SM-AHNMD (myelodysplastic syndrome) with 5% MCs, but improved symptomatically and achieved a complete pathological bone marrow CR (with no evidence of MDS and MCs) on decitabine. However, decitabine therapy did not change serum tryptase levels (44 ng/dL to 45 ng/dL). Thus, in spite of these pathological single time point bone marrow CRs, the levels of serum tryptase did not vary significantly, and an improvement in symptoms was not always observed. These data suggest that in addition to the less than perfect correlation of serum tryptase levels and MC burden in patients with SM, measurement of the former may be hampered by inherent technical problems, as previously recognized [19]. In addition, patients who fail to respond to different therapies may exhibit improvement or worsening in tryptase levels and MC burden [Figures 2(A)–2(D)].
Figure 2.
Representative examples of variation in serum tryptase (□) and bone marrow mast cell (●) burden in patients with aggressive systemic mastocytosis not responding to mastocytosis-directed therapy. FLAG denotes fludarabine, ara-C, G-CSF.
Discussion
Current response criteria for patients with ASM are based on the recommendations set forth by the consensus group [13,14], which involve the assessment of improvement of B/C-findings (MC marrow infiltrates, serum tryptase level, cytopenias, hepatomegaly with ascites and impaired liver function, splenomegaly with hypersplenism, malabsorption with hypoalbuminuria and weight loss, skeletal lesions) [13,14]. Three types of response are typified: major (complete disappearance of at least one C-finding and no progression in other C-findings), partial (incomplete disappearance of one or more C-findings) and no response. A major response is further categorized as complete (elimination of MC infiltrates and decrease of serum tryptase level to < 20 ng/mL), incomplete (decrease of both MC infiltrates and serum tryptase level) and pure clinical response (no decrease of MC infiltrates and tryptase) [13,14]. Thus, response in ASM is a markedly fragmented concept in that single time point improvement (“incomplete disappearance” or “decrease”) or single time point resolution of isolated signs or symptoms of the disease suffice to fulfill the criteria for specific response categories.
MC-derived mediators such as tryptase are frequently elevated in urine and/or blood in patients with SM. A serum tryptase level ≥ 20 ng/mL is a minor criterion for the diagnosis of SM. However, the use of single time point serum tryptase level as a response criterion in SM is hampered by several factors. First, in our experience, a serum tryptase level ≥ 20 ng/mL is detected in only 85% of patients with SM [10]. Second, tryptase levels can also be markedly elevated in patients with severe allergic reactions, and, in patients with SM, can be elevated above baseline by triggers of acute mast cell degranulation [10]. Some have indeed suggested alternative and perhaps more accurate measures of MC burden, such as soluble KIT and soluble CD25 levels. They have been reported to correlate with bone marrow involvement and therefore could be proposed as surrogate markers of disease severity, aid in diagnosis, and be used in documenting disease progression, and monitoring response to SM-directed therapy [19].
It is worth emphasizing several aspects regarding response criteria in SM. First, well-defined criteria for several WHO-defined categories of SM (for instance ISM) are not available. Second, the frequent lack of connection between C-findings, tryptase levels and bone marrow MC infiltrates must be recognized. Frequently, improvement or even resolution of one of these factors is accompanied by no change in any of the other parameters, as illustrated in our patients, among which three who achieved a bone marrow CR experienced no improvement in serum tryptase levels and/or disease-specific symptoms. The possibility of sampling bias in these three cases of bone marrow CR due to patchy MC infiltration cannot be ruled out. Importantly, most patients included in this study had a relatively low marrow mast cell burden, which might limit the extension of our conclusions to patients with high mast cell marrow burden. Alternatively, the assumption is customarily made that the bone marrow MC burden and tryptase level should correlate, when in fact the total body MC burden, including skin, could be more important than the bone marrow MC burden. In fact, bone marrow changes did not correlate with skin involvement in our series. A factor that might potentially influence the wide variability of tryptase level and bone marrow MC burden in our patient cohort is the absence of a uniform treatment for all patients studied. The counter argument to this proposition is the fact that when subsets of patients treated with the same therapy were analyzed (data not shown), similar results were observed. In other words, whether patients were on therapy or not, or on different therapies, the results were similar. Third, although a decrease or normalization of serum tryptase levels and a decrease or resolution of MC infiltrates have been sanctioned as important response criteria, there are no data supporting that such single time point improvements are associated with improved clinical outcomes. In this regard, we observed that serum tryptase levels vary significantly at different time points when measured in the same patient in the absence of intervention, which may reflect time-dependent variability in the extent of MC activation. Alternatively, triggers of acute MC degranulation might be partly responsible for such variability. Thus, tryptase release could reflect a reaction to environmental stimuli and/or disease-associated fluctuations and/or SM therapy. Tryptase levels can also vary in patients with SM-AHNMD in concert with progression or response to therapy of the associated hematological malignancy. Furthermore, the lack of correlation between tryptase levels and MC burden may be partly due to important marrow sampling bias given the patchy MC infiltration observed in many cases of SM. Finally, it must be noted that a shortcoming of the test used to monitor tryptase levels is its upper limit of detection set at 200 ng/mL, which makes the monitoring of patients with tryptase elevations well beyond such a limit of detection difficult. In such cases, dilution methods should be used to more accurately quantify baseline and serial serum tryptase levels.
In conclusion, albeit appealing from a practical point of view, the use of single time point improvement in bone marrow MC percentage and serum tryptase levels as response criteria in SM is limited by the fact that their prognostic value is not supported by clinical data, the correlation with each other and SM-related symptoms is poor, and their determination is subject to multiple biases. While most patients fail to respond to currently available SM-directed therapies, for those who do respond, more clinically meaningful and reliable response criteria are warranted. We suggest starting with the incorporation of an assessment of the degree in decrease/improvement, and assessment of the durability of response. With these simple additions to response assessment, potentially clinically relevant improvements would be properly recorded.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Valent P, Akin C, Sperr WR, et al. Mastocytosis: pathology, genetics, and current options for therapy. Leuk Lymphoma. 2005;46:35–48. doi: 10.1080/10428190400010775. [DOI] [PubMed] [Google Scholar]
- 2.Pardanani A. Systemic mastocytosis: bone marrow pathology, classification, and current therapies. Acta Haematol. 2005;114:41–51. doi: 10.1159/000085561. [DOI] [PubMed] [Google Scholar]
- 3.Valent P, Horny HP, Li CY, et al. Mastocytosis (mast cell disease) In: Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization (WHO) classification of tumors: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 291–302. [Google Scholar]
- 4.Horny H, Metcalfe DD, Bennett JM, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumors of hematopoietic and lymphoid tissues. 4th. Lyon: IARC; 2008. pp. 54–63. [Google Scholar]
- 5.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 6.Akin C. Molecular diagnosis of mast cell disorders: a paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn. 2006;8:412–419. doi: 10.2353/jmoldx.2006.060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens EC, Rosenthal NS. Bone marrow mast cell morphologic features and hematopoietic dyspoiesis in mast cell disease. Am J Clin Pathol. 2001;116:177–182. doi: 10.1309/Q2WJ-46CL-YRFT-M5JF. [DOI] [PubMed] [Google Scholar]
- 8.Pardanani A, Kimlinger T, Reeder T, et al. Bone marrow mast cell immunophenotyping in adults with mast cell disease: a prospective study of 33 patients. Leuk Res. 2004;28:777–783. doi: 10.1016/j.leukres.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Valent P, Akin C, Metcalfe DD. FIP1L1/PDGFRA is a molecular marker of chronic eosinophilic leukaemia but not for systemic mastocytosis. Eur J Clin Invest. 2007;37:153–154. doi: 10.1111/j.1365-2362.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- 10.Tefferi A, Verstovsek S, Pardanani A. How we diagnose and treat WHO-defined systemic mastocytosis in adults. Haematologica. 2008;93:6–9. doi: 10.3324/haematol.12324. [DOI] [PubMed] [Google Scholar]
- 11.Quintas-Cardama A, Jain N, Verstovsek S. Advances and controversies in the diagnosis, pathogenesis, and treatment of systemic mastocytosis. Cancer. 2011;117:5439–5449. doi: 10.1002/cncr.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennessy B, Giles F, Cortes J, et al. Management of patients with systemic mastocytosis: review of M. D. Anderson Cancer Center experience. Am J Hematol. 2004;77:209–214. doi: 10.1002/ajh.20211. [DOI] [PubMed] [Google Scholar]
- 13.Valent P, Akin C, Sperr WR, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27:635–641. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 14.Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–453. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 15.Irani AA, Schechter NM, Craig SS, et al. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz LB, Irani AM, Roller K, et al. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611–2615. [PubMed] [Google Scholar]
- 17.He S, Gaca MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- 18.Vega-Ruiz A, Cortes JE, Sever M, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33:1481–1484. doi: 10.1016/j.leukres.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akin C, Schwartz LB, Kitoh T, et al. Soluble stem cell factor receptor (CD117) and IL-2 receptor alpha chain (CD25) levels in the plasma of patients with mastocytosis: relationships to disease severity and bone marrow pathology. Blood. 2000;96:1267–1273. [PubMed] [Google Scholar]